The Critical Role of Vitamin D Supplementation for Skeletal and Neurodevelopmental Outcomes in Preterm Neonates
Abstract
1. Introduction
2. Organization of the Review
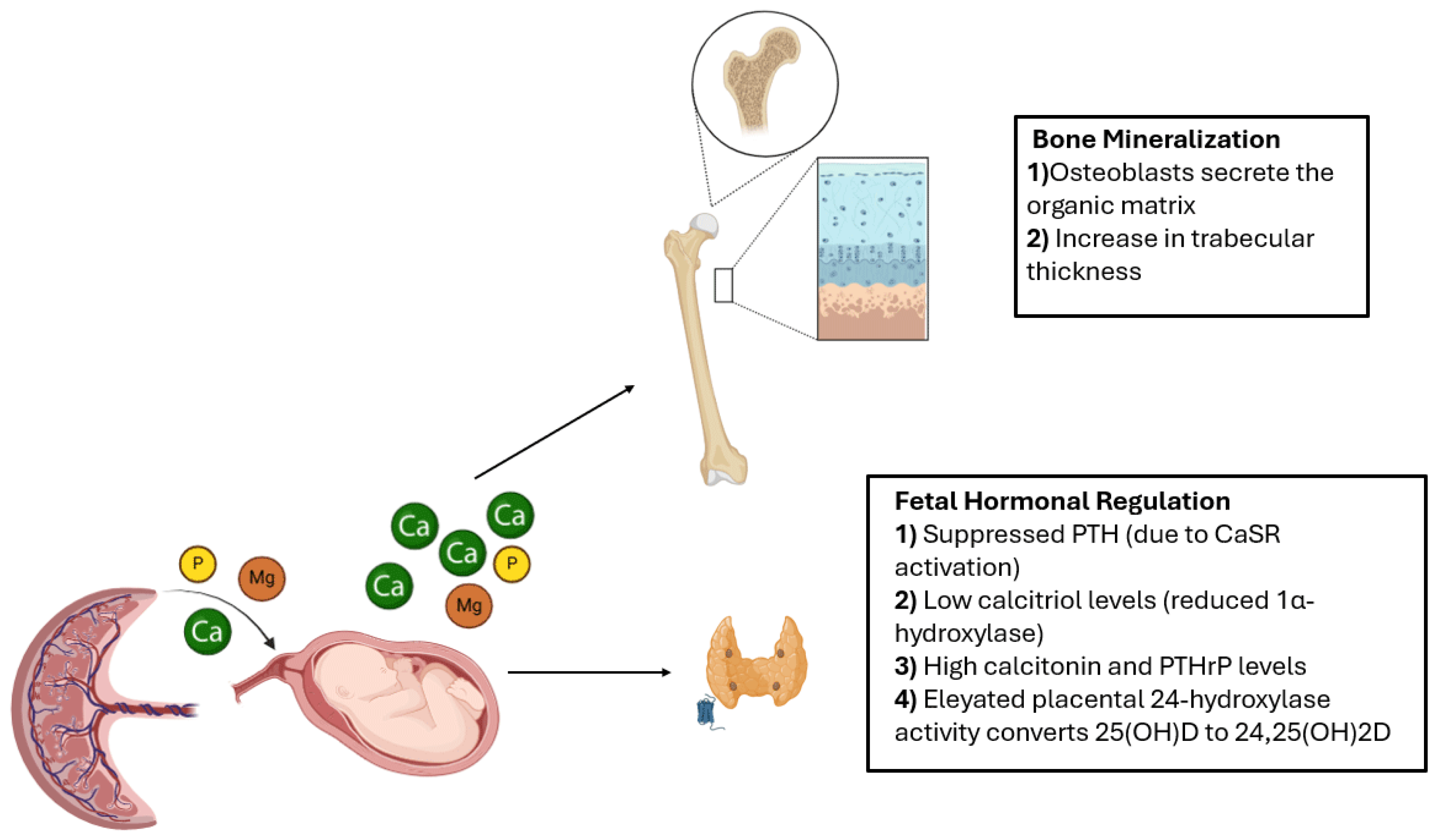
3. Pathophysiology and Risk Factors of MBDP
4. Early Diagnostic Biomarkers and Predictive Models
5. Preventive Supplementation Strategies
6. Neurological Effects of Osteopenia in Preterm Infants and Preventive Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MBDP | Metabolic Bone Disease of Prematurity |
| NEC | Necrotizing Enterocolitis |
| BPD | Bronchopulmonary Dysplasia |
| NICU | Neonatal Intensive Care Unit |
| QUS | Quantitative Ultrasound |
| VLBW | Very Low Birth Weight |
| ESPGHAN | European Society for Paediatric Gastroenterology, Hepatology, and Nutrition |
| Ca | Calcium |
| P | Phosphorus |
| ALP | Alkaline Phosphatase |
| PTH | Parathyroid Hormone |
| FGF23 | Fibroblast Growth Factor-23 |
| TRP | Tubular Reabsorption of Phosphate |
| BIA | Bioelectrical Impedance Analysis |
| TPN | Total Parenteral Nutrition |
| SMA | Spinal Muscular Atrophy |
| BSID | Bayley Scales of Infant Development |
| MDI | Mental Development Index |
| PDI | Psychomotor Development Index |
| VDD | Vitamin D Deficiency |
| 25(OH)D | 25-Hydroxyvitamin D |
| IU | International Units |
| D2 | Ergocalciferol |
| D3 | Cholecalciferol |
| DPDIA3 | Protein Disulfide Isomerase A3 |
| CNS | Central Nervous System |
| MPS | Mucopolysaccharidosis |
| DBP | Vitamin D Binding Protein |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| EEG | Electroencephalogram |
| MRI | Magnetic resonance Imaging |
| B-ALP | Bone-Specific Alkaline Phosphatase |
| PTHrP | Parathyroid Hormone-Related Protein |
| CaSR | Calcium-Sensing Receptor |
| Mg | Magnesium |
References
- Rehman, M.U.; Narchi, H. Metabolic bone disease in the preterm infant: Current state and future directions. World J. Methodol. 2015, 5, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, M.; Lecoq, L.; Wachter, P.-Y.; Durandy, A.; Boileau, P.; Motte-Signoret, E. Early Diagnosis of Ongoing Metabolic Bone Disease of Prematurity with Elevated Serum Alkaline Phosphatase. Indian J. Pediatr. 2023, 90, 305. [Google Scholar] [CrossRef] [PubMed]
- Groves, H.T.; Cuthbertson, L.; James, P.; Moffatt, M.F.; Cox, M.J.; Tregoning, J.S. Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota. Front. Immunol. 2018, 9, 182. [Google Scholar] [CrossRef]
- Dos Santos, E.S.R.; dos Santos Silveira, R.D.C.; Pérsico, R.S.; Escovar, L.R.; Nery, M.B.R.; Schneider, M.A.O.; de Mello, P.P.; Viana, L.V. Metabolic bone disease in premature infants receiving parenteral nutrition: Incidence, clinical, laboratory and nutritional profile. Early Hum. Dev. 2025, 200, 106153. [Google Scholar] [CrossRef]
- Takada, M.; Shimada, M.; Hosono, S.; Tauchi, M.; Minato, S.; Takahashi, M.; Okuni, S.; Takeuchi, S. Trace elements and mineral requirements for very low birth weight infants in rickets of prematurity. Early Hum. Dev. 1992, 29, 333–338. [Google Scholar] [CrossRef]
- Chen, W.; Yang, C.; Chen, H.; Zhang, B. Risk factors analysis and prevention of metabolic bone disease of prematurity. Medicine 2018, 97, e12861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosseinzadeh, V.; Rad, E.M.; Alirezaee, A. A case report of osteopenia of prematurity. Radiol. Case Rep. 2024, 19, 6369–6372. [Google Scholar] [CrossRef]
- Viswanathan, S.; Khasawneh, W.; McNelis, K.; Dykstra, C.; Amstadt, R.; Super, D.M.; Groh-Wargo, S.; Kumar, D. Metabolic Bone Disease: A continued challenge in extremely low birth weight infants. J. Parenter. Enter. Nutr. 2014, 38, 982–990. [Google Scholar] [CrossRef]
- Faienza, M.F.; D’Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D’Amato, G. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front. Pediatr. 2019, 7, 143. [Google Scholar] [CrossRef]
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity—National survey of current neonatal and paediatric endocrine approaches. Acta Paediatr. 2021, 110, 1855–1862. [Google Scholar] [CrossRef]
- Fewtrell, M.S.D.; Prentice, A.; Jones, S.C.; Bishop, N.J.; Stirling, D.; Buffenstein, R.; Lunt, M.; Cole, T.J.; Lucas, A. Bone mineralization and turnover in preterm infants at 8–12 years of age: The effect of early diet. J. Bone Miner. Res. 1999, 14, 810–820. [Google Scholar] [CrossRef] [PubMed]
- García-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 2468–2481. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chang, Y.-J.; Chen, L.-J.; Lee, C.-H.; Hsiao, C.-C.; Chen, J.-Y.; Chen, H.-N. Neurodevelopment Outcomes in Very-Low-Birth-Weight Infants with Metabolic Bone Disease at 2 Years of Age. Children 2024, 11, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, L.; Zhuo, R.; Zhu, H.; Xie, Q.; Yang, M.; Liu, Y.; Lin, J. Establishment of a nomogram model for predicting metabolic bone disease in preterm infants: A case–control study. Eur. J. Pediatr. 2023, 182, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Bone Development and Mineral Homeostasis in the Fetus and Neonate: Roles of the Calciotropic and Phosphotropic Hormones. Physiol. Rev. 2014, 94, 1143–1218. [Google Scholar] [CrossRef]
- Brunetti, G.; Tummolo, A.; D’Amato, G.; Gaeta, A.; Ortolani, F.; Piacente, L.; Giordano, P.; Colucci, S.; Grano, M.; Papadia, F.; et al. Mechanisms of Enhanced Osteoclastogenesis in Alkaptonuria. Am. J. Pathol. 2018, 188, 1059–1068. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum. Dev. 2015, 91, 623–628. [Google Scholar] [CrossRef]
- Wagner, C.L.; McNeil, R.B.; Johnson, D.D.; Hulsey, T.C.; Ebeling, M.; Robinson, C.; Hamilton, S.A.; Hollis, B.W. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. J. Steroid Biochem. Mol. Biol. 2013, 136, 313–320. [Google Scholar] [CrossRef]
- von Websky, K.; Hasan, A.A.; Reichetzeder, C.; Tsuprykov, O.; Hocher, B. Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J. Steroid Biochem. Mol. Biol. 2018, 180, 51–64. [Google Scholar] [CrossRef]
- Funke, S.; Morava, É.; Czakó, M.; Vida, G.; Ertl, T.; Kosztolányi, G. Influence of genetic polymorphisms on bone disease of preterm infants. Pediatr. Res. 2006, 60, 607–612. [Google Scholar] [CrossRef]
- Senterre, J.; Salle, B. Renal aspects of calcium and phosphorus metabolism in preterm infants. Neonatology 1988, 53, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Rustico, S.E.; Calabria, A.C.; Garber, S.J. Metabolic bone disease of prematurity. J. Clin. Transl. Endocrinol. 2014, 1, 85–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamkin, D.; Radmacher, P. Current trends and future challenges in neonatal parenteral nutrition. J. Neonatal-Perinat. Med. 2014, 7, 157–164. [Google Scholar] [CrossRef]
- Chin, L.K.; Doan, J.; Teoh, Y.S.; Stewart, A.; Forrest, P.; Simm, P.J. Outcomes of standardised approach to metabolic bone disease of prematurity. J. Paediatr. Child Health 2018, 54, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, F.B.; Mandel, D.; Lubetzky, R.; Senterre, T. Calcium, phosphorus, magnesium and vitamin D requirements of the preterm infant. World Rev. Nutr. Diet. 2014, 110, 140–151. [Google Scholar]
- Ukarapong, S.; Venkatarayappa, S.K.B.; Navarrete, C.; Berkovitz, G. Risk factors of metabolic bone disease of prematurity. Early Hum. Dev. 2017, 112, 29–34. [Google Scholar] [CrossRef]
- Lee, S.M.; Namgung, R.; Park, M.S.; Eun, H.S.; Park, K.I.; Lee, C. High incidence of rickets in extremely low birth weight infants with severe parenteral nutrition-associated cholestasis and bronchopulmonary dysplasia. J. Korean Med. Sci. 2012, 27, 1552–1555. [Google Scholar] [CrossRef]
- Okumura, A.; Hayakawa, M.; Arai, H.; Maruo, Y.; Kusaka, T.; Kunikata, T.; Iwatani, S.; Sato, Y.; Morioka, I. Clinical factors related to bilirubin encephalopathy in preterm infants: A case-control study. Brain Dev. 2025, 47, 104342. [Google Scholar] [CrossRef]
- Yeh, J.K.; Liu, C.C.; Aloia, J.F. Effects of exercise and immobilization on bone formation and resorption in young rats. Am. J. Physiol. Metab. 1993, 264 Pt 1, E182–E189. [Google Scholar] [CrossRef]
- Giovanni, V. Manuale di Neonatologia. Guida Alla Buona Pratica Clinica; Capitolo 11, «Osteopenia della prematurità»; Edizioni Scientifiche Falco: Cosenza, Italy, 2024; Volume 1, pp. 577–579. [Google Scholar]
- Hung, Y.; Chen, P.; Jeng, S.; Hsieh, C.; Peng, S.S.; Yen, R.; Chou, H.; Chen, C.; Tsao, P.; Hsieh, W. Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J. Paediatr. Child Health 2011, 47, 134–139. [Google Scholar] [CrossRef]
- Backström, M.C.; Kouri, T.; Kuusela, A.L.; Sievänen, H.; Koivisto, A.M.; Ikonen, R.S.; Mäki, M. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr. 2000, 89, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Lü, K.-L.; Xie, S.-S.; Hu, Q.; Yang, Z.-Y.; Fan, Q.-L.; Liu, E.; Zhang, Y.-P. Diagnostic markers of metabolic bone disease of prematurity in preterm infants. Bone 2023, 169, 116656. [Google Scholar] [CrossRef]
- Costa, R.; Franco, C.; Santos, N.; Maio, P.; Vieira, F.; Antunes, S.; Martins, L.; Tuna, M.L. Metabolic Bone Disease of Prematurity in Very Low Birthweight Infants: Retrospective Observational Study. Acta Medica Port. 2019, 32, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Pelayo, S.; Docio, P.; Arriola, S.; Lavín-Gómez, B.A.; García-Unzueta, M.T.; Ballesteros, M.Á.; Cabero-Pérez, M.J.; González-Lamuño, D. Role of fibroblast growth factor-23 as an early marker of metabolic bone disease of prematurity. BMC Pediatr. 2024, 24, 418. [Google Scholar] [CrossRef]
- McCarter, D.L.; Morgan, C.; Bray, L.; Tume, L. How is bioelectrical impedance used in neonatal intensive care? A scoping review. Eur. J. Pediatr. 2024, 183, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.C.d.S.; Kuhl, A.M.; Coradine, A.V.P.; Rabito, E.I.; Sarquis, A.L. Application of Bioelectrical Impedance in Newborns: An Integrative Review. Nutr Hosp [Internet]. 2023. Available online: https://www.nutricionhospitalaria.org/articles/04365/show (accessed on 7 February 2025).
- Tortorella, C.C.D.S.; Chao, B.M.P.; Rabito, E.I.; Lima, M.N.; Sarquis, A.L.F. Bioelectrical Impedance in Premature Newborns and Its Relationship with Diet Therapy in a Neonatal Intensive Care Unit. Nutrients 2024, 16, 601. [Google Scholar] [CrossRef]
- Dung, N.Q.; Fusch, G.; Armbrust, S.; Jochum, F.; Fusch, C. Body composition of preterm infants measured during the first months of life: Bioelectrical impedance provides insignificant additional information compared to anthropometry alone. Eur. J. Pediatr. 2007, 166, 215–222. [Google Scholar] [CrossRef]
- Núñez-Ramos, R.; Escuder-Vieco, D.; Cruz, C.R.; De Diego-Poncela, C.; Vázquez-Román, S.; Germán-Díaz, M.; García-Lara, N.R.; Pallás-Alonso, C. Bioelectrical Impedance Vector Analysis in Extremely Low-Birth-Weight Infants to Assess Nutritional Status: Breakthroughs and Insights. Nutrients 2024, 16, 4348. [Google Scholar] [CrossRef]
- Peng, J.; Yang, J.; Li, F. Association of bioimpedance analysis parameters trajectories with clinical outcomes in neurocritical patients. Heliyon 2024, 10, e32948. [Google Scholar] [CrossRef]
- Ito, Y.; Ito, T.; Narahara, S.; Sugiura, H.; Sugiyama, Y.; Hattori, T.; Kidokoro, H.; Tsuji, T.; Kubota, T.; Natsume, J.; et al. Body composition and motor function in children born large for gestational age at term. Pediatr. Res. 2024, 96, 1030–1036. [Google Scholar] [CrossRef]
- Xu, X.; Ma, H.; Cheng, S.; Xue, J. Effect of early preventive supplementation with calcium and phosphorus on metabolic bone disease in premature infants. BMC Pediatr. 2024, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Barekatain, B.; Hamidipour, S.; Badiei, Z.; Farghadani, M. Comparison of Daily Dose of 400 and 600 Units of Vitamin D in the Prevention of Osteopenia of Prematurity in Infants with a Gestational Age of Less Than and Equal to 32 Weeks. Int. J. Prev. Med. 2024, 15, 55. [Google Scholar] [PubMed]
- Romero-Lopez, M.; Tyson, J.E.; Naik, M.; Pedroza, C.; Holzapfel, L.F.; Avritscher, E.; Mosquera, R.; Khan, A.; Rysavy, M. Randomized controlled trial of enteral vitamin D supplementation (ViDES) in infants <28 weeks gestational age or <1000 g birth weight: Study protocol. Trials 2024, 25, 423. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Menkovic, I.; Williams, M.; Makhijani, N.; Wei, R.; Young, S.P.; El-Gharbawy, A.; Stiles, A.R. Persistent elevations of alkaline phosphatase as an early indicator of GM1 gangliosidosis. Mol. Genet. Metab. Rep. 2025, 42, 101191. [Google Scholar] [CrossRef]
- Mogilner, B.M.; Barak, Y.; Amitay, M.; Zlotogora, J. Hyperphosphatasemia in infantile GM1 gangliosidosis: Possible association with microscopic bone marrow osteoblastosis. J. Pediatr. 1990, 117, 758–761. [Google Scholar] [CrossRef]
- Caciotti, A.; Garman, S.C.; Rivera-Colón, Y.; Procopio, E.; Catarzi, S.; Ferri, L.; Guido, C.; Martelli, P.; Parini, R.; Antuzzi, D.; et al. GM1 gangliosidosis and Morquio B disease: An update on genetic alterations and clinical findings. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 782–790. [Google Scholar] [CrossRef]
- Gray-Edwards, H.L.; Regier, D.S.; Shirley, J.L.; Randle, A.N.; Salibi, N.; Thomas, S.E.; Latour, Y.L.; Johnston, J.; Golas, G.; Maguire, A.S.; et al. Novel Biomarkers of Human GM1 Gangliosidosis Reflect the Clinical Efficacy of Gene Therapy in a Feline Model. Mol. Ther. 2017, 25, 892–903. [Google Scholar] [CrossRef]
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Newborn Screening Programmes for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 49. [Google Scholar] [CrossRef]
- Praticò, A.D.; Bianco, M.L.; Leonardi, R.; Polizzi, A.; Ruggieri, M. The Impact of Vitamin D Supplementation Duration on Early Childhood Developmental Milestones: A Retrospective Study. Nutrients 2024, 16, 4395. [Google Scholar] [CrossRef]
- Gáll, Z.; Székely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, S.; Ha, T.T.; Simpson, J.A.; Thuy, T.T.; Khuong, N.C.; Thoang, D.D.; Tran, T.D.; Tuan, T.; Fisher, J.; Biggs, B.-A. Maternal vitamin D status and infant outcomes in rural Vietnam: A prospective cohort study. PLoS ONE 2014, 9, e99005. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.; Halldorsson, T.I.; Hansen, S.; Granström, C.; Maslova, E.; Petersen, S.B.; Cohen, A.S.; Olsen, S.F. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: A prospective study with long-term follow-up. Ann. Nutr. Metab. 2014, 64, 254–261. [Google Scholar] [CrossRef]
- Zhu, P.; Tong, S.-L.; Hao, J.-H.; Tao, R.-X.; Huang, K.; Hu, W.-B.; Zhou, Q.-F.; Jiang, X.-M.; Tao, F.-B. Cord blood vitamin D and neurocognitive development are non-linearly related in toddlers. J. Nutr. 2015, 145, 1232–1238. [Google Scholar] [CrossRef]
| Title, Author, Year, Reference | Type of Study | Findings | Neurological Findings |
|---|---|---|---|
| Metabolic Bone Disease in the Preterm Infant: Current State and Future Directions (Rehman & Narchi, 2015) [1] | Review | Highlights the importance of early biochemical and radiological monitoring and nutritional interventions in preterm infants with MBD | Identifies neuromuscular disorders as risk factors that, via prolonged immobilization, contribute to impaired cortical bone growth and subsequent rickets/fractures. |
| Persistent Elevations of ALP as an Early Indicator of GM1 Gangliosidosis (Menkovic et al., 2025) [47] | Case report | Suggests persistent ALP elevation as early marker for GM1 gangliosidosis, linking skeletal dysplasia with neurodegeneration | Progressive neurodegenerative signs including central hypotonia, head lag, hypertonia, and hearing loss. |
| Neurodevelopment Outcomes in Very-Low-Birth-Weight Infants with Metabolic Bone Disease at 2 Years of Age (Chen et al., 2024) [13] | Retrospective study | Assessed neurodevelopmental outcomes at 6, 12, and 24 months of corrected age in 749 VLBW infants (<1350 g), comparing those with and without radiographic signs of MBD. | Infants diagnosed with MBD showed significantly lower scores in cognitive, motor, and language domains at 2 years of age, even after adjusting for confounders. Strong association between MBD and neurodevelopmental delay was demonstrated. |
| The Impact of Vitamin D Supplementation Duration on Early Childhood Developmental Milestones: A retrospective study (Praticò et al., 2024) [52] | Retrospective study | Indicates that extended vitamin D supplementation may confer modest yet significant neurodevelopmental advantages, likely via enhanced skeletal, neuromuscular, and brain function. | The 12-month supplementation group achieved certain motor (e.g., walking), fine motor (object tracking), and language milestones earlier, with higher Griffiths developmental scores at 1 and 2 years. |
| Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings (Gáll et al., 2021) [53] | Review | Highlights the complex role of vitamin D signaling in the CNS and underscores that maintaining adequate vitamin D is vital for cognitive development, with systemic implications for skeletal health. | Links vitamin D deficiency with cognitive dysfunction, neurodevelopmental disorders (e.g., ADHD, autism, and schizophrenia), and neurodegenerative diseases; discusses VDR expression in key brain regions. |
| Maternal Vitamin D Status and Infant Outcomes in Rural Vietnam: a prospective cohort study (Hanieh et al., 2014) [54] | Prospective cohort study | Indicates low maternal vitamin D during late pregnancy is associated with impaired language development in infancy, supporting antenatal vitamin D supplementation to improve neurodevelopmental outcomes. | Infants born to vitamin-D-deficient mothers (<37.5 nmol/L) had significantly lower language composite scores and smaller head circumferences at birth, indicating an adverse neurodevelopmental impact. |
| Neurodevelopmental Effects of Prenatal Vitamin D in Humans: Systematic Review and Meta-analysis (García-Serna et al., 2020) [12] | Systematic review and Meta-analysis | Evaluated prenatal 25(OH)D (maternal and cord blood) as a marker of vitamin D exposure; reinforced the importance of adequate prenatal vitamin D for neurodevelopment. | Higher prenatal 25(OH)D levels were associated with improved cognitive development (borderline significance) and significantly lower risks of ADHD and autism-related traits. |
| Cord Blood Vitamin D and Neurocognitive Development Are Nonlinearly Related in Toddlers (Zhu et al., 2015) [57] | Prospective follow-up study | Suggests that there is an optimal cord blood vitamin D level (approximately 30–50 nmol/L) for neurocognitive outcomes; both deficient and excessive vitamin D levels may impair development, highlighting the need for further research on optimal thresholds in early life. | Demonstrated an inverted U-shaped relationship between cord blood 25(OH)D concentrations and neurocognitive development: both the lowest and highest quintiles were associated with significantly lower mental (MDI) and psychomotor (PDI) scores compared to mid-range values. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, R.; Mattia, C.; Decembrino, N.; Polizzi, A.; Ruggieri, M.; Betta, P. The Critical Role of Vitamin D Supplementation for Skeletal and Neurodevelopmental Outcomes in Preterm Neonates. Nutrients 2025, 17, 1381. https://doi.org/10.3390/nu17081381
Leonardi R, Mattia C, Decembrino N, Polizzi A, Ruggieri M, Betta P. The Critical Role of Vitamin D Supplementation for Skeletal and Neurodevelopmental Outcomes in Preterm Neonates. Nutrients. 2025; 17(8):1381. https://doi.org/10.3390/nu17081381
Chicago/Turabian StyleLeonardi, Roberta, Carmine Mattia, Nunzia Decembrino, Agata Polizzi, Martino Ruggieri, and Pasqua Betta. 2025. "The Critical Role of Vitamin D Supplementation for Skeletal and Neurodevelopmental Outcomes in Preterm Neonates" Nutrients 17, no. 8: 1381. https://doi.org/10.3390/nu17081381
APA StyleLeonardi, R., Mattia, C., Decembrino, N., Polizzi, A., Ruggieri, M., & Betta, P. (2025). The Critical Role of Vitamin D Supplementation for Skeletal and Neurodevelopmental Outcomes in Preterm Neonates. Nutrients, 17(8), 1381. https://doi.org/10.3390/nu17081381







