Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications
Abstract
1. Introduction
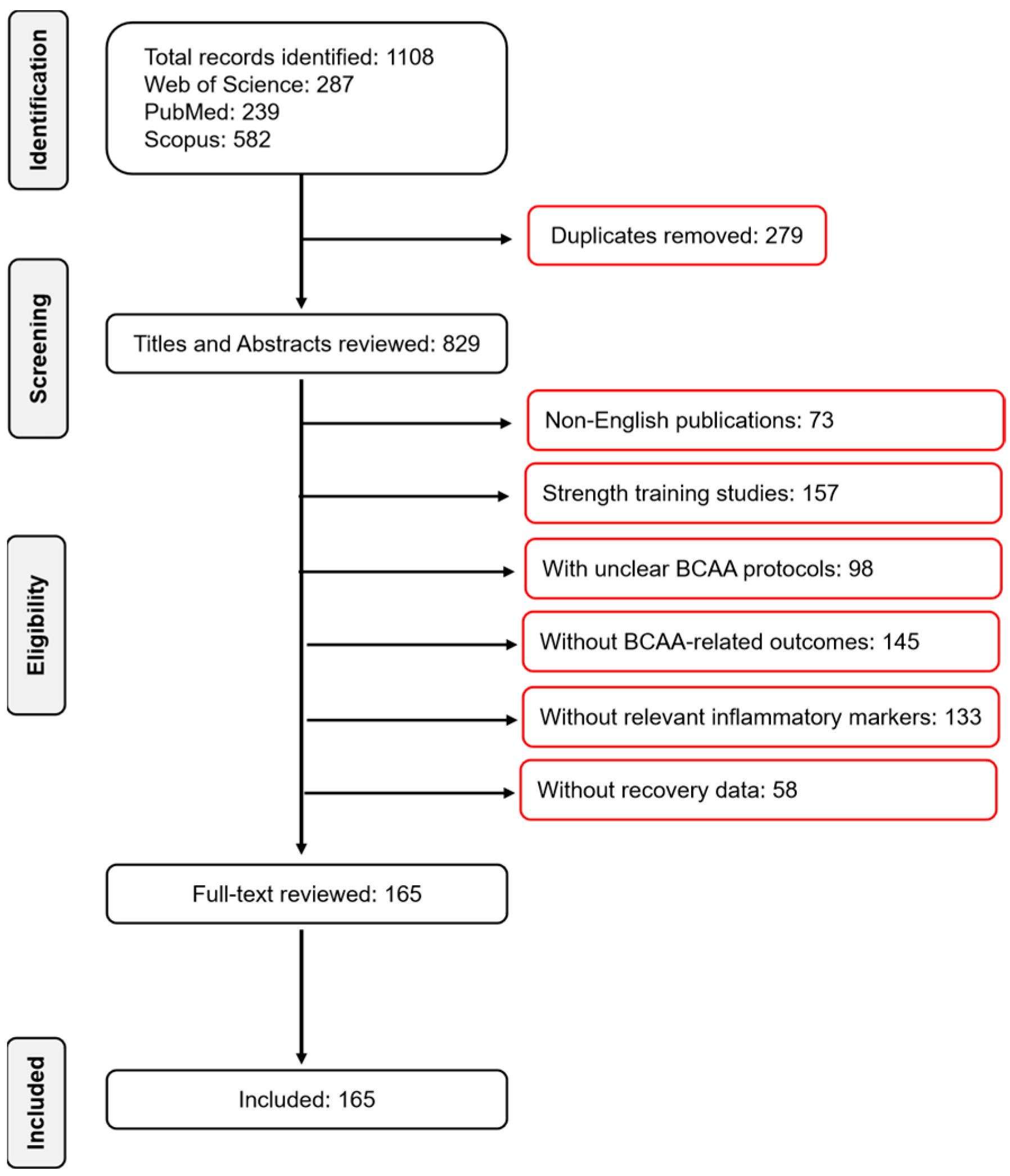
2. Methods
3. Exercise-Induced Muscle Damage (EIMD) and Inflammation in Endurance Athletes
3.1. Muscle Damage and Inflammation Response
3.2. Physiological Mechanisms of EIMD
| Inflammatory Marker | Description | Role Post-Exercise | Normal/Baseline Values | Typical Changes Post-Exercise | References |
|---|---|---|---|---|---|
| IL-6 | Cytokine involved in immune response and inflammation | Released during exercise; promotes muscle repair and adaptation | ~1–5 pg/mL | Increases post-exercise, peaks within 1–2 h, returns to baseline after a few hours | Fernández-Lázaro et al. [40] |
| TNF-α | Pro-inflammatory cytokine | Induces inflammation; excessive levels can cause muscle damage | ~1–3 pg/mL | Peaks 1–3 h post-exercise, elevated for several hours | Mallett et al. [29] |
| CRP | Protein produced by the liver in response to inflammation | Marker of systemic inflammation; elevated after intense exercise | ~1–3 mg/L | Significant increase post-exercise, peaks after 24 h | Zhao et al. [50] |
| IL-1β | Pro-inflammatory cytokine | Plays a role in tissue damage response and muscle inflammation | ~2–5 pg/mL | Increases immediately post-exercise, returns to baseline within 24–48 h | Notbohm et al. [37] |
| MCP-1 | Chemokine that attracts immune cells to inflammation sites | Mediates macrophage recruitment to injured muscle tissue | ~100–300 pg/mL | Increases 2–6 h post-exercise, declines within 24 h | Lagzdina et al. [51] |
| IL-8 | Chemokine involved in attracting neutrophils to inflammation sites | Facilitates neutrophil recruitment; supports repair and inflammation | ~5–10 pg/mL | Peaks 2–4 h post-exercise, returns to baseline within 24 h | Małkowska et al. [42] |
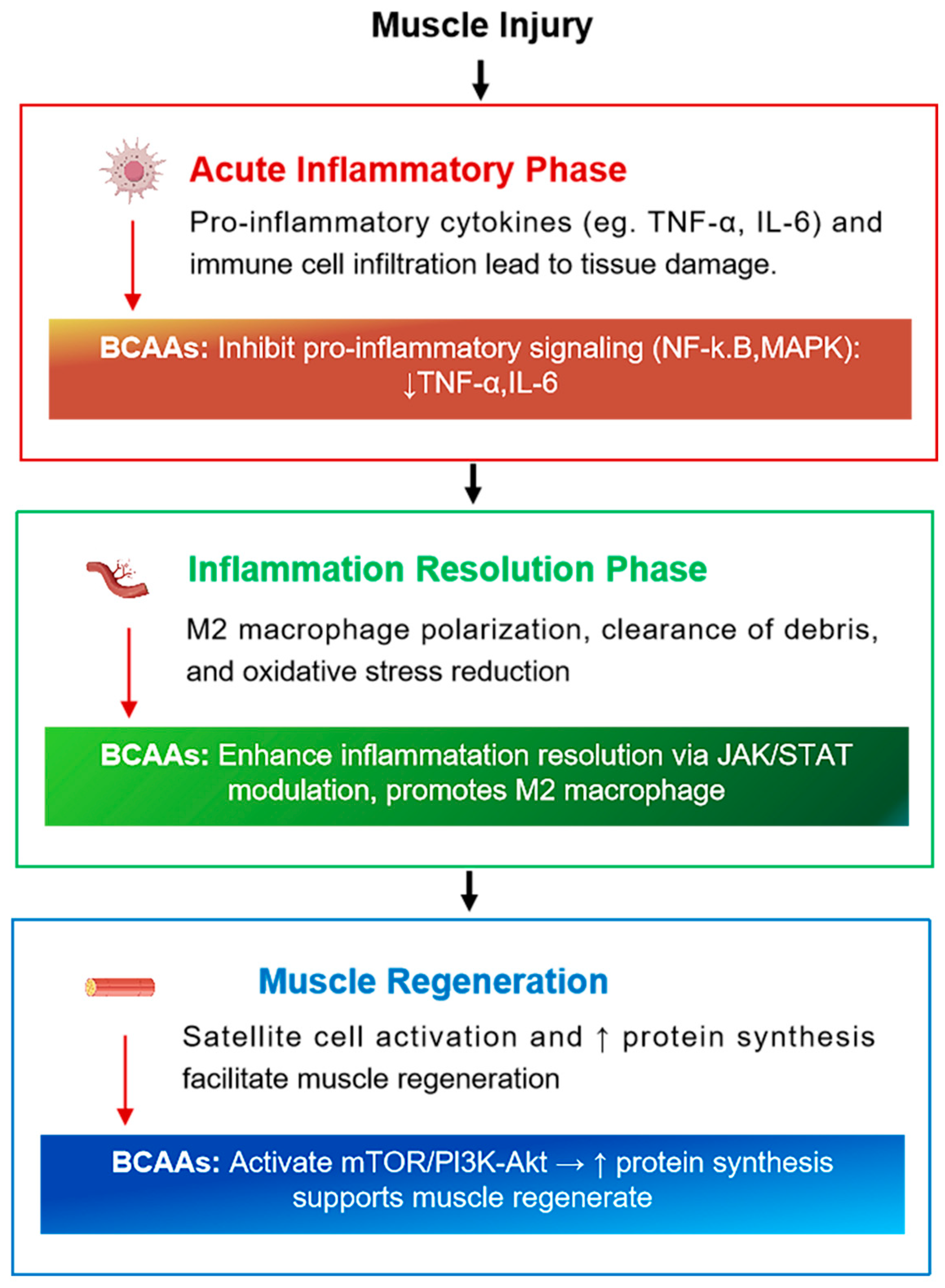
3.3. Inflammatory Response and Recovery
- (1)
- Acute Inflammatory Phase—Neutrophils activate M1 macrophages, which release pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) to initiate the repair cascade [54];
- (2)
- Resolution Phase—A shift occurs toward M2 macrophage activity, characterized by anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor beta (TGF-β), which promote healing and regeneration [55];
- (3)
4. Role of BCAAs in Muscle Recovery
4.1. Mechanisms of Action
| BCAA | Effect on Protein Synthesis | Mechanism of Action | Relative Potency/Optimal Ratio | References |
|---|---|---|---|---|
| Leucine | Strongly stimulates protein synthesis, activates mTOR pathway | Activates mTORC1 signaling pathway, increasing protein synthesis rate | Highest potency for protein synthesis; typically found in a 2:1:1 ratio with isoleucine and valine | Ma et al. [67] |
| Isoleucine | Promotes muscle protein synthesis, weaker mTOR activation effect | Increases energy availability, promotes protein synthesis, involved in muscle metabolism regulation | Moderate potency; often combined with leucine and valine in a 2:1:1 ratio to enhance effects | Mao et al. [83] |
| Valine | Weaker effect on protein synthesis | Regulates amino acid balance, influences synthesis of other amino acids | Least potent for protein synthesis; part of the 2:1:1 ratio for optimal effect | Beigi et al. [84] |
4.2. BCAAs and Muscle Damage
4.3. BCAAs and Fatigue Reduction
| BCAA | Effect on Fatigue | Mechanism of Action | References |
|---|---|---|---|
| Leucine | Reduces central fatigue and improves endurance performance | Inhibits serotonin production in the brain by competing with tryptophan for transport into the brain | Wen et al. [114] |
| Isoleucine | Enhances energy production and reduces fatigue during prolonged exercise | Increases glucose uptake into muscles, enhancing energy availability and reducing perceived fatigue | Baraniuk et al. [115] |
| Valine | Reduces perceived exertion and delays fatigue onset | Competes with tryptophan to lower serotonin levels, reducing central fatigue during prolonged exercise | Duttagupta et al. [116] |
5. BCAAs and Inflammation Management in Endurance Athletes
5.1. BCAAs Modulating Inflammatory Cytokines
5.2. Immune System Regulation
| BCAA | Effect | Impact on Immune Function | References |
|---|---|---|---|
| Leucine, Isoleucine, and Valine | Immune Cell Proliferation | Stimulates immune cell growth | Yahsi et al. [138] |
| Leucine | Cytokine Production | Increases production of IL-6 and TNF-α | Brown et al. [117] |
| All BCAAs | T-Cell Activation | Enhances T-cell activation | Yao et al. [130] |
| Isoleucine | Macrophage Function | Modulates macrophage activity | Mao et al. [83] |
| Valine | Inflammation | Reduces chronic inflammation | Gart et al. [139] |
| Leucine and Isoleucine | Overall Immune Response | Improves immune response during stress | Wen et al. [114] |
5.3. Research Evidence on Inflammation Management
6. Practical Considerations for BCAA Supplementation in Endurance Athletes
6.1. Dosage and Timing
| Athlete Profile | BCAA Dosage (Grams per Day) | Timing Options | Comments | Study Type | References |
|---|---|---|---|---|---|
| Ultra-marathon runners | 5–10 g | Pre- and post-race | Reduces muscle soreness, speeds recovery by 20% | Observational study | Zhang et al. [146] |
| Triathletes | 15 g | Post-training | Decreases muscle damage markers by 25% | Randomized controlled trial | Durkalec-Michalski et al. [147] |
| Swimmers | 18–20 g | Pre- and post-training | Improves recovery, reduces fatigue by 18% | Cross-sectional study | Cai et al. [148] |
| Cyclists | 84 mg/kg | During training | Improves endurance, reduces fatigue during sessions | Randomized controlled trial | Fa et al. [149] |
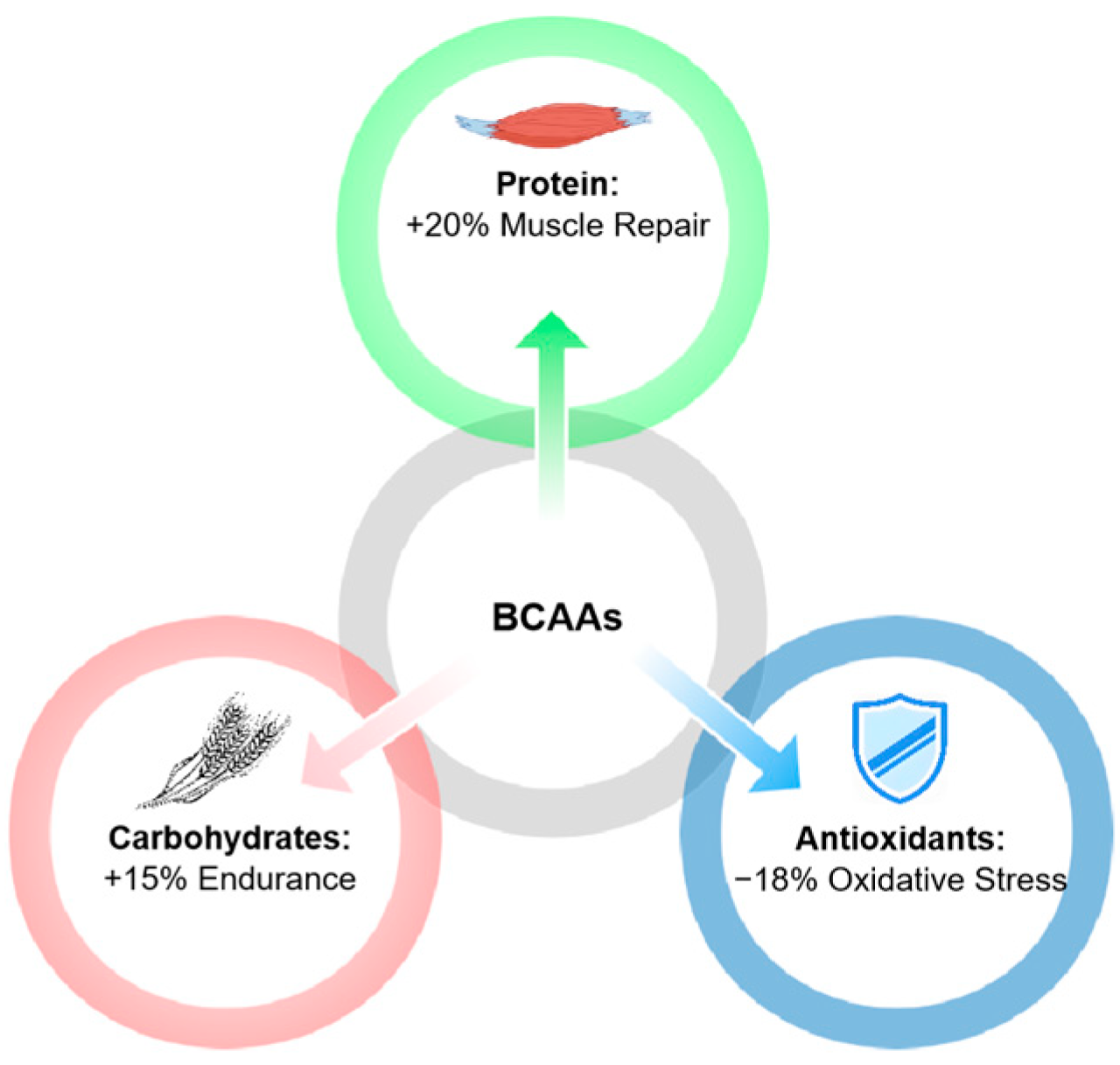
6.2. Synergistic Effects with Other Supplements
6.3. Application
7. Limitations and Future Directions
7.1. Limitations of Current Research
7.2. Future Research Directions
- (1)
- Standardization of Dosage and Protocols—There is a need for large-scale, well-controlled trials to determine optimal BCAA dosages, ratios, and timing specific to endurance athletes. Personalized supplementation strategies should be explored based on training intensity, metabolic demands, and genetic predisposition. Methodological recommendations include the use of double-blind, placebo-controlled designs and the establishment of standardized protocols to account for variations in exercise modalities and recovery periods.
- (2)
- Long-Term Effects on Recovery and Performance—While the short-term benefits of BCAA supplementation are well-documented, more research is needed to assess its long-term impact on muscle adaptation, chronic inflammation, and endurance capacity over extended training cycles. Future studies should implement longitudinal designs with repeated measures to capture changes over time and ensure more reliable conclusions about the long-term effects.
- (3)
- Integration with Other Nutritional Strategies—Investigating the synergistic effects of BCAAs when combined with other nutrients (e.g., carbohydrates, protein, omega-3s, and antioxidants) could provide valuable insights into optimal recovery protocols. Methodologically, studies should employ a multi-variable approach, controlling for nutrient interactions, and utilize metabolomic and proteomic analyses to explore synergistic effects at the molecular level.
- (4)
- Mechanistic Insights into Muscle and Immune Function—Advanced molecular and omics-based approaches (e.g., proteomics, metabolomics) should be employed to uncover the precise biochemical pathways through which BCAAs influence muscle repair, immune regulation, and mitochondrial function. It would be beneficial to combine these omics-based approaches with in vivo animal models and human trials to validate findings and provide a more comprehensive understanding of underlying mechanisms.
- (5)
- Personalized Nutrition and Genetic Factors—Future research should examine how genetic variations in amino acid metabolism affect BCAA utilization and efficacy in different athlete populations. Such findings could pave the way for more individualized and evidence-based supplementation strategies. Studies should incorporate genetic screening alongside phenotypic assessments to create a more robust understanding of individual variability and its impact on BCAA supplementation outcomes.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ostapiuk-Karolczuk, J.; Kasperska, A.; Dziewiecka, H.; Cieślicka, M.; Zawadka-Kunikowska, M.; Zaleska-Posmyk, I. Changes in the Hormonal and Inflammatory Profile of Young Sprint- and Endurance-Trained Athletes Following a Sports Camp: A Nonrandomized Pretest-Posttest Study. BMC Sports Sci. Med. Rehabil. 2024, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari Rad, R. The Impact of Different Training Intensities on Athletes’ Immune System Function and the Management of Upper Respiratory Traction Infections: A Narrative Review. Sport Sci. Health 2024, 20, 415–426. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Belinchón-deMiguel, P.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F.; Martínez-Guardado, I.; Villanueva-Tobaldo, C.V.; Clemente-Suárez, V.J. Advances in Understanding the Interplay between Dietary Practices, Body Composition, and Sports Performance in Athletes. Nutrients 2024, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Injury Prevention and Talent Retention: A Coach’s Role in Sustaining Athletic Potential in Young Athletes. Stud. Sports Sci. Phys. Educ. 2024, 2, 24–31. [Google Scholar] [CrossRef]
- Mardiana, M.; Kartini, A.; Sutiningsih, D.; Suroto, S.; Muhtar, M.S. Literature Review: Nutrition Supplementation for Muscle Fatigue in Athletes. J. Keolahragaan 2023, 11, 10–23. [Google Scholar] [CrossRef]
- Wang, L.; Meng, Q.; Su, C.-H. From Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L.; et al. Attenuating Muscle Damage Biomarkers and Muscle Soreness after an Exercise-Induced Muscle Damage with Branched-Chain Amino Acid (BCAA) Supplementation: A Systematic Review and Meta-Analysis with Meta-Regression. Sports Med.—Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The Effects of Branched-Chain Amino Acids on Muscle Protein Synthesis, Muscle Protein Breakdown and Associated Molecular Signalling Responses in Humans: An Update. Nutr. Res. Rev. 2024, 37, 273–286. [Google Scholar] [CrossRef]
- Orhan, B.E. The Role of Supplementation in Enhancing Recovery and Endurance among Fitness Trainers. IgMin Res. 2024, 2, 752–758. [Google Scholar] [CrossRef]
- Buonfiglio, V.; Pertici, I.; Marcello, M.; Morotti, I.; Caremani, M.; Reconditi, M.; Linari, M.; Fanelli, D.; Lombardi, V.; Bianco, P. Force and Kinetics of Fast and Slow Muscle Myosin Determined with a Synthetic Sarcomere–like Nanomachine. Commun. Biol. 2024, 7, 361. [Google Scholar] [CrossRef]
- Squire, J. The Actin-Myosin Interaction in Muscle: Background and Overview. Int. J. Mol. Sci. 2019, 20, 5715. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Hou, Y.; Lei, T.; Wei, D.; Zhao, Y. Multiple Roles of the Stress Sensor GCN2 in Immune Cells. Int. J. Mol. Sci. 2023, 24, 4285. [Google Scholar] [CrossRef]
- Dent, J.R.; Stocks, B.; Campelj, D.G.; Philp, A. Transient Changes to Metabolic Homeostasis Initiate Mitochondrial Adaptation to Endurance Exercise. Semin. Cell Dev. Biol. 2023, 143, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Z.; Liu, J. Amino Acids Regulating Skeletal Muscle Metabolism: Mechanisms of Action, Physical Training Dosage Recommendations and Adverse Effects. Nutr. Metab. 2024, 21, 41. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Delcastillo, K.; Every, D.W.V.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Gao, B. Integrative Effects of Resistance Training and Endurance Training on Mitochondrial Remodeling in Skeletal Muscle. Eur. J. Appl. Physiol. 2024, 124, 2851–2865. [Google Scholar] [CrossRef]
- Ma, X.; Liu, B.; Jiang, Z.; Rao, Z.; Zheng, L. Physical Exercise: A Promising Treatment against Organ Fibrosis. Int. J. Mol. Sci. 2025, 26, 343. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Z.; Zhang, H.; Lu, J.; Tian, Y.; Piao, S.; Lin, Z.; Bai, L. Moderate-Intensity Exercise Alleviates Pyroptosis by Promoting Autophagy in Osteoarthritis via the P2X7/AMPK/mTOR Axis. Cell Death Discov. 2021, 7, 346. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Song, K.; Liu, Z.-Y.; Ke, Y.-F.; Shi, Y.; Cai, K.; Zhao, R.; Sun, X.; Tao, H.; Zhao, J.-Y. Branched-Chain Amino Acids Deficiency Promotes Diabetic Cardiomyopathy by Activating Autophagy of Cardiac Fibroblasts. Theranostics 2024, 14, 7333–7348. [Google Scholar] [CrossRef]
- Choi, B.H.; Hyun, S.; Koo, S.-H. The Role of BCAA Metabolism in Metabolic Health and Disease. Exp. Mol. Med. 2024, 56, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Jang, Y.; Lee, Y. Endurance Exercise-Induced Autophagy/Mitophagy Coincides with a Reinforced Anabolic State and Increased Mitochondrial Turnover in the Cortex of Young Male Mouse Brain. J. Mol. Neurosci. 2021, 71, 42–54. [Google Scholar] [CrossRef]
- Corsetti, G.; Pasini, E.; Scarabelli, T.M.; Romano, C.; Singh, A.; Scarabelli, C.C.; Dioguardi, F.S. Importance of Energy, Dietary Protein Sources, and Amino Acid Composition in the Regulation of Metabolism: An Indissoluble Dynamic Combination for Life. Nutrients 2024, 16, 2417. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, B.; Yong, S.S.; Wu, X.; Tian, Z. Exercise and Nutrition Benefit Skeletal Muscle: From Influence Factor and Intervention Strategy to Molecular Mechanism. Sports Med. Health Sci. 2024, 6, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Debnath, M.; Das, M.; Bandyopadhyay, A.; Dey, S.K.; Datta, G. Effect of High Intensity Interval Training on Antioxidant Status, Inflammatory Response and Muscle Damage Indices in Endurance Team Male Players. Apunt. Sports Med. 2021, 56, 100352. [Google Scholar] [CrossRef]
- Leite, C.D.F.C.; Zovico, P.V.C.; Rica, R.L.; Barros, B.M.; Machado, A.F.; Evangelista, A.L.; Leite, R.D.; Barauna, V.G.; Maia, A.F.; Bocalini, D.S. Exercise-Induced Muscle Damage after a High-Intensity Interval Exercise Session: Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 7082. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E. Exercise-Induced Oxidative Stress and Melatonin Supplementation: Current Evidence. J. Physiol. Sci. 2021, 71, 27. [Google Scholar] [CrossRef]
- Mallett, G.S.; McGrath, K. Effect of Endurance Exercise on Markers of Oxidative Stress: A Systematic Review. J. Sci. Sport Exerc. 2024. [Google Scholar] [CrossRef]
- Stožer, A.; Vodopivc, P.; Križančić Bombek, L. Pathophysiology of Exercise-Induced Muscle Damage and Its Structural, Functional, Metabolic, and Clinical Consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Goldstein, N.; Amedi, R.; Bornstein, Y.; Stolkovsky, Y.; Vidal, M.; Lev-Ari, S.; Balaban, R.; Leibou, S.; et al. Age Differences in Recovery Rate Following an Aerobic-Based Exercise Protocol Inducing Muscle Damage among Amateur, Male Athletes. Front. Physiol. 2022, 13, 916924. [Google Scholar] [CrossRef]
- D’Amico, A.; Fossati, C.; Pigozzi, F.; Borrione, P.; Peruzzi, M.; Bartimoccia, S.; Saba, F.; Pingitore, A.; Biondi-Zoccai, G.; Petramala, L.; et al. Natural Activators of Autophagy Reduce Oxidative Stress and Muscle Injury Biomarkers in Endurance Athletes: A Pilot Study. Nutrients 2023, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. [Google Scholar] [CrossRef]
- Muire, P.J.; Mangum, L.H.; Wenke, J.C. Time Course of Immune Response and Immunomodulation during Normal and Delayed Healing of Musculoskeletal Wounds. Front. Immunol. 2020, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Nasir, Y.; Rahimi, M.H. Effect of Omega-3 Fatty Acids Supplementation on Inflammatory Markers Following Exercise-Induced Muscle Damage: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Clin. Métabolisme 2024, 38, 158–167. [Google Scholar] [CrossRef]
- Sözlü, U.; Başar, S.; Şemsi, R.; Akaras, E.; Dinçel, A. Effectiveness of neurodynamic mobilization technique on delayed onset muscle soreness-associated muscle damage and inflammatory biomarkers. In International Studies and Evaluation in the Field of Health Sciences; Serüven: Ankara, Turkey, 2024. [Google Scholar]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 Signaling in Acute Exercise and Chronic Training: Potential Consequences for Health and Athletic Performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Brzezinska, P.; Mieszkowski, J.; Kochanowicz, A.; Niespodzinski, B.; Surmiak, M.; Reczkowicz, J.; Borkowska, A.; Antosiewicz, J. Single and Consecutive 10-Day Remote Ischemic Preconditioning Modify Physical Performance, Post-Exercise Exerkine Levels, and Inflammation. Front. Physiol. 2024, 15, 1428404. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; James, L.S.; Hussey, B.; Ferguson, R.A.; Lindley, M.R.; Mastana, S.S. Impacts of Eccentric Resistance Exercise on DNA Methylation of Candidate Genes for Inflammatory Cytokines in Skeletal Muscle and Leukocytes of Healthy Males. Genes 2023, 14, 478. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef]
- Notbohm, H.L.; Umlauff, L.; Bloch, W.; Schumann, M. Comparison of the Cytokine Responses to Acute Strength Exercise between Oral Contraceptive Users and Naturally Cycling Women. Eur. J. Appl. Physiol. 2024, 124, 257–267. [Google Scholar] [CrossRef]
- Małkowska, P.; Sawczuk, M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11156. [Google Scholar] [CrossRef] [PubMed]
- Kusnanik, N.W.; Komaini, A.; Adi, S.; Pradana, P.Y.; Ayubi, N.; Bird, S.P. Acute Effect of Curcumin on Interleukin-6 (IL-6) Levels and C-Reactive Protein (CRP) Levels after High-Intensity Physical Exercises. Trends Sport Sci. 2024, 31, 161–167. [Google Scholar] [CrossRef]
- Jamurtas, A.Z. Exercise-Induced Muscle Damage and Oxidative Stress. Antioxidants 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Wojciuk, B.; Frulenko, I.; Brodkiewicz, A.; Kita, D.; Baluta, M.; Jędrzejczyk, F.; Budkowska, M.; Turkiewicz, K.; Proia, P.; Ciechanowicz, A.; et al. The Complement System as a Part of Immunometabolic Post-Exercise Response in Adipose and Muscle Tissue. Int. J. Mol. Sci. 2024, 25, 11608. [Google Scholar] [CrossRef]
- Di, C.; Jia, W. Food-Derived Bioactive Peptides as Momentous Food Components: Can Functional Peptides Passed through the PI3K/Akt/mTOR Pathway and NF-κB Pathway to Repair and Protect the Skeletal Muscle Injury? Crit. Rev. Food Sci. Nutr. 2024, 64, 9210–9227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Luo, S.; Zhang, Y.; Feng, Z.; Li, S. The Roles and Mechanisms of the NF-κB Signaling Pathway in Tendon Disorders. Front. Vet. Sci. 2024, 11, 1382239. [Google Scholar] [CrossRef]
- Puengpan, S.; Phetrungnapha, A.; Sattayakawee, S.; Tunsophon, S. Phycocyanin Attenuates Skeletal Muscle Damage and Fatigue via Modulation of Nrf2 and IRS-1/AKT/mTOR Pathway in Exercise-Induced Oxidative Stress in Rats. PLoS ONE 2024, 19, e0310138. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, J.; Xu, Z.; Liu, Z.; Bao, L.; Xue, L. ROS-Induced Autophagy of Skeletal Muscle Confers Resistance of Rice Flower Carp (Cyprinus Carpio) to Short-Term Fasting. Genes 2024, 15, 840. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, H.; Yun, H.; Liu, J.; Song, G.; Teng, J.; Zou, D.; Lu, N.; Liu, C. Assessment of Urolithin a Effects on Muscle Endurance, Strength, Inflammation, Oxidative Stress, and Protein Metabolism in Male Athletes with Resistance Training: An 8-Week Randomized, Double-Blind, Placebo-Controlled Study. J. Int. Soc. Sports Nutr. 2024, 21, 2419388. [Google Scholar] [CrossRef]
- Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Levels of IL-8 and MCP-1 in Healthy Adults: Changes after an Acute Aerobic Exercise and Association with Body Composition and Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 14725. [Google Scholar] [CrossRef]
- Egan, B.; Sharples, A.P. Molecular Responses to Acute Exercise and Their Relevance for Adaptations in Skeletal Muscle to Exercise Training. Physiol. Rev. 2023, 103, 2057–2170. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Sánchez, N.; Alonso-Alonso, S.; Nagy, L. Regenerative Inflammation: When Immune Cells Help to Re-Build Tissues. FEBS J. 2024, 291, 1597–1614. [Google Scholar] [CrossRef] [PubMed]
- Khuu, S.; Fernandez, J.W.; Handsfield, G.G. Delayed Skeletal Muscle Repair Following Inflammatory Damage in Simulated Agent-Based Models of Muscle Regeneration. PLoS Comput. Biol. 2023, 19, e1011042. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Lin, Y.; Liu, F.; Tao, J.; Zhang, W.; Xu, J.; Zhang, M. Extracellular Vesicles Derived from Human ESC–MSCs Target Macrophage and Promote Anti-Inflammation Process, Angiogenesis, and Functional Recovery in ACS-Induced Severe Skeletal Muscle Injury. Stem Cell Res. Ther. 2023, 14, 331. [Google Scholar] [CrossRef]
- Dias, K.A.; Conceição, A.R.d.; Oliveira, L.A.; Pereira, S.M.S.; Paes, S.d.S.; Monte, L.F.; Sarandy, M.M.; Novaes, R.D.; Gonçalves, R.V.; Lucia, C.M.D. Effects of Curcumin Supplementation on Inflammatory Markers, Muscle Damage, and Sports Performance during Acute Physical Exercise in Sedentary Individuals. Oxidative Med. Cell. Longev. 2021, 2021, 9264639. [Google Scholar] [CrossRef]
- Marquez, K. Effects of Branched Chain Amino Acid Supplementation on Post-Exercise Muscle Recovery and Muscle Growth; Honors Projects; Grand Valley State University: Allendale, MI, USA, 2022. [Google Scholar]
- Salem, A.; Trabelsi, K.; Jahrami, H.; AlRasheed, M.M.; Boukhris, O.; Puce, L.; Bragazzi, N.L.; Ammar, A.; Glenn, J.M.; Chtourou, H. Branched-Chain Amino Acids Supplementation and Post-Exercise Recovery: An Overview of Systematic Reviews. J. Am. Nutr. Assoc. 2024, 43, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.; Myles, A. BCAAs as Ergogenic Aids: Exploring Their Impact on Exercise-Induced Muscle Damage. Stud. Sports Sci. Phys. Educ. 2024, 2, 1–10. [Google Scholar] [CrossRef]
- Mann, G.; Adegoke, O.A.J. Elevated BCAA Catabolism Reverses the Effect of Branched-Chain Ketoacids on Glucose Transport in mTORC1-Dependent Manner in L6 Myotubes. J. Nutr. Sci. 2024, 13, e66. [Google Scholar] [CrossRef]
- Song, M.-Q.; Yu, Q.-R.; Li, E.-C.; Song, Y.; Cai, X.-Y.; Huang, Y.-X.; Qin, C.-J.; Wang, X.-D.; Qin, J.-G.; Chen, L.-Q. Leucine Improves Dietary Protein Use Efficiency by Regulating Protein Synthesis by Activating Amino Acid Transporters and the mTORC1 Pathways in Chinese Mitten Crab (Eriocheir Sinensis). Aquaculture 2024, 581, 740423. [Google Scholar] [CrossRef]
- Shan, C.; Liu, Y.; Ma, C.; Li, C.; Liu, Q.; Liu, S.; Jiang, G.; Tian, J. Dietary Supplementation with Clostridium Autoethanogenum Protein Improves Growth Performance and Promotes Muscle Protein Synthesis by Activating the mTOR Signaling Pathway of the Broiler. Front. Vet. Sci. 2024, 11, 1389738. [Google Scholar] [CrossRef]
- Jiang, C.; Tan, X.; Liu, N.; Yan, P.; Hou, T.; Wei, W. Nutrient Sensing of mTORC1 Signaling in Cancer and Aging. Semin. Cancer Biol. 2024, 106–107, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Kumar, A.; Piprode, V.; Dasgupta, A.; Singh, S.; Khalique, A. Regulatory-Associated Protein of mTOR-Mediated Signaling: A Nexus between Tumorigenesis and Disease. Targets 2024, 2, 341–371. [Google Scholar] [CrossRef]
- Hur, H.; Kim, H.-J.; Lee, D.; Jo, C. Beef Peptides Mitigate Skeletal Muscle Atrophy in C2C12 Myotubes through Protein Degradation, Protein Synthesis, and the Oxidative Stress Pathway. Food Funct. 2024, 15, 4564–4574. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Fujii, J. Primary Roles of Branched Chain Amino Acids (BCAAs) and Their Metabolism in Physiology and Metabolic Disorders. Molecules 2025, 30, 56. [Google Scholar] [CrossRef]
- Ma, C.; Teng, L.; Lin, G.; Guo, B.; Zhuo, R.; Qian, X.; Guan, T.; Wu, R.; Liu, Y.; Liu, M. L-Leucine Promotes Axonal Outgrowth and Regeneration via mTOR Activation. FASEB J. 2021, 35, e21526. [Google Scholar] [CrossRef]
- Son, S.M.; Park, S.J.; Stamatakou, E.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Leucine Regulates Autophagy via Acetylation of the mTORC1 Component Raptor. Nat. Commun. 2020, 11, 3148. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.S. Branched-Chain Amino Acid Catabolism: Regulation and Effect on Insulin Resistance. Ph.D. Thesis, York University, Toronto, ON, Canada, 2024. [Google Scholar]
- Abdualkader, A.M.; Karwi, Q.G.; Lopaschuk, G.D.; Al Batran, R. The Role of Branched-Chain Amino Acids and Their Downstream Metabolites in Mediating Insulin Resistance. J. Pharm. Pharm. Sci. 2024, 27, 13040. [Google Scholar] [CrossRef]
- Posey, E.A.; Bazer, F.W.; Wu, G. Amino Acids and Their Metabolites for Improving Human Exercising Performance. Adv. Exp. Med. Biol. 2021, 1332, 151–166. [Google Scholar] [CrossRef]
- Hwang, D.-J.; Yang, H.-J. Nutritional Strategies for Enhancing Performance and Training Adaptation in Weightlifters. Int. J. Mol. Sci. 2025, 26, 240. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Cabre, H.E.; Gould, L.M.; Blue, M.N.M.; Smith-Ryan, A.E. Effects of Essential Amino Acids on High-Intensity Interval Training Performance, Fatigue Outcomes, and Workload Progression. J. Am. Nutr. Assoc. 2023, 42, 411–417. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Reifenberg, P.; Zimmer, A. Branched-Chain Amino Acids: Physico-Chemical Properties, Industrial Synthesis and Role in Signaling, Metabolism and Energy Production. Amino Acids 2024, 56, 51. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.; Sisti, D.; Amatori, S.; Donati Zeppa, S.; Annibalini, G.; Piccoli, G.; Vallorani, L.; Benelli, P.; Rocchi, M.B.L.; Barbieri, E.; et al. Effects of a Commercially Available Branched-Chain Amino Acid-Alanine-Carbohydrate-Based Sports Supplement on Perceived Exertion and Performance in High Intensity Endurance Cycling Tests. J. Int. Soc. Sports Nutr. 2020, 17, 6. [Google Scholar] [CrossRef]
- Holeček, M. Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H. Possible Role of Exercise Therapy on Depression: Effector Neurotransmitters as Key Players. Behav. Brain Res. 2024, 459, 114791. [Google Scholar] [CrossRef]
- AbuMoh’d, M.F.; Matalqah, L.; Al-Abdulla, Z. Effects of Oral Branched-chain Amino Acids (BCAAs) Intake on Muscular and Central Fatigue during an Incremental Exercise. J. Hum. Kinet. 2020, 72, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gharakhanlou, R.; Fasihi, L. The Role of Neurotransmitters (Serotonin and Dopamine) in Central Nervous System Fatigue during Prolonged Exercise. New Approaches Exerc. Physiol. 2023, 5, 138–160. [Google Scholar] [CrossRef]
- Martinho, D.V.; Nobari, H.; Faria, A.; Field, A.; Duarte, D.; Sarmento, H. Oral Branched-Chain Amino Acids Supplementation in Athletes: A Systematic Review. Nutrients 2022, 14, 4002. [Google Scholar] [CrossRef]
- Yamashita, M. Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit. Int. J. Tryptophan Res. 2020, 13, 1178646920936279. [Google Scholar] [CrossRef]
- Mao, X.; Sun, R.; Wang, Q.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Luo, Y.; Yan, H.; et al. L-Isoleucine Administration Alleviates DSS-Induced Colitis by Regulating TLR4/MyD88/NF-κB Pathway in Rats. Front. Immunol. 2022, 12, 817583. [Google Scholar] [CrossRef]
- Beigi, M.-H. Valine Consumption and Endurance Exercise Modified the Inflammation in Rat Model of Anxiety/Depression. Exerc. Physiol. Perform. 2023, 1, 81–90. [Google Scholar] [CrossRef]
- Wang, S. Influence of Branched-Chain Amino Acid Ingestion on Creatine Kinase Post of Eccentric Exercise on Recovery: A Systematic Review and Meta-Analysis. Braz. Arch. Biol. Technol. 2024, 67, e24220879. [Google Scholar] [CrossRef]
- de Sousa Fernandes, M.S.; Gomes, J.M.; Aidar, F.J.; Thuany, M.; Filgueira, T.O.; de Souza, R.F.; Badicu, G.; Yagin, F.H.; Greco, G.; Cataldi, S.; et al. Impacts of Different Triathlon Races on Systemic Cytokine Profile and Metabolic Parameters in Healthy Individuals: A Systematic Review. BMC Sports Sci. Med. Rehabil. 2023, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Madzar, T.; Masina, T.; Zaja, R.; Kastelan, S.; Cvetkovic, J.P.; Brborovic, H.; Dvorski, M.; Kirin, B.; Barisic, A.V.; Cehok, I.; et al. Overtraining Syndrome as a Risk Factor for Bone Stress Injuries among Paralympic Athletes. Medicina 2024, 60, 52. [Google Scholar] [CrossRef] [PubMed]
- Kusy, K.; Ciekot-Sołtysiak, M.; Matysiak, J.; Klupczyńska-Gabryszak, A.; Plewa, S.; Zarębska, E.A.; Kokot, Z.J.; Dereziński, P.; Zieliński, J. Changes in Plasma Free Amino Acid Profile in Endurance Athletes over a 9-Month Training Cycle. Metabolites 2024, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Jiang, M.; Li, X.; Yuan, J.; Zhang, H. Precision Medicine in Inflammatory Bowel Disease. Precis. Clin. Med. 2023, 6, pbad033. [Google Scholar] [CrossRef]
- Weber, M.G.; Dias, S.S.; de Angelis, T.R.; Fernandes, E.V.; Bernardes, A.G.; Milanez, V.F.; Jussiani, E.I.; de Paula Ramos, S. The Use of BCAA to Decrease Delayed-Onset Muscle Soreness after a Single Bout of Exercise: A Systematic Review and Meta-Analysis. Amino Acids 2021, 53, 1663–1678. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, Y.; Zhang, C.; Zhao, X.; Qiu, J. Consumption of a Branched-Chain Amino Acids-Containing Sports Beverage during 21 Km of Running Reduces Dehydration, Lowers Muscle Damage, and Prevents a Decline in Lower Limb Strength. Nutrients 2024, 16, 3799. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, X.; Azhar, G.; Patyal, P.; Verma, A.; KC, G.; Wei, J.Y. Valine Improves Mitochondrial Function and Protects against Oxidative Stress. Biosci. Biotechnol. Biochem. 2024, 88, 168–176. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, Z.; Qu, P. NAD+-A Hub of Energy Metabolism in Heart Failure. Int. J. Med. Sci. 2024, 21, 369–375. [Google Scholar] [CrossRef]
- Gupta, S.; Afzal, M.; Agrawal, N.; Almalki, W.H.; Rana, M.; Gangola, S.; Chinni, S.V.; Kumar, K.B.; Ali, H.; Singh, S.K.; et al. Harnessing the FOXO-SIRT1 Axis: Insights into Cellular Stress, Metabolism, and Aging. Biogerontology 2025, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fan, D.; Liu, F.-Y.; Ma, S.-Q.; An, P.; Yang, D.; Wang, M.-Y.; Yang, Z.; Tang, Q.-Z. NEU1 Regulates Mitochondrial Energy Metabolism and Oxidative Stress Post-Myocardial Infarction in Mice via the SIRT1/PGC-1 Alpha Axis. Front. Cardiovasc. Med. 2022, 9, 821317. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Hinkle, J.S.; Rivera, C.N.; Vaughan, R.A. Branched-Chain Amino Acids and Mitochondrial Biogenesis: An Overview and Mechanistic Summary. Mol. Nutr. Food Res. 2022, 66, 2200109. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef]
- Pellicer-Caller, R.; Vaquero-Cristóbal, R.; González-Gálvez, N.; Abenza-Cano, L.; Horcajo, J.; de la Vega-Marcos, R. Influence of Exogenous Factors Related to Nutritional and Hydration Strategies and Environmental Conditions on Fatigue in Endurance Sports: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 2700. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.B.; Melhem, M.B.; Al-Ababneh, H. The Impact of Some Types of Physical Activity on the Level of Releasing Serotonin Hormone (a Comparative Study). Educ. Psychol. Sci. Ser. 2023, 2, 119–134. [Google Scholar] [CrossRef]
- Mallea, A.P.; Espinosa, C.D.; Lee, S.A.; Cristobal, M.A.; Torrez-Mendoza, L.J.; Stein, H.H. Dietary Supplementation of Valine, Isoleucine, and Tryptophan May Overcome the Negative Effects of Excess Leucine in Diets for Weanling Pigs Containing Corn Fermented Protein. J. Anim. Sci. Biotechnol. 2024, 15, 125. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Yamamoto, S.; Katano, Y.; Narita, I. The Combined Effect of Leucine-Enriched Essential Amino Acid Supplements and Locomotion Training on Physical Functions and Quality of Life in Hemodialysis Patients. Ren. Replace. Ther. 2024, 10, 33. [Google Scholar] [CrossRef]
- Falabrègue, M.; Boschat, A.-C.; Jouffroy, R.; Derquennes, M.; Djemai, H.; Sanquer, S.; Barouki, R.; Coumoul, X.; Toussaint, J.-F.; Hermine, O.; et al. Lack of Skeletal Muscle Serotonin Impairs Physical Performance. Int. J. Tryptophan Res. 2021, 14, 11786469211003109. [Google Scholar] [CrossRef]
- Stefańska, O.; Rudnicki, J.; Szczepocki, M.; Jurek, J.M. Narrative Literature Review: Effect of Branched-Chain Amino Acids (BCAAs) on Muscle Hypertrophy and Athletic Performance. Tanjungpura J. Coach. Res. 2024, 2, 46–59. [Google Scholar] [CrossRef]
- De Salles Painelli, V.; Lienbenberger, C.A.; Zorek, L.; Pires, F.O. Mental Fatigue Impairs Strength Endurance Performance in Trained Individuals. Sport Sci. Health 2024, 20, 789–796. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. Mechanisms of Physical Fatigue and Its Applications in Nutritional Interventions. J. Agric. Food Chem. 2021, 69, 6755–6768. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.J.A.; Best, R.; Best, A.W.; Lane, A.M.; Millet, G.Y.; Barwood, M.; Marcora, S.; Wilson, P.; Bearden, S. Limits of Ultra: Towards an Interdisciplinary Understanding of Ultra-Endurance Running Performance. Sports Med. 2024, 54, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Genovesi, F.; Nemmer, M.; Carling, C.; Alberti, G.; Howatson, G. Nutritional Interventions for Reducing the Signs and Symptoms of Exercise-Induced Muscle Damage and Accelerate Recovery in Athletes: Current Knowledge, Practical Application and Future Perspectives. Eur. J. Appl. Physiol. 2020, 120, 1965–1996. [Google Scholar] [CrossRef]
- Parthimos, T.P.; Schulpis, K.H.; Loukas, Y.L.; Dotsikas, Y. Increased Blood Concentrations of Neurotransmission Amino Acids and Modulation of Specific Enzyme Activities after Resistance and Endurance Exercise. Sport Sci. Health 2020, 16, 217–226. [Google Scholar] [CrossRef]
- Li, H.; Seugnet, L. Decoding the Nexus: Branched-Chain Amino Acids and Their Connection with Sleep, Circadian Rhythms, and Cardiometabolic Health. Neural Regen. Res. 2025, 20, 1350. [Google Scholar] [CrossRef] [PubMed]
- Majdizadeh, G.; Beytollahi, M.; Djazayery, A.; Movahedi, A. Role of Branched and Aromatic Amino Acids, Diet Inflammatory Index, and Anthropometric Indices on Mental Health. Int. J. Prev. Med. 2024, 15, 23. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Doma, K.; Singh, U.; Boullosa, D.; Connor, J.D. The Effect of Branched-Chain Amino Acid on Muscle Damage Markers and Performance Following Strenuous Exercise: A Systematic Review and Meta-Analysis. Appl. Physiol. Nutr. Metab. 2021, 46, 1303–1313. [Google Scholar] [CrossRef]
- Wen, J.; Fan, C.; Liu, M.; Li, Q.; Shi, C.; Wu, X.; Wang, C.; Liu, K.; Wu, W. Leucine-enriched Essential Amino Acids Promote Muscle Protein Synthesis and Ameliorate Exercise-Induced Exhaustion in Prolonged Endurance Exercise in Rats. Nutrire 2022, 47, 7. [Google Scholar] [CrossRef]
- Baraniuk, J.N. Exertional Exhaustion (Post-Exertional Malaise, PEM) Evaluated by the Effects of Exercise on Cerebrospinal Fluid Metabolomics–Lipidomics and Serine Pathway in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2025, 26, 1282. [Google Scholar] [CrossRef]
- Duttagupta, S.; Krishna Roy, N.; Dey, G. Efficacy of Amino Acids in Sports Nutrition- Review of Clinical Evidences. Food Res. Int. 2024, 187, 114311. [Google Scholar] [CrossRef]
- Brown, A.D.; Grammenou, M.; Tee, C.C.; Chong, M.C.; Stewart, C.E. Exercise on Cytokine Responses in Males and Females: Effect of Leucine, HMB and BCAA. bioRxiv 2023. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The Effect of Exercise on Cytokines: Implications for Musculoskeletal Health: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Wu, Y.; Zhang, F. Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism. Nutrients 2023, 15, 2161. [Google Scholar] [CrossRef]
- An, Y.H.; Kim, J.; Kim, H.-J.; Lim, K. Effects of Leucine-Enriched Essential Amino Acid Supplementation on Muscular Fatigue and Inflammatory Cytokines in Wheelchair Basketball Players. Phys. Act. Nutr. 2020, 24, 38–46. [Google Scholar] [CrossRef]
- Sadri, S.; Sharifi, G.; Jalali Dehkordi, K. Nano Branched-Chain Amino Acids Enhance the Effect of Uphill (Concentric) and Downhill (Eccentric) Treadmill Exercise on Muscle Gene Expression of Akt and mTOR on Aged Rats. Sport Sci. Health 2022, 18, 481–490. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can Exercise Affect Immune Function to Increase Susceptibility to Infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; de Araujo, R.B.; Woods, J.A.; Lord, J.M.; Chacon-Mikahil, M.P.T. Higher Risk of Upper Respiratory Tract Infection Post Marathon Running: When Physical Exercise Becomes a Threat to the Immune System. Exerc. Immunol. Rev. 2024, 30, 6–13. [Google Scholar]
- Schumm, B.; Heiber, M.; Grätz, F.; Stabile, L.; Buonanno, G.; Schönfelder, M.; Hain, R.; Kähler, C.J.; Wackerhage, H. Respiratory Aerosol Particle Emission and Simulated Infection Risk Is Greater during Indoor Endurance than Resistance Exercise. Proc. Natl. Acad. Sci. USA 2023, 120, e2220882120. [Google Scholar] [CrossRef]
- Roh, H.-T. Effects of Acute Endurance Exercise at 65% and 85% of VO2max on Markers of Immune Function in Healthy Subjects. Iran. J. Public Health 2023, 52, 1303–1305. [Google Scholar] [CrossRef]
- Chien, Y.-J.; Tsao, J.-P.; Tsai, C.-T.; Cheng, I.-S.; Hsu, C.-L. Antifatigue Effect of Okara Protein Hydrolysate Supplementation during Cycling Exercise in Men: A Pre-Post Uncontrolled Pilot Study. J. Int. Soc. Sports Nutr. 2024, 21, 2416479. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Koksoy, S.; Vojdani, E.; Engelman, M.; Benzvi, C.; Lerner, A. Natural Killer Cells and Cytotoxic T Cells: Complementary Partners against Microorganisms and Cancer. Microorganisms 2024, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-Cell Subsets: Heterogeneity, Functions, and Therapeutic Potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef]
- Yao, C.; Sun, R.; Yang, Y.; Zhou, H.; Meng, Z.; Chi, R.; Xia, L.; Ji, P.; Chen, Y.; Zhang, G.; et al. Accumulation of Branched-Chain Amino Acids Reprograms Glucose Metabolism in CD8+ T Cells with Enhanced Effector Function and Anti-Tumor Response. Cell Rep. 2023, 42, 112186. [Google Scholar] [CrossRef]
- Tj, W.; Sc, E.; Ea, A. The Emerging Role of the Branched Chain Aminotransferases, BCATc and BCATm, for Anti-Tumor T-Cell Immunity. Immunometabolism 2023, 5, e00014. [Google Scholar]
- Liu, S.; Mochizuki, M.; Suzuki, Y.; Takemasa, E.; Yano, A.; Imai, M.; Mogi, M. Dietary Leucine Supplementation Restores T-Cell Mitochondrial Respiration and Regulates T-Lineage Differentiation in Denervation-Induced Sarcopenic Mice. J. Nutr. Biochem. 2024, 124, 109508. [Google Scholar] [CrossRef]
- Garcia, B.R.E.V.; Makiyama, E.N.; Sampaio, G.R.; Soares-Freitas, R.A.M.; Bonvini, A.; Amaral, A.G.; Bordin, S.; Fock, R.A.; Rogero, M.M. Effects of Branched-Chain Amino Acids on the Inflammatory Response Induced by LPS in Caco-2 Cells. Metabolites 2024, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Wang, N.; Zhang, X.; Liu, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. Comprehensive Study on the Effect of Dietary Leucine Supplementation on Intestinal Physiology, TOR Signaling and Microbiota in Juvenile Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2023, 141, 109060. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Luo, D.; Zhang, Z.; Ouyang, F.; Shi, Y.; Hu, C.; Su, H.; Li, Y.; Zhang, J.; Gui, Q.; et al. Branched-Chain Amino Acid Catabolism Promotes M2 Macrophage Polarization. Front. Immunol. 2024, 15, 1469163. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.Y.; Chi, H.; Ng, S.; Ng, D.H.; Shelat, V.G. Effect of Perioperative Branched Chain Amino Acids Supplementation in Liver Cancer Patients Undergoing Surgical Intervention: A Systematic Review. World J. Gastrointest. Surg. 2023, 15, 2596–2618. [Google Scholar] [CrossRef]
- Wiącek, J.; Karolkiewicz, J. Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review. Nutrients 2023, 15, 1541. [Google Scholar] [CrossRef]
- Yahsi, B.; Gunaydin, G. Immunometabolism—The Role of Branched-Chain Amino Acids. Front. Immunol. 2022, 13, 886822. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Caspers, M.P.M.; van Trigt, N.; Snabel, J.; Menke, A.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R. Intervention with Isoleucine or Valine Corrects Hyperinsulinemia and Reduces Intrahepatic Diacylglycerols, Liver Steatosis, and Inflammation in Ldlr−/−.Leiden Mice with Manifest Obesity-Associated NASH. FASEB J. 2022, 36, e22435. [Google Scholar] [CrossRef]
- Khemtong, C.; Kuo, C.-H.; Chen, C.-Y.; Jaime, S.J.; Condello, G. Does Branched-Chain Amino Acids (BCAAs) Supplementation Attenuate Muscle Damage Markers and Soreness after Resistance Exercise in Trained Males? A Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1880. [Google Scholar] [CrossRef]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Costa Rosa, L.F. The Effect of BCAA Supplementation upon the Immune Response of Triathletes. Med. Sci. Sports Exerc. 2000, 32, 1214–1219. [Google Scholar] [CrossRef]
- Alhebshi, A.; Alsharif, N.; Thorley, J.; James, L.J.; Clifford, T. The Effects of Dietary Protein Supplementation on Exercise-Induced Inflammation and Oxidative Stress: A Systematic Review of Human Trials. Antioxidants 2022, 11, 13. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Mu, H.; Zhang, W.; Zeng, J.; Li, H.; Wang, S.; Zhao, X.; Chen, W.; Dong, J.; et al. Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice. Nutrients 2022, 14, 5065. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Effects of Branched-Chain Amino Acid Supplementation on Exercise Induced Muscle Damage and Delayed Onset of Muscle Soreness After a Bout of Eccentric Exercise. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2021. Electronic Thesis and Dissertation Repository. [Google Scholar]
- Bird, S.P.; Nienhuis, M.; Biagioli, B.; De Pauw, K.; Meeusen, R. Supplementation Strategies for Strength and Power Athletes: Carbohydrate, Protein, and Amino Acid Ingestion. Nutrients 2024, 16, 1886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, X.; Dai, Z.; Guo, J. Usage and Trends of Dietary Supplements in Ultra-Long Distance Endurance Events in China. Stud. Sports Sci. Phys. Educ. 2023, 1, 1–7. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Kusy, K.; Ciekot-Sołtysiak, M.; Zieliński, J. The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study. Nutrients 2019, 11, 1961. [Google Scholar] [CrossRef]
- Cai, M.; Wu, C.; Jing, C.; Shen, X.; He, M.; Wang, L.; Guo, Q.; Yan, Y.; Yan, X.; Yang, R. Blood Metabolomics Analysis Identifies Differential Serum Metabolites in Elite and Sub-Elite Swimmers. Front. Physiol. 2022, 13, 858869. [Google Scholar] [CrossRef]
- Manaf, F.A.; Peiffer, J.J.; Maker, G.L.; Fairchild, T.J. Branched-Chain Amino Acid Supplementation Improves Cycling Performance in Untrained Cyclists. J. Sci. Med. Sport 2021, 24, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Yanagimoto, K.; Sunagawa, N.; Ueda, H.; Tsuji, K.; Ochi, E. Omega-3 Fatty Acids Enhance the Beneficial Effect of BCAA Supplementation on Muscle Function Following Eccentric Contractions. J. Int. Soc. Sports Nutr. 2022, 19, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Saoud, H.A.; et al. Athletes’ Nutritional Demands: A Narrative Review of Nutritional Requirements. Front. Nutr. 2023, 10, 1331854. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef]
- Rostami, A.; Damirchi, A.; Ebrahimi, M. The Effect of Branched Chain Amino Acids and Vitamin E on Muscle Damage. Complement. Med. J. 2022, 12, 160–171. [Google Scholar] [CrossRef]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef]
- Walters, J. The Effects of Branched Chained Amino Acid Supplementation on Acute Markers of Fatigue and Performance. Ph.D. Thesis, East Tennessee State University, Johnson City, TN, USA, 2019. Electronic Theses and Dissertations. [Google Scholar]
- Kaufman, M.W.; Roche, M.; Fredericson, M. The Impact of Supplements on Sports Performance for the Trained Athlete: A Critical Analysis. Curr. Sport Med. Rep. 2022, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Khoonin, W.; Shantavasinkul, P.C.; Santivarangkna, C.; Praengam, K.; Trachootham, D. Eicosapentaenoic Acid and Branched-Chain Amino Acids Fortified Complete Nutrition Drink Improved Muscle Strength in Older Individuals with Inadequate Protein Intake. Front. Nutr. 2023, 10, 1164469. [Google Scholar] [CrossRef] [PubMed]
- Martinez Galan, B.S.; Giolo De Carvalho, F.; Carvalho, S.C.S.; Cunha Brandao, C.F.; Morhy Terrazas, S.I.; Abud, G.F.; Meirelles, M.S.S.; Sakagute, S.; Ueta Ortiz, G.; Marchini, J.S.; et al. Casein and Whey Protein in the Breast Milk Ratio: Could It Promote Protein Metabolism Enhancement in Physically Active Adults? Nutrients 2021, 13, 2153. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Hu, D.; Liu, X.; Li, Z.; Lu, L. Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications. Nutrients 2025, 17, 1335. https://doi.org/10.3390/nu17081335
Xu M, Hu D, Liu X, Li Z, Lu L. Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications. Nutrients. 2025; 17(8):1335. https://doi.org/10.3390/nu17081335
Chicago/Turabian StyleXu, Miaomiao, Danting Hu, Xiaoguang Liu, Zhaowei Li, and Liming Lu. 2025. "Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications" Nutrients 17, no. 8: 1335. https://doi.org/10.3390/nu17081335
APA StyleXu, M., Hu, D., Liu, X., Li, Z., & Lu, L. (2025). Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications. Nutrients, 17(8), 1335. https://doi.org/10.3390/nu17081335






