Frailty and Energy Intake Deficiency Reduce the Efficiency of Activities of Daily Living in Patients with Musculoskeletal Disorders: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants and Setting
2.3. Assessment of Frailty and Energy Intake
2.4. Rehabilitation Outcome
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | activities of daily living |
| ANCOVA | analysis of covariance |
| ANOVA | analysis for a one-way analysis of variance |
| BEE | basal energy expenditure |
| BI | Barthel Index |
| BMI | body mass index |
| BNP | Brain (B-type) Natriuretic Peptide |
| CFS | Clinical Frailty Scale |
| CRP | C-reactive protein |
| FIM | Functional Independence Measure |
| FOIS | Functional Oral Intake Scale |
| GNRI | Geriatric Nutritional Risk Index |
| HBE | Harris–Benedict equation |
| MNA-SF | Mini Nutritional Assessment-Short Form |
| RE | rehabilitation effectiveness |
References
- Iida, H.; Seki, T.; Sakai, Y.; Watanabe, T.; Wakao, N.; Matsui, H.; Imagama, S. Low muscle mass affect hip fracture treatment outcomes in older individuals: A single-institution case-control study. BMC Musculoskelet. Disord. 2021, 22, 259. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Min. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef]
- Ritsuno, Y.; Kawado, M.; Morita, M.; Yamada, H.; Kanaji, A.; Nakamura, M.; Matsumoto, M.; Hashimoto, S.; Fujita, N. Impact of musculoskeletal disorders on healthy life expectancy in Japan. BMC Musculoskelet. Disord. 2021, 22, 661. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.X.; Hu, Y.; Wu, C. Association of Frailty with recovery from disability among community-dwelling Chinese older adults: China health and retirement longitudinal study. BMC Geriatr. 2020, 20, 119. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Eyigor, S.; Kutsal, Y.G.; Duran, E.; Huner, B.; Paker, N.; Durmus, B.; Sahin, N.; Civelek, G.M.; Gokkaya, K.; Doğan, A.; et al. Turkish Society of Physical Medicine and Rehabilitation, Geriatric Rehabilitation Working Group. Frailty prevalence and related factors in the older adult-FrailTURK Project. Age 2015, 37, 9791. [Google Scholar] [CrossRef]
- Kohler, S.; Rametta, R.; Poulter, M.; Vogrin, S.; Yates, P. Resilience, frailty and outcomes in geriatric rehabilitation. Australas. J. Ageing 2020, 39, e205–e209. [Google Scholar] [CrossRef]
- Calle, A.; Onder, G.; Morandi, A.; Bellelli, G.; Ortolani, E.; Pérez, L.M.; Mesas, M.; Sanniti, A.; Mazzanti, P.; Platto, C.N.; et al. Frailty related factors as predictors of functional recovery in geriatric rehabilitation: The Sarcopenia and Function in Aging Rehabilitation (SAFARI) multi-centric study. J. Nutr. Health Aging 2018, 22, 1099–1106. [Google Scholar] [CrossRef]
- Tröster, T.; Thalmann, M.; Fischer, K.; Bieri-Brüning, G.; Beeler, P.E.; Bischoff-Ferrari, H.A.; Gagesch, M. Frailty, underweight and impaired mobility are associated with institutionalisation after post-acute care. Swiss Med. Wkly. 2020, 150, w20276. [Google Scholar] [CrossRef]
- Afilalo, J.; Mottillo, S.; Eisenberg, M.J.; Alexander, K.P.; Noiseux, N.; Perrault, L.P.; Morin, J.F.; Langlois, Y.; Ohayon, S.M.; Monette, J.; et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 222–228. [Google Scholar] [CrossRef]
- Kawamura, K.; Osawa, A.; Tanimoto, M.; Kagaya, H.; Matsuura, T.; Arai, H. Clinical frailty scale is useful in predicting return-to-home in patients admitted due to coronavirus disease. BMC Geriatr. 2023, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Yamamoto, M.; Kano, S.; Kagase, A.; Kodama, A.; Koyama, Y.; Tsuchikane, E.; Suzuki, T.; Otsuka, T.; Kohsaka, S.; et al. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation 2017, 135, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ido, Y.; Yoshimura, Y.; Mutai, H. Relationship of Malnutrition During Hospitalization with Functional Recovery and Postdischarge Destination in Elderly Stroke Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Nii, M.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Tanaka, A. Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J. Stroke Cerebrovasc. Dis. 2016, 25, 57–62. [Google Scholar] [CrossRef]
- Milte, R.; Miller, M.D.; Crotty, M.; Mackintosh, S.; Thomas, S.; Cameron, I.D.; Whitehead, C.; Kurrle, S.; Ratcliffe, J. Cost-effectiveness of individualized nutrition and exercise therapy for rehabilitation following hip fracture. J. Rehabil. Med. 2016, 48, 378–385. [Google Scholar] [CrossRef]
- Flodin, L.; Cederholm, T.; Sääf, M.; Samnegård, E.; Ekström, W.; Al-Ani, A.N.; Hedström, M. Effects of protein-rich nutritional supplementation and bisphosphonates on body composition, handgrip strength and health-related quality of life after hip fracture: A 12-month randomized controlled study. BMC Geriatr. 2015, 15, 149. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of nutritional supplementation on sarcopenia and re- covery in hip fracture patients: A multi-centre randomized trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef]
- Kaipainen, T.; Hartikainen, S.; Tiihonen, M.; Nykänen, I. Effect of individually tailored nutritional counselling on protein and energy intake among older people receiving home care at risk of or having malnutrition: A non-randomised intervention study. BMC Geriatr. 2022, 22, 391. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Matsuura, M.; Shiba, S.; Nishikimi, T. Effect of heart failure and malnutrition, alone and in combination, on rehabilitation effectiveness in patients with hip fracture. Clin. Nutr. ESPEN 2021, 44, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Matsuura, M.; Shiba, S.; Nishikimi, T. Effect of comorbid heart failure assessed by plasma B-type natriuretic peptide level on the activities of daily living in patients with hospitalization-associated disability after aspiration pneumonia. Eur. Geriatr. Med. 2024, 15, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D. Functional evaluation: The Barthel Index. Md. Med. J. 1965, 14, 56–61. [Google Scholar]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the clinical Frailty Scale in allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 210–215. [Google Scholar] [CrossRef]
- Tokuda, T.; Yamamoto, M.; Kagase, A.; Shimura, T.; Yamaguchi, R.; Saji, M.; Asami, M.; Enta, Y.; Nakashima, M.; Shirai, S.; et al. Clinical Impact of Baseline Frailty Status and Residual Mitral Regurgitation After Transcatheter Edge-to-Edge Repair: Insights from the OCEAN-Mitral Registry. J. Am. Heart Assoc. 2024, 13, e035109. [Google Scholar] [CrossRef]
- Hamana, T.; Iwasaki, M.; Shinke, T.; Kokawa, T.; Fukuishi, Y.; Masaki, R.; Odajima, S.; Fujimoto, W.; Kuroda, K.; Hatani, Y.; et al. Prognostic Impact of the Clinical Frailty Scale After Balloon Aortic Valvuloplasty. Circ. Rep. 2020, 2, 322–329. [Google Scholar] [CrossRef]
- Wang, H.T.; Fafard, J.; Ahern, S.; Vendittoli, P.A.; Hebert, P. Frailty as a predictor of hospital length of stay after elective total joint replacements in elderly patients. BMC Musculoskelet. Disord. 2018, 19, 14. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Jesus, P.; Achamrah, N.; Grigioni, S.; Charles, J.; Rimbert, A.; Folope, V.; Petit, A.; Dechelotte, P.; Coeffier, M. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin. Nutr. 2015, 34, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shoji, S.; Shiraishi, Y.; Kawana, M.; Kohno, T.; Inoue, K.; Fukuda, K.; Heidenreich, P.A.; Kohsaka, S. Hospital meal intake in acute heart failure patients and its association with long-term outcomes. Open Heart 2020, 7, e001248. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.C.; Chen, C.H.; Petrella, R.; Thind, A. Rehabilitation impact indices and their independent predictors: A systematic review. BMJ Open 2013, 3, e003483. [Google Scholar] [CrossRef]
- Tamamura, Y.; Matsuura, M.; Shiba, S.; Nishikimi, T. Heart failure assessed based on plasma B-type natriuretic peptide (BNP) levels negatively impacts activity of daily living in patients with hip fracture. PLoS ONE 2020, 15, e0237387. [Google Scholar] [CrossRef]
- Tamamura, Y.; Hachiuma, C.; Matsuura, M.; Shiba, S.; Nishikimi, T. Relationship between Improvement in Physical Activity and Three Nutritional Assessment Indicators in Patients Admitted to a Convalescent Rehabilitation Ward. Nutrients 2024, 16, 2531. [Google Scholar] [CrossRef]
- García-Peña, C.; García-Fabela, L.C.; Gutiérrez-Robledo, L.M.; García-González, J.J.; Arango-Lopera, V.E.; Pérez-Zepeda, M.U. Handgrip strength predicts functional decline at discharge in hospitalized male elderly: A hospital cohort study. PLoS ONE 2013, 8, e69849. [Google Scholar] [CrossRef]
- Olguín, T.; Bunout, D.; Pia de la Maza, M.P.; Barrera, G.; Hirsch, S. Admission Handgrip strength predicts functional decline in hospitalized patients. Clin. Nutr. ESPEN 2017, 17, 28–32. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of fractures among community-dwelling older people: A systematic review and meta-analysis. Bone 2016, 90, 116–122. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Association between frailty and quality of life among community-dwelling older people: A systematic review and meta- analysis. J. Epidemiol. Community Health 2016, 70, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Singh, I.; Gallacher, J.; Davis, K.; Johansen, A.; Eeles, E.; Hubbard, R.E. Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing 2012, 41, 242–246. [Google Scholar] [CrossRef]
- Hiesmayr, M.; Schindler, K.; Pernicka, E.; Schuh, C.; Schoeniger-Hekele, A.; Bauer, P.; Laviano, A.; Lovell, A.D.; Mouhieddine, M.; Schuetz, T.; et al. Decreased food intake is a risk factor for mortality in hospitalised patients: The Nutrition Day survey 2006. Clin. Nutr. 2009, 28, 484–491. [Google Scholar] [CrossRef]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Inadequate postoperative energy intake relative to total energy requirements diminishes acute phase functional recovery from hip fracture. Arch. Phys. Med. Rehabil. 2019, 100, 32–38. [Google Scholar] [CrossRef]
- Pansarasa, O.; Mimmi, M.C.; Davin, A.; Giannini, M.; Guaita, A.; Cereda, C. Inflammation and cell-to-cell communication, two related aspects in frailty. Immun. Ageing 2022, 19, 49. [Google Scholar] [CrossRef]
- Nishikimi, T.; Nakagawa, Y. B-Type Natriuretic Peptide (BNP) Revisited-Is BNP Still a Biomarker for Heart Failure in the Angiotensin Receptor/Neprilysin Inhibitor Era? Biology 2022, 11, 1034. [Google Scholar] [CrossRef]
- Nishikimi, T.; Yoshihara, F.; Morimoto, A.; Ishikawa, K.; Ishimitsu, T.; Saito, Y.; Kangawa, K.; Matsuo, H.; Omae, T.; Matsuoka, H. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension 1996, 28, 22–30. [Google Scholar] [CrossRef]
- Nishikimi, T.; Kuwahara, K.; Nakao, K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J. Cardiol. 2011, 57, 131–140. [Google Scholar] [CrossRef]
- Nishikimi, T.; Mori, Y.; Ishimura, K.; Tadokoro, K.; Yagi, H.; Yabe, A.; Horinaka, S.; Matsuoka, H. Association of plasma atrial natriuretic peptide, N-terminal proatrial natriuretic peptide, and brain natriuretic peptide levels with coronary artery stenosis in patients with normal left ventricular systolic function. Am. J. Med. 2004, 116, 517–523. [Google Scholar] [CrossRef]
- Nishikimi, T.; Ikeda, M.; Takeda, Y.; Ishimitsu, T.; Shibasaki, I.; Fukuda, H.; Kinoshita, H.; Nakagawa, Y.; Kuwahara, K.; Nakao, K. The effect of glycosylation on plasma N-terminal proBNP-76 levels in patients with heart or renal failure. Heart 2012, 98, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.; Moppett, I.K.; Juurlink, I.; Nightingale, J.; Moran, C.G. Devonald MA. Acute and chronic kidney disease in elderly patients with hip fracture: Prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Oldmeadow, L.B.; Edwards, E.R.; Kimmel, L.A.; Kipen, E.; Robertson, V.J.; Bailey, M.J. No rest for the wounded: Early ambulation after hip surgery accelerates recovery. ANZ J. Surg. 2006, 76, 607–611. [Google Scholar] [CrossRef]
- Husby, V.S.; Foss, O.A.; Husby, O.S.; Winther, S.B. Randomized controlled trial of maximal strength training vs. standard rehabilitation following total knee arthroplasty. Eur. J. Phys. Rehabil. Med. 2018, 54, 371–379. [Google Scholar] [CrossRef]
- Shonkoff, E.; Cara, K.C.; Pei, X.; Chung, M.; Kamath, S.; Panetta, K.; Hennessy, E. AI-based digital image dietary assessment methods compared to humans and ground truth: A systematic review. Ann. Med. 2023, 55, 2273497. [Google Scholar] [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The application of digital technologies and artificial intelligence in healthcare: An overview on nutrition assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef]
- Tagi, M.; Hamada, Y.; Shan, X.; Ozaki, K.; Kubota, M.; Amano, S.; Sakaue, H.; Suzuki, Y.; Konishi, T.; Hirose, J. A Food Intake Estimation System Using an Artificial Intelligence-Based Model for Estimating Leftover Hospital Liquid Food in Clinical Environments: Development and Validation Study. JMIR Form. Res. 2024, 8, e55218. [Google Scholar] [CrossRef]
- Ferguson, C.E.; Tatucu-Babet, O.A.; Amon, J.N.; Chapple, L.S.; Malacria, L.; Myint Htoo, I.; Hodgson, C.L.; Ridley, E.J. Dietary assessment methods for measurement of oral intake in acute care and critically ill hospitalized patients: A scoping review. Nutr. Res. Rev. 2023, 1, 14. [Google Scholar]
- Phalle, A.; Gokhale, D. Navigating next-gen nutrition care using artificial intelligence-assisted dietary assessment tools-a scoping review of potential applications. Front. Nutr. 2025, 12, 1518466. [Google Scholar] [CrossRef]

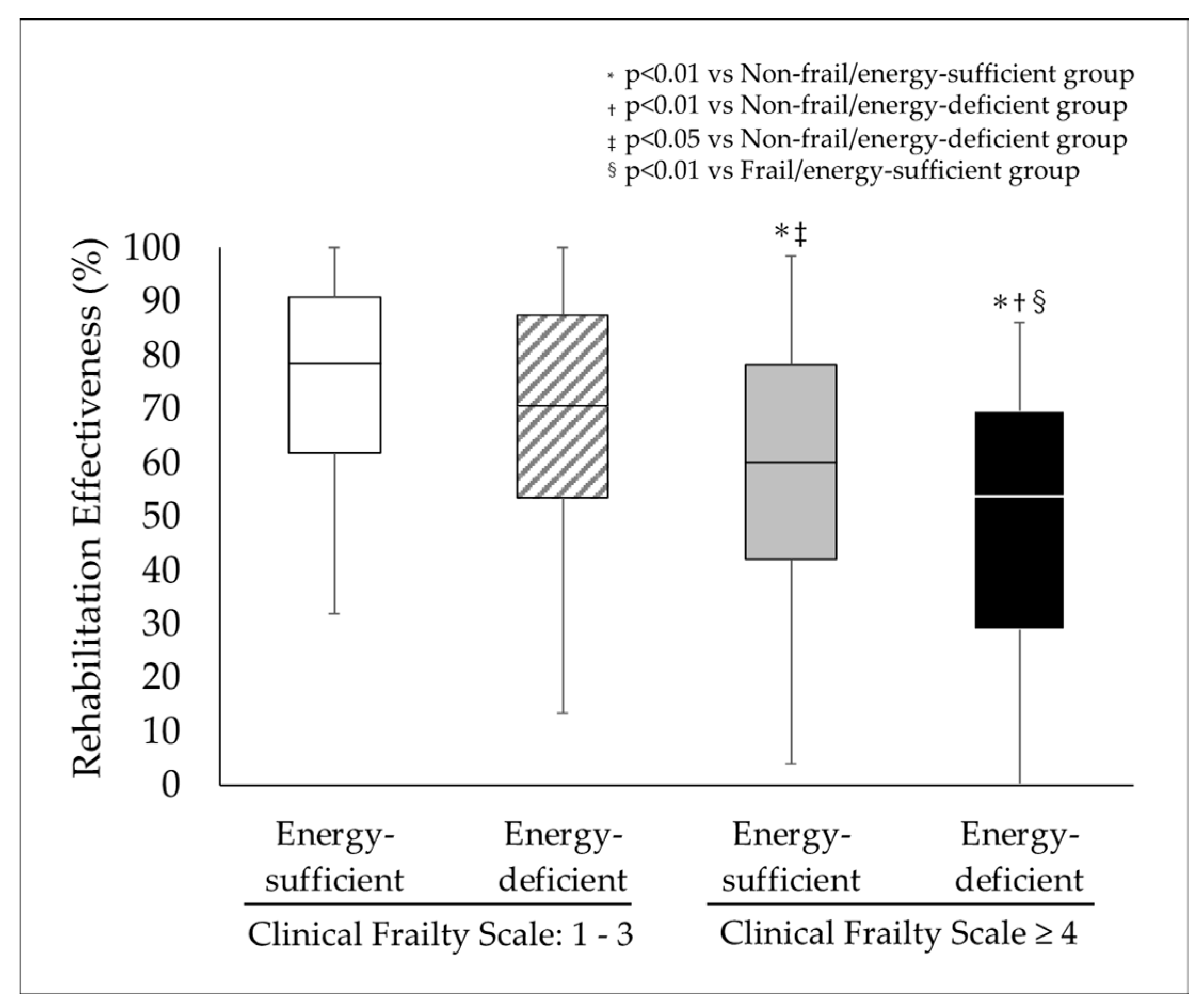
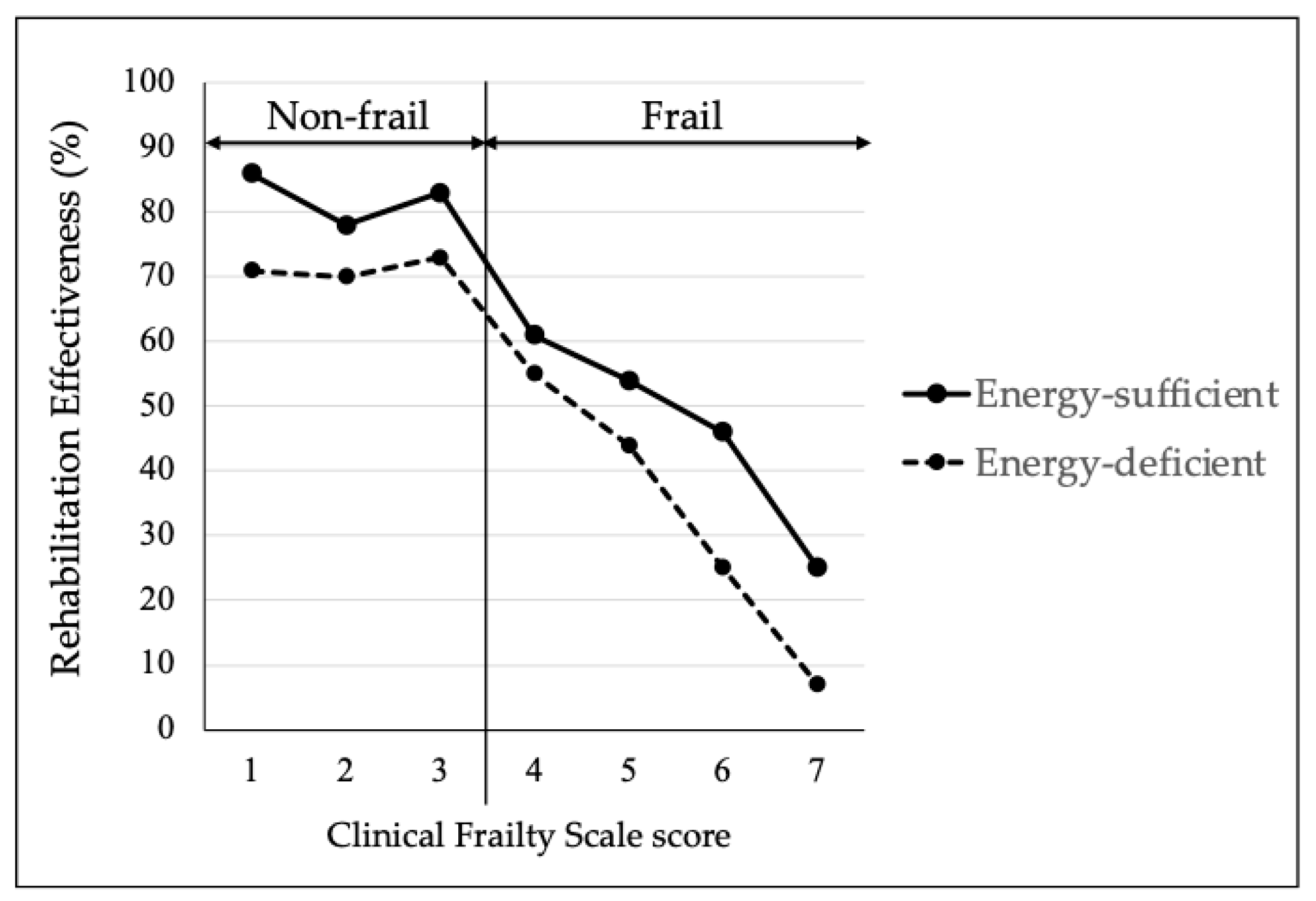
| Total | Non-Frail/Energy-Sufficient Group | Non-Frail/Energy-Deficient Group | Frail/Energy-Sufficient Group | Frail/Energy-Deficient Group | p | |
|---|---|---|---|---|---|---|
| n | 735 | 184 | 86 | 230 | 235 | <0.001 |
| Age, year | 81 ± 10 | 77 ± 12 | 81 ± 10 * | 81 ± 9 * | 85 ± 7 **†¶ | <0.001 |
| Male, n (%) | 202 (27.5) | 62 (33.7) | 20 (23.3) | 77 (33.5) | 43 (18.3) *§ | <0.001 |
| CFS, score | 3.8 ± 1.2 | 2.6 ± 0.7 | 2.8 ± 0.6 | 4.4 ± 0.8 * | 4.5 ± 0.9 *† | <0.001 |
| Energy intake ratio, (%) | 84.9± 21.3 | 100 ± 0 | 72.3 ± 19.9 * | 100 ± 0 † | 63.7 ± 19.1 *†§ | <0.001 |
| Height, m | 1.52 ± 0.1 | 1.54 ± 0.1 | 1.52 ± 0.1 | 1.52 ± 0.1 | 1.49 ± 0.1 *‡¶ | <0.001 |
| Weight, kg | 49.0 ± 10.3 | 52.5 ± 10.1 | 50.5 ± 10.2 | 49.1 ± 10.6 * | 45.6 ± 9.1 *†§ | <0.001 |
| Body mass index kg/m2 | 21.3 ± 3.6 | 22.1 ± 3.6 | 21.7 ± 3.3 | 21.1 ± 3.6 | 20.6 ± 3.4 * | <0.001 |
| Comorbidity n (%) | ||||||
| Hypertension | 446 (60.7) | 114 (60.2) | 51 (59.3) | 132 (57.4) | 149 (63.4) | 0.580 |
| Diabetes mellitus | 162 (22.0) | 51 (27.7) | 14 (16.3) | 58 (25.2) | 39 (16.6) * | 0.014 |
| Dyslipidemia | 162 (22.0) | 42 (22.8) | 15 (17.4) | 51 (22.2) | 54 (23.0) | 0.728 |
| Atrial fibrillation | 55 (7.5) | 12 (6.5) | 8 (9.3) | 17 (7.4) | 18 (7.7) | 0.884 |
| Handgrip strength, kg | 15.5 ± 7.9 | 19.0 ± 9.1 | 16.1 ± 7.4 ** | 15.2 ± 7.7 * | 12.6 ± 5.9 *†§ | <0.001 |
| Quadriceps strength, kg | 12.3 ± 6.8 | 13.7 ± 7.4 | 12.8 ± 6.7 | 12.4 ± 6.8 | 10.7 ± 5.8 * | 0.001 |
| Thigh circumference, cm | 35.9 ± 5.1 | 37.7 ± 4.8 | 36.7 ± 6.1 | 35.5 ± 5.2 * | 34.6 ± 4.5 *‡ | <0.001 |
| Calf circumference, cm | 28.7 ± 3.8 | 30.3 ± 3.3 | 28.8 ± 4.4 ** | 28.5 ± 3.9 * | 27.6 ± 3.2 * | <0.001 |
| FOIS | 6.4 ± 1.0 | 6.8 ± 0.5 | 6.5 ± 0.8 | 6.3 ± 1.2 * | 6.2 ± 1.0 *‡ | <0.001 |
| Barthel Index, score | 50 (35–70) | 70 (55–80) | 58 (40–70) * | 50 (35–60) * | 40 (25–50) *†§ | <0.001 |
| FIM, score | ||||||
| Motor | 37 (25–47) | 47 (39–56) | 40 (27–48) * | 36 (24–45) * | 30 (22–37) *†§ | <0.001 |
| Cognitive | 23 (18–27) | 26 (22–31) | 25 (20–29) ** | 22 (17–27) * | 21 (16–25) *† | <0.001 |
| Total | 59 (45–75) | 74 (63–86) | 66 (48–77) * | 57 (42–70) * | 49 (40–61) *†§ | <0.001 |
| MNA-SF | 6.7 ± 2.5 | 7.8 ± 2.1 | 6.6 ± 2.3 * | 6.7 ± 2.6 * | 5.7 ± 2.5 *‡§ | <0.001 |
| GNRI | 90.6 ± 11.1 | 95.5 ± 11.1 | 91.1± 11.1 | 89.1 ± 11.4 * | 88.2 ± 9.5 * | <0.001 |
| Energy intake, kcal | 1365 ± 376 | 1619 ± 262 | 1151 ± 313 * | 1554 ± 202 | 1059 ± 343 *†§ | <0.001 |
| Medication, n | 5 ± 3 | 5 ± 3 | 5 ± 3 | 6 ± 3 | 6 ± 3 | 0.487 |
| BNP, pg/mL | 41 (28–86) | 31 (16–73) | 43 (24–83) | 43 (25–86) | 48 (22–99) | 0.929 |
| Albumin, g/dL | 3.5 ± 0.5 | 3.8 ± 0.4 | 3.5 ± 0.5 * | 3.4 ± 0.5 | 3.3 ± 0.5 *‡ | <0.001 |
| CRP, mg/dL | 0.0 (0.0–1.0) | 0.0 (0.0–0.9) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.2 (0.0–1.0) | 0.123 |
| Creatinine, mg/dL | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.903 |
| Total cholesterol, mg/dL | 185 ± 40 | 188 ± 44 | 189 ± 38 | 180 ± 36 | 185 ± 40 | 0.111 |
| Hemoglobin, g/dL | 11.5 ± 1.6 | 11.9 ± 1.6 | 11.6 ± 1.4 | 11.4 ± 1.6 * | 11.4 ± 1.6 * | 0.006 |
| Total | Non-Frail/Energy-Sufficient Group | Non-Frail/Energy-Deficient Group | Frail/Energy-Sufficient Group | Frail/Energy-Deficient Group | p | |
|---|---|---|---|---|---|---|
| n | 735 | 184 | 86 | 230 | 235 | |
| Energy intake ratio, (%) | 92.9 ± 13.3 | 99.9 ± 2.2 | 86.0 ± 15.0 * | 96.3 ± 11.1 *† | 86.9 ± 15.6 *§ | <0.001 |
| Weight, kg | 48.7 ± 10.0 | 52.5 ± 9.8 | 49.9 ± 9.8 * | 48.7 ± 10.4 *† | 45.2 ± 8.7 *†§ | <0.001 |
| Body mass index kg/m2 | 21.1 ± 3.4 | 22.1 ± 3.5 | 21.5 ± 3.2 * | 20.9 ± 3.5 * | 20.4 ± 3.3 * | <0.001 |
| Handgrip strength, kg | 15.9 ± 7.8 | 19.3 ± 8.8 | 15.8 ± 8.0 * | 15.9 ± 7.6 * | 13.1 ± 5.8 *‡§ | <0.001 |
| Quadriceps strength, kg | 14.4 ± 7.1 | 16.4 ± 7.3 | 14.2 ± 7.1 | 14.5 ± 7.0 | 12.7 ± 6.5 *¶ | <0.001 |
| Thigh circumference, cm | 36.1± 4.8 | 37.8 ± 4.4 | 37.1 ± 4.8 | 35.8 ± 5.1 * | 34.7 ± 4.5 *† | <0.001 |
| Calf circumference, cm | 29.3 ± 3.7 | 30.8 ± 3.4 | 29.8 ± 3.8 | 29.1 ± 3.7 * | 28.0 ± 3.2 *†§ | <0.001 |
| FOIS | 6.4 ± 1.1 | 6.8 ± 0.5 | 6.5 ± 1.0 | 6.3 ± 1.2 * | 6.0 ± 1.3 *† | <0.001 |
| MNA-SF | 9.7 ± 2.7 | 11.0 ± 2.1 | 10.1 ± 2.2 ** | 9.4 ± 2.7 * | 8.7 ± 2.7 *¶ | <0.001 |
| GNRI | 91.0 ± 10.5 | 95.8 ± 10.2 | 91.1 ± 10.7 | 89.9 ± 10.3 * | 88.2 ± 9.6 * | <0.001 |
| Energy intake, kcal | 1509 ± 309 | 1653 ± 278 | 1432 ± 316 * | 1555 ± 264 *† | 1378 ± 312 *§ | <0.001 |
| Barthel Index, score | 90 (70–100) | 100 (90–100) | 90 (85–100) | 90 (70–100) * | 80 (55–90) *†§ | <0.001 |
| Changes during hospitalization | ||||||
| Change in body weight | −0.4 ± 2.4 | 0.0 ± 2.2 | −0.6 ± 2.1 | −0.4 ± 2.8 | −0.4 ± 2.3 | 0.354 |
| Change in handgrip strength | 0.6 ± 3.9 | 0.6 ± 3.2 | −0.1 ± 5.0 | 0.8 ± 3.8 | 0.6 ± 4.0 | 0.683 |
| Change in quadriceps strength | 2.3 ± 4.8 | 2.7 ± 5.0 | 1.4 ± 4.5 | 2.5 ± 5.1 | 2.1 ± 4.2 | 0.999 |
| Change in FOIS | 0.0 ± 0.8 | 0.0 ± 0.3 | 0.0 ± 0.9 | 0.0 ± 0.7 | −0.2 ± 1.2¶ | <0.001 |
| Change in MNA-SF | 3.0 ± 2.6 | 3.3 ± 2.3 | 3.4 ± 2.5 | 2.7 ± 2.6 | 3.0 ± 2.8 | 0.139 |
| Change in GNRI | 0.4 ± 4.7 | 0.4 ± 5.0 | 0.1 ± 5.0 | 0.8 ± 4.4 | 0.0 ± 4.8 | 0.687 |
| FIM, score | ||||||
| Motor | 75 (62–84) | 85 (77–88) | 80 (67–85) ** | 74 (61–83) * | 67 (52–77) *†§ | <0.001 |
| Cognitive | 28 (22–33) | 33 (28–35) | 30 (23–35) | 26 (19–32) *† | 25 (20–30) *† | <0.001 |
| Total | 103 (84–117) | 117 (106–122) | 108 (91–119) ** | 98 (80–115) *‡ | 92 (74–105) *†§ | <0.001 |
| Barthel Index gain, score | 30 (20–45) | 25 (15–40) | 30 (20–45) | 30 (20 -45) | 35(20–50) | 0.067 |
| FIM gain, score | ||||||
| Motor | 35(24–43) | 35 (25–42) | 37(29–44) | 34(25–43) | 35(21–43) | 0.096 |
| Cognitive | 3 (0–6) | 3 (1–6) | 4 (0–7) | 3 (0–6) | 3 (0–6) | 0.921 |
| Total | 39 (27–48) | 38 (28–46) | 41 (31–49) | 38 (26–47) | 40 (23–49) | 0.116 |
| Length of hospital stay, day | 78 (56–87) | 62 (44–81) | 74 (52–87) | 79 (59–88) * | 84 (64–88) *‡ | <0.001 |
| FIM efficiency, score/day | 0.54 (0.37–0.74) | 0.61 (0.44–0.83) | 0.60 (0.42–0.87) | 0.51 (0.36–0.70) *† | 0.51 (0.28–0.64)*† | 0.002 |
| Univariate Linear Regression Analysis | Multiple Linear Regression Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | β | p | 95%CI | B | β | p | 95%CI | |||
| Lower | Higher | Lower | Higher | |||||||
| 11.748 | 0.419 | −16.809 | 40.304 | |||||||
| Age | −0.961 | −0.345 | <0.001 | −1.150 | −0.771 | −0.361 | −0.134 | <0.001 | −0.552 | −0.169 |
| Male | 1.990 | 0.033 | 0.370 | −2.366 | 6.345 | −10.949 | −0.184 | <0.001 | −15.621 | −6.277 |
| CFS | −7.989 | −0.346 | <0.001 | −9.559 | −6.420 | −3.047 | −0.131 | <0.001 | −4.615 | −1.480 |
| Energy intake ratio | 0.471 | 0.357 | <0.001 | 0.387 | 0.556 | 0.281 | 0.220 | <0.001 | 0.195 | 0.368 |
| Handgrip strength | 1.318 | 0.395 | <0.001 | 1.085 | 1.551 | 0.751 | 0.227 | <0.001 | 0.467 | 1.036 |
| FOIS | 10.915 | 0.402 | <0.001 | 9.044 | 12.786 | 5.319 | 0.192 | <0.001 | 3.371 | 7.267 |
| MNA-SF | 4.457 | 0.423 | <0.001 | 3.761 | 5.153 | 1.268 | 0.120 | 0.001 | 0.503 | 2.033 |
| BNP | −0.027 | −0.108 | 0.004 | −0.045 | −0.009 | −0.017 | −0.073 | 0.041 | −0.033 | −0.001 |
| Creatinine | 5.945 | 0.083 | 0.024 | 0.769 | 11.121 | 9.687 | 0.141 | <0.001 | 4.728 | 14.645 |
| Hemoglobin | 3.315 | 0.197 | <0.001 | 2.115 | 4.514 | 0.776 | 0.045 | 0.201 | −0.415 | 1.966 |
| CRP | −1.940 | −0.108 | 0.003 | −3.231 | −0.648 | −0.088 | −0.005 | 0.877 | −1.207 | 1.031 |
| Total cholesterol | 0.052 | 0.076 | 0.040 | 0.002 | 0.101 | −0.009 | −0.013 | 0.706 | −0.054 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamamura, Y.; Hachiuma, C.; Matsuura, M.; Shiba, S.; Nishikimi, T. Frailty and Energy Intake Deficiency Reduce the Efficiency of Activities of Daily Living in Patients with Musculoskeletal Disorders: A Retrospective Cohort Study. Nutrients 2025, 17, 1334. https://doi.org/10.3390/nu17081334
Tamamura Y, Hachiuma C, Matsuura M, Shiba S, Nishikimi T. Frailty and Energy Intake Deficiency Reduce the Efficiency of Activities of Daily Living in Patients with Musculoskeletal Disorders: A Retrospective Cohort Study. Nutrients. 2025; 17(8):1334. https://doi.org/10.3390/nu17081334
Chicago/Turabian StyleTamamura, Yusuke, Chihiro Hachiuma, Michiko Matsuura, Sumiko Shiba, and Toshio Nishikimi. 2025. "Frailty and Energy Intake Deficiency Reduce the Efficiency of Activities of Daily Living in Patients with Musculoskeletal Disorders: A Retrospective Cohort Study" Nutrients 17, no. 8: 1334. https://doi.org/10.3390/nu17081334
APA StyleTamamura, Y., Hachiuma, C., Matsuura, M., Shiba, S., & Nishikimi, T. (2025). Frailty and Energy Intake Deficiency Reduce the Efficiency of Activities of Daily Living in Patients with Musculoskeletal Disorders: A Retrospective Cohort Study. Nutrients, 17(8), 1334. https://doi.org/10.3390/nu17081334







