Non-Severe Hypophosphatemia in Older Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
- Study characteristics: first author, publication year, country, observation period, study design;
- Setting: hospital vs. outpatient department;
- Inclusion criteria;
- Sample size;
- Patient’s mean or median age;
- Patients’ sex;
- No. of patients with HP;
- HP cut-off at diagnosis;
- Phosphorus mean/median value;
- Comorbidities;
- Primary and secondary outcomes.
2.4. Risk of Bias-Quality Assessment
3. Results
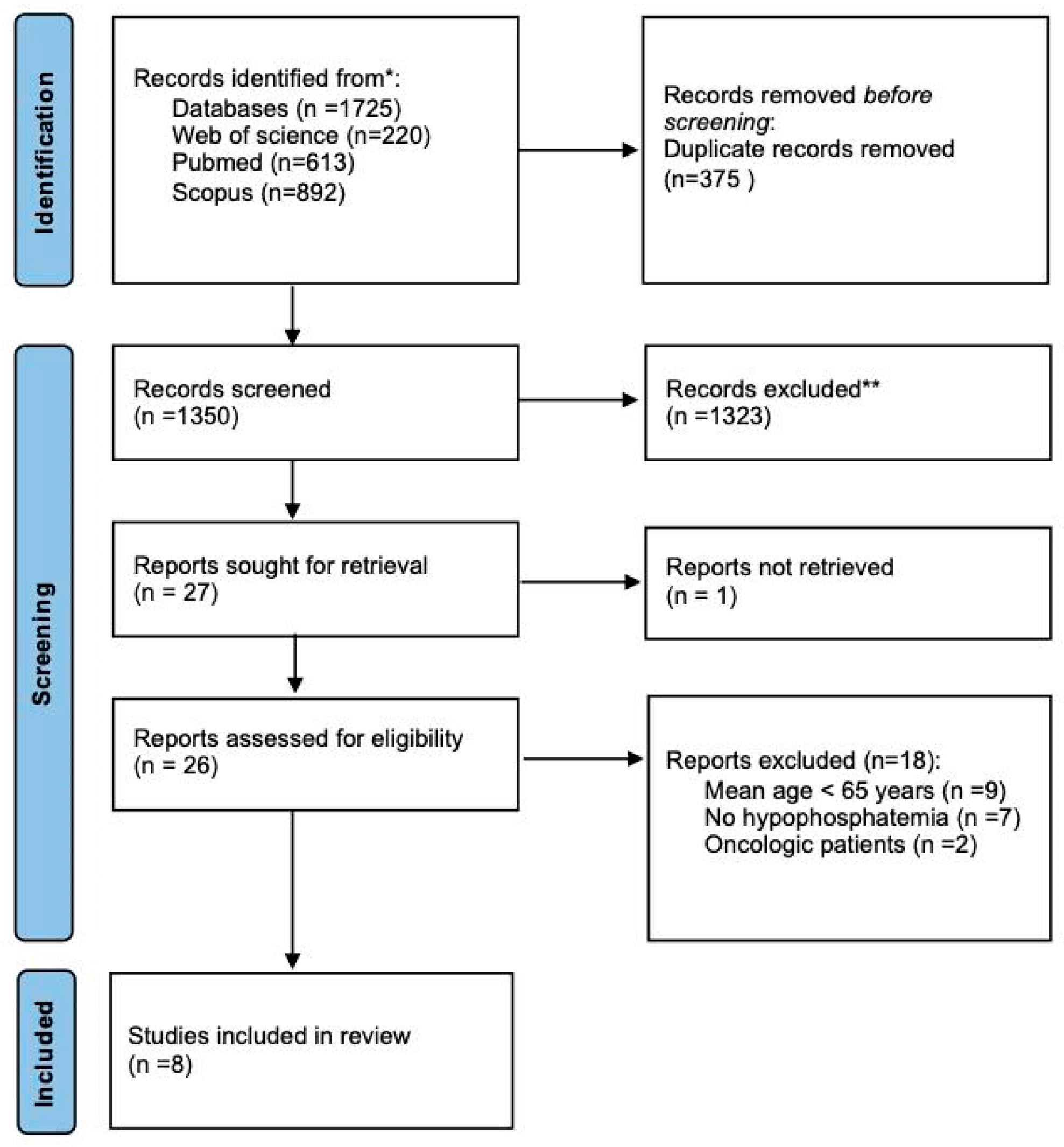
3.1. Search Results
3.2. Studies Characteristics
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Authors | Country | Period | Design | Setting | Sample | S-P Level (mg/dL) | HP pz. |
| Fujisawa et al. (2022) [30] | Japan | 2010–2017 | Cross-sectional study | Outpatient department | 4204 | 3.0 | 370 |
| Haglin et al. (2010) [34] | Sweden | 1992–1994 | Retrospective | Hospital | 76 * | 2.5 | 24 |
| Heybeli et al. (2022) [35] | Turkey | 2016–2020 | NR | Outpatient department | 464 | 2.5 | 23 |
| Jang et al. (2022) [32] | South Korea | 2018–2021 | Retrospective | Hospital | 15,485 | 2.8 | 2406 |
| Morimoto et al. (2022) [31] | Japan | 2009–2018 | Retrospective | Hospital | 600 | 2.0 | 72 |
| Park et al. (2019) [33] | South Korea | 2010–2014 | Retrospective | Hospital | 4782 | 2.5 | 238 |
| Pourhassan et al. (2018) [36] | Germany | NR | Retrospective | Hospital | NR | 335 | 2.1 |
| Sankaran et al. (1997) [37] | USA | 1993 | Retrospective | Hospital | NR | 602 | 2.4 |
| (b) | |||||||
| Authors | Inclusion Criteria | Age | Female (%) | Comorbidities (%) | |||
| Fujisawa et al. (2022) [30] | Patients aged 70 years or older visited forassessment of a memory disorder who present at least one among serumNa, K, Ca, P and answered for at least 30 items on the 50-item FI. | Non-frail group: 75 (73–79); Mildly frail group: 78 (75–82); Moderate frail group: 80 (77–84); Severe frail group: 83 (79–87) | 563 (61.1) in the frail group 691 (60.3) in the mildly frail group 698 (67.2) in the moderately frail group 764 (69.5) in the severely frail group | Diabetes 1500 (35.7); Hypertension 2129 (50.6); Heart disease 531 (12.6); Liver disease 101 (2.4); Lung disease 177 (4.2); Cancer 30 (7.8); Stroke 253 (6.0); Estimated glomerular filtration rate <60 mL/min/1.73 m2: 1504 (35.8); Insomnia complaint 154 (3.7) | |||
| Haglin et al. (2010) [34] | Patients with virologically confirmed acute influenza | Unclear | 25 (50) | Diabetes: 13 (17.1); Bronchial asthma: 4 (5.3); CVD: 4 (5.3); COPD: 4 (5.3); Other: 10 (13.2) | |||
| Heybeli et al. (2022) [35] | Patients aged ≥ 65 years | 78 (72–83) | 321 (69.2) | Diabetes: 167 (36); Hypertension: 320 (69); Chronic kidney disease: 181 (39); Heart failure: 51 (11); Ischemic heart disease: 70 (15); Cerebrovascular disease: 60 (13) | |||
| Jang et al. (2022) [32] | Hospitalized patients | 70.0 (60.0–79.0) | 7971 (52.1) | NR | |||
| Morimoto et al. (2022) [31] | Hospitalized patients with community-acquired pneumonia | 67.9 ± 15.2 | 175 (29.2) | Pulmonary disease: 321 (53.5); Non-pulmonary disease: 312 (52) | |||
| Park et al. (2019) [33] | Patients undergoing CABG | Normal: 63.1 ± 9.8 HP: 65.1 ± 9.6 | 1237 (25.9) | Hypertension: 2957 (61.8) ; Diabetes: 2212 (46.3) ; Ejection fraction < 40%: 1398 (29.2) ; Dyslipidemia: 1598 (33.4) ; Stroke: 721(15.1); Chronic kidney disease: 243 (5.1); COPD: 1479 (30.9); PAOD: 360 (7.5) ; ACS: 2387 (49.9); Old MI: 587 (12.3); Carotid arterial disease: 1082 (22.6) | |||
| Pourhassan et al. (2018) [36] | Older hospitalized patients | 83.1 ± 6.8 | NR | NR | |||
| Sankaran et al. (1997) [37] | Hospitalized patients | HP: 67.5 ± 1. 9; Control group: 60.7 ± 1.1 years; Normophosphatemia: 63.4± 1.5 years | NR | NR | |||
3.3. Risk of Bias
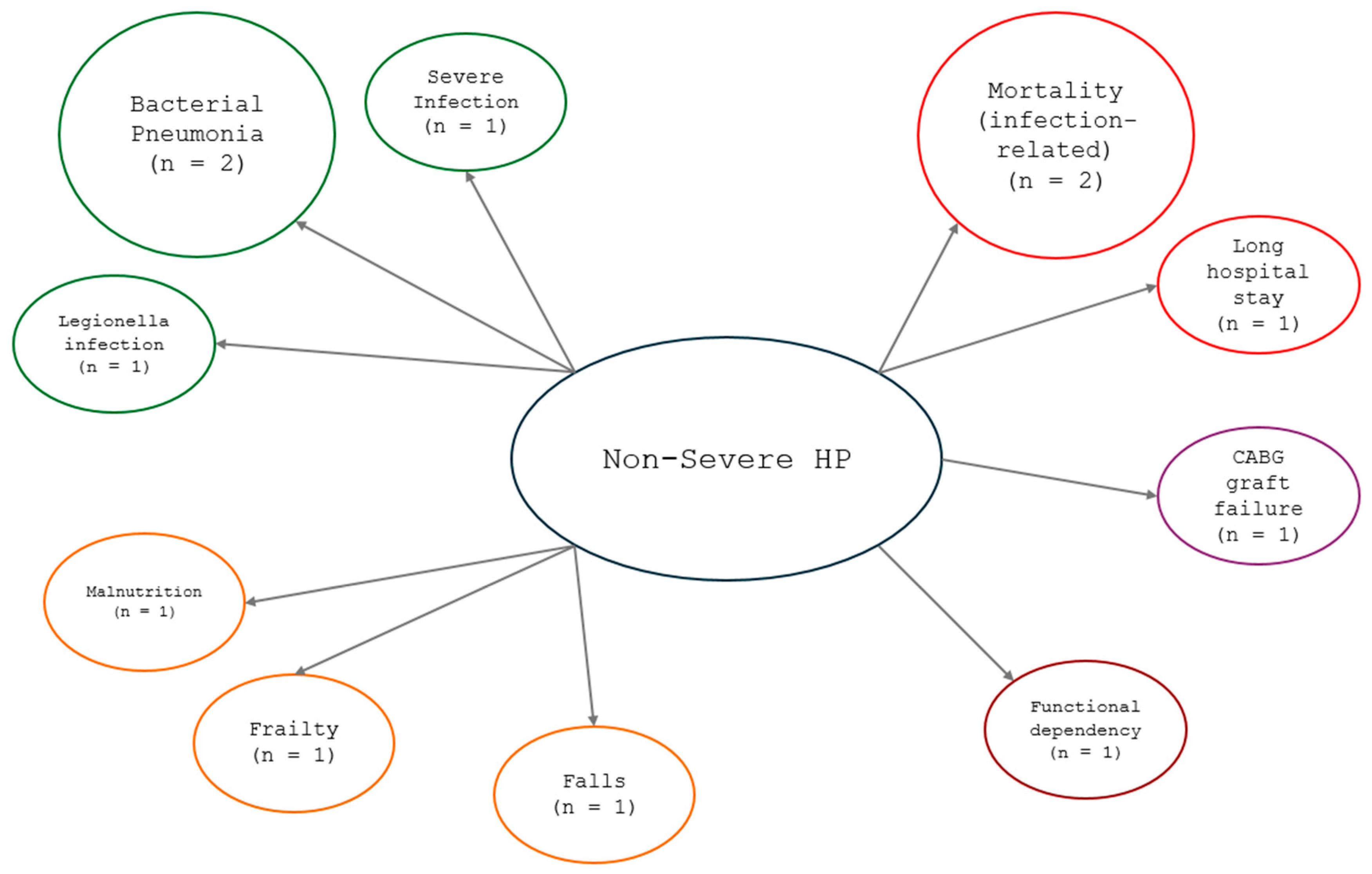
3.4. Hypophosphatemia and Related Comorbidities
3.5. Infectious or Bacterial Diseases
3.6. Risk of Falls
3.7. Malnutrition
3.8. Risk of Negative Outcome After Risk of Falls
4. Discussion
4.1. Frailty
4.2. Risk of Falls
4.3. Infectious or Bacterial Diseases
4.4. Malnutrition
4.5. Risk of Negative Outcome After Coronary Artery Bypass Graft
4.6. Hypophosphatemia Management
5. Conclusions
Key Findings and Clinical Implications
6. Future Research Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Penido, M.G.M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, J.; Reilly, R.F., Jr. Hypophosphatemia: An evidence-based approach to its clinical consequences and management. Nat. Clin. Pract. Nephrol. 2006, 2, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M. Disorders involving calcium, phosphorus, and magnesium. Prim. Care 2008, 35, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Knochel, J.P. Neuromuscular manifestations of electrolyte disorders. Am. J. Med. 1982, 72, 521–535. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef]
- Manghat, P.; Sodi, R.; Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 2014, 51 Pt 6, 631–656. [Google Scholar] [CrossRef]
- Tenenhouse, H.S. Phosphate transport: Molecular basis, regulation and pathophysiology. J. Steroid Biochem. Mol. Biol. 2007, 103, 572–577. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ganz, T.; Trumbo, H.; Seid, M.H.; Goodnough, L.T.; Levine, M.A. Parenteral iron therapy and phosphorus homeostasis: A review. Am. J. Hematol. 2021, 96, 606–616. [Google Scholar] [CrossRef]
- Gaasbeek, A.; Meinders, A.E. Hypophosphatemia: An update on its etiology and treatment. Am. J. Med. 2005, 118, 1094–1101. [Google Scholar] [CrossRef]
- Guy, J.M.; Stewart, M.F.; Olukoga, A.; Horsman, G.; McMurray, J.R. Hypophosphataemia in general practice patients. Ann. Clin. Biochem. 1999, 36 Pt 1, 37–42. [Google Scholar] [CrossRef]
- Broman, M.; Carlsson, O.; Friberg, H.; Wieslander, A.; Godaly, G. Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol. Scand. 2011, 55, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Marinella, M.A. Refeeding syndrome and hypophosphatemia. J. Intensive Care Med. 2005, 20, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Hasselstrom, L.; Wimberley, P.D.; Nielsen, V.G. Hypophosphatemia and acute respiratory failure in a diabetic patient. Intensive Care Med. 1986, 12, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Datta, B.N.; Stone, M.D. Hyperventilation and hypophosphataemia. Ann. Clin. Biochem. 2009, 46 Pt 2, 170–171. [Google Scholar] [CrossRef]
- Witteveen, J.E.; Van Thiel, S.; Romijn, J.A.; Hamdy, N.A. Hungry bone syndrome: Still a challenge in the post-operative management of primary hyperparathyroidism: A systematic review of the literature. Eur. J. Endocrinol. 2013, 168, R45–R53. [Google Scholar] [CrossRef]
- Murer, H. Homer Smith Award. Cellular mechanisms in proximal tubular Pi reabsorption: Some answers and more questions. J. Am. Soc. Nephrol. 1992, 2, 1649–1665. [Google Scholar] [CrossRef]
- De Marchi, S.; Cecchin, E.; Basile, A.; Bertotti, A.; Nardini, R.; Bartoli, E. Renal tubular dysfunction in chronic alcohol abuse--effects of abstinence. N. Engl. J. Med. 1993, 329, 1927–1934. [Google Scholar] [CrossRef]
- Lotz, M.; Zisman, E.; Bartter, F.C. Evidence for a phosphorus-depletion syndrome in man. N. Engl. J. Med. 1968, 278, 409–415. [Google Scholar] [CrossRef]
- Tebben, P.J. Hypophosphatemia: A Practical Guide to Evaluation and Management. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2022, 28, 1091–1099. [Google Scholar] [CrossRef]
- Boland, J.M.; Tebben, P.J.; Folpe, A.L. Phosphaturic mesenchymal tumors: What an endocrinologist should know. J. Endocrinol. Investig. 2018, 41, 1173–1184. [Google Scholar] [CrossRef]
- Fernández-Fernández, F.J.; Martín-Fernández, A. Parenteral iron as a cause of hypophosphataemia. Bmj 2014, 349, g4616. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Hatfield, A.; Letvak, L. Imatinib and altered bone and mineral metabolism. N. Engl. J. Med. 2006, 355, 627. [Google Scholar] [PubMed]
- Kempe, D.S.; Dërmaku-Sopjani, M.; Fröhlich, H.; Sopjani, M.; Umbach, A.; Puchchakayala, G.; Capasso, A.; Weiss, F.; Stübs, M.; Föller, M.; et al. Rapamycin-induced phosphaturia. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2010, 25, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Pia, A.; Brizzi, M.P.; Tampellini, M.; Torta, M.; Terzolo, M.; Dogliotti, L.; Berruti, A. Sorafenib may induce hypophosphatemia through a fibroblast growth factor-23 (FGF23)—Independent mechanism. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 988–990. [Google Scholar] [CrossRef]
- Kichloo, A.; Chugh, S.S.; Gupta, S.; Panday, J.; Goldar, G.E. Tenofovir and Severe Symptomatic Hypophosphatemia. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619848796. [Google Scholar] [CrossRef]
- Baeg, S.I.; Lee, K.; Jeon, J.; Jang, H.R. Management for Electrolytes Disturbances during Continuous Renal Replacement Therapy. Electrolyte Blood Press. E BP 2022, 20, 64–75. [Google Scholar] [CrossRef]
- Subramanian, R.; Khardori, R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Fujisawa, C.; Umegaki, H.; Sugimoto, T.; Huang, C.H.; Fujisawa, H.; Sugimura, Y.; Kuzuya, M.; Toba, K.; Sakurai, T. Older adults with a higher frailty index tend to have electrolyte imbalances. Exp. Gerontol. 2022, 163, 111778. [Google Scholar] [CrossRef]
- Morimoto, Y.; Ishiguro, T.; Uozumi, R.; Takano, K.; Kobayashi, Y.; Kobayashi, Y.; Shimizu, Y.; Takayanagi, N. Significance of Hypophosphatemia in Patients with Pneumonia. Intern. Med. 2022, 61, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Kwon, S.J.; Kim, C.S.; Park, S.W.; Kim, K.M. Association Between Low Serum Phosphate Level and Risk of Falls in Hospitalized Patients Over 50 Years of Age: A Retrospective Observational Cohort Study. Clin. Interv. Aging 2022, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hong, K.Y.; Min, J.J.; Kwon, E.; Lee, Y.T.; Kim, W.S.; Kim, H.S.; Kim, K.; Lee, J.H. Clinically-defined preoperative serum phosphorus abnormalities and outcomes of coronary artery bypass grafting: Retrospective analysis using inverse probability weighting adjustment. PLoS ONE 2019, 14, e0225720. [Google Scholar] [CrossRef] [PubMed]
- Håglin, L.M.; Burman, L.A.; Nilsson, M. Predisposing chronic diseases and hypophosphatemia in patients with influenza. Arch. Gerontol. Geriatr. 2010, 51, 26–30. [Google Scholar] [CrossRef]
- Heybelİ, C.; Tan, S.G.; KazancioĞLu, R.; Smith, L.; Soysal, P. Prevalence of Electrolyte Impairments Among Outpatient Elderly Subjects. Bezmialem Sci. 2022, 10, 305–311. [Google Scholar] [CrossRef]
- Pourhassan, M.; Müller, M.J.; Volkert, D.; Wirth, R. Hypophosphatemia as a sign of malnutrition in older hospitalized patients. Eur. J. Clin. Nutr. 2019, 73, 634–636. [Google Scholar] [CrossRef]
- Sankaran, R.T.; Mattana, J.; Pollack, S.; Bhat, P.; Ahuja, T.; Patel, A.; Singhal, P.C. Laboratory abnormalities in patients with bacterial pneumonia. Chest 1997, 111, 595–600. [Google Scholar] [CrossRef]
- Lan, X.; Li, H.; Wang, Z.; Chen, Y. Frailty as a predictor of future falls in hospitalized patients: A systematic review and meta-analysis. Geriatr. Nurs. 2020, 41, 69–74. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Temedda, M.N.; Garnier-Crussard, A.; Mouchoux, C.; Dauphinot, V. Association between Comorbidity Indices and Functional Autonomy in Individuals with Cognitive Impairment: A Systematic Review. J. Prev. Alzheimers Dis. 2024, 11, 1047–1054. [Google Scholar] [CrossRef]
- Tornero-Quinones, I.; Saez-Padilla, J.; Espina Diaz, A.; Abad Robles, M.T.; Sierra Robles, A. Functional Ability, Frailty and Risk of Falls in the Elderly: Relations with Autonomy in Daily Living. Int. J. Environ. Res. Public Health 2020, 17, 1006. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; Murphy, T.E.; Gahbauer, E.A.; Allore, H.G. Association of injurious falls with disability outcomes and nursing home admissions in community-living older persons. Am. J. Epidemiol. 2013, 178, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, M.; Ioannou, P.; Tsioutis, C.; Persynaki, G.S.; Pediaditis, E.; Koutserimpas, C.; Kontakis, G.; Alpantaki, K.; Samonis, G.; Panagiotakis, S.H. Age, Comorbidities and Fear of Fall: Mortality predictors associated with fall-related fractures. Maedica 2020, 15, 18–23. [Google Scholar] [PubMed]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef]
- Koziel, H.; Koziel, M.J. Pulmonary complications of diabetes mellitus. Pneumonia. Infect. Dis. Clin. N. Am. 1995, 9, 65–96. [Google Scholar] [CrossRef]
- Craddock, P.R.; Yawata, Y.; VanSanten, L.; Gilberstadt, S.; Silvis, S.; Jacob, H.S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N. Engl. J. Med. 1974, 290, 1403–1407. [Google Scholar] [CrossRef]
- Peng, A.; Wu, T.; Zeng, C.; Rakheja, D.; Zhu, J.; Ye, T.; Hutcheson, J.; Vaziri, N.D.; Liu, Z.; Mohan, C.; et al. Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS ONE 2011, 6, e23268. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Heames, R.M.; Cope, R.A. Hypophosphataemia causing profound cardiac failure after cardiac surgery. Anaesthesia 2006, 61, 1211–1213. [Google Scholar] [CrossRef]
- UpToDate. Hypophosphatemia: Evaluation and Treatment. 2024. Available online: https://www.uptodate.com/contents/hypophosphatemia-evaluation-and-treatment (accessed on 8 October 2024).


| Topic | Authors | Outcome | Main Findings | Conclusion |
|---|---|---|---|---|
| Hypophosphatemia and related comorbidities | Fujisawa et al. (2022) [30] | Electrolyte imbalance, comorbidities, cognitive function and mood, ADL (both basic and instrumental), physical function, nutrition, fall risks from physical weakness, fall risks from comorbidities | Comparison HP risk between non-frailty group and mildly frail group: OR: 1.52; 95% CI, 1.07–2.14; p = 0.02; Comparison HP risk between non-frailty group and moderate frail group: OR: 1.56; 95% CI, 1.08–2.24; p = 0.02; Comparison HP risk between non-frail group and severe frail group: OR: 2.00; 95% CI, 1.37–2.89; p < 0.001). For every increase of 0.1 in the FI score produced an increase in HP risk of 16% (exp(β) = 1.16, 95%CI: 1.06–1.27). HP correlated with cognitive function and mood (OR: 1.07, 95%CI: 1.03–10.12, p < 0.01), fall risks from physical weakness (OR: 1.05, 95%CI: 1.01–1.10, p < 0.05), and fall risks from comorbidities (OR: 1.05, 95%CI: 1.00–1.10, p < 0.05). | Compared with the non-frail group, the mildly and moderately frail groups tended to have HP. |
| Heybeli et al. (2022) [35] | Electrolyte abnormalities in geriatric population with attention to age, comorbidities, drug exposure, and malnutrition | A significantly lower prevalence of diabetes mellitus in patients with HP (13.6%) than in normophosphatemia patients (37%, p = 0.026). A greater, but not statistically significant, prevalence of HP in patients over 80 years (6.7%) compared to those in the range 65–79 years (3.7%). | DM was more common in patients with normophosphatemia (p = 0.026), while CKD was more present in HP patients (p = n.s.). | |
| Infectious or bacterial diseases | Haglin et al. (2010) [34] | Prevalence of HP and role of HP in virus-infected patients in chronic disease and/or bacterial infection and longer hospitalization stay | Prevalence of HP (S-P ≤ 0.82 mmol/L) in 34% of patients with a greater prevalence of mild-severe HP (S-P < 0.70 mmol/L) in 13% of women and 15% of men. Influenza and chronic disease (i.e., DM, asthma bronchiale, CVD, or COPD); double the risk of HP. | A chronic disease in old patients indicates a high prevalence of hypophosphatemia, and this in turn might exacerbate morbidity and mortality. |
| Morimoto et al. (2022) [31] | Causative organisms of pneumonia, patient factors, disease severity, and mortality | Non-pulmonary disease and the severity of pneumonia were significantly higher in the patients with HP than in those without it. Polymicrobial infections were lower in HP patients (n = 9, 12.5%) than in non-HP patients (n = 44, 8.3%). Legionella was more frequent in the HP patients than in the non-HP patients (11.1% vs. 3.6%, p = 0.010). Legionella (OR, 2.89; 95% CI, 1.19 to 6.99; p = 0.019), diabetes mellitus (OR, 2.53; 95% CI, 1.41 to 4.56; p = 0.002), and severe pneumonia (OR, 2.86; 95% CI, 1.57 to 5.22; p = 0.001) were independent factors for HP. | HP was not associated with the prognosis in patients with community-acquired pneumonia, although HP could predict abnormal glucose metabolism, Legionella infection, and severe disease. | |
| Sankaran et al. (1997) [37] | Bacterial pneumonia at discharge, morbidity, and mortality | HP was more prevalent in patients with a diagnosis of bacterial pneumonia at discharge (44.7%) (p < 0.001); HP patients had lower levels of potassium, calcium, and albumin compared to their normophosphatemic counterparts, but for different normophosphatemic patients, they had higher glucose levels. Hospitalization was longer for HP patients (HP: 24.6 ± 2.1 days vs. normophosphatemia, 14.1 ± 1.0; p < 0.0001), and HP patients had higher mortality rates (p < 0.001) than their normophosphatemic counterparts. | HP may be a predictor of the severity of illness in patients admitted to the hospital with bacterial pneumonia. | |
| Risk of falls | Jang et al. (2022) [32] | Risk of falls in hospitalized patients | Fall risk in patients with a lower S-P level (≤2.8 mg/dL) was more than double that of patients with S-P level above 4.5 mg/dL. | Lower s-phosphate level on admission was independently associated with an increased risk of in-hospital falls. |
| Malnutrition | Pourhassan et al. (2018) [36] | Malnutrition | HP patients had significantly more unintentional weight loss than non-HP patients; malnutrition or at risk of malnutrition was present in 86% of HP patients, values were significantly higher (p = 0.003) than reported among patients without HP (56%) among participants. | Older patients with HP are likely to have experienced unintentional weight loss and to have nutritional difficulties compared to non-HP patients. |
| Risk of negative outcome after CABG | Park et al. (2019) [33] | CABG failure (all-cause death, cardiovascular death, graft failure, composite of major adverse cardiovascular and cerebral events MACE) | A 3.7% incidence of all-cause death in patients with normal S-P levels, and 9.7% in patients with HP (S-P lower than 2.5 mg/dL); the HP was significantly associated with risk of all-cause (HR 1.76; 95% CI 1.13–2.76; p = 0.01) in patients with HP; a higher incidence of graft failure was confirmed hypophosphatemia patients (multivariate Cox regression HR 2.14; 95% CI 1.22–3.75; p = 0.01, IWR analysis HR 2.51; 95% CI 1.37–4.61; p = 0.003). | Preoperative serum phosphorus abnormalities were not associated with outcomes after CABG except for graft failure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarossa, L.; Zandonà, M.; Garo, M.L.; Mjahed, R.B.; D’Amelio, P. Non-Severe Hypophosphatemia in Older Patients: A Systematic Review. Nutrients 2025, 17, 1354. https://doi.org/10.3390/nu17081354
Barbarossa L, Zandonà M, Garo ML, Mjahed RB, D’Amelio P. Non-Severe Hypophosphatemia in Older Patients: A Systematic Review. Nutrients. 2025; 17(8):1354. https://doi.org/10.3390/nu17081354
Chicago/Turabian StyleBarbarossa, Luca, Martina Zandonà, Maria Luisa Garo, Ribal Bou Mjahed, and Patrizia D’Amelio. 2025. "Non-Severe Hypophosphatemia in Older Patients: A Systematic Review" Nutrients 17, no. 8: 1354. https://doi.org/10.3390/nu17081354
APA StyleBarbarossa, L., Zandonà, M., Garo, M. L., Mjahed, R. B., & D’Amelio, P. (2025). Non-Severe Hypophosphatemia in Older Patients: A Systematic Review. Nutrients, 17(8), 1354. https://doi.org/10.3390/nu17081354







