Taste Plasticity in Nutrition and Health: A Scoping Review
Abstract
1. Introduction
2. Anatomy of Taste System
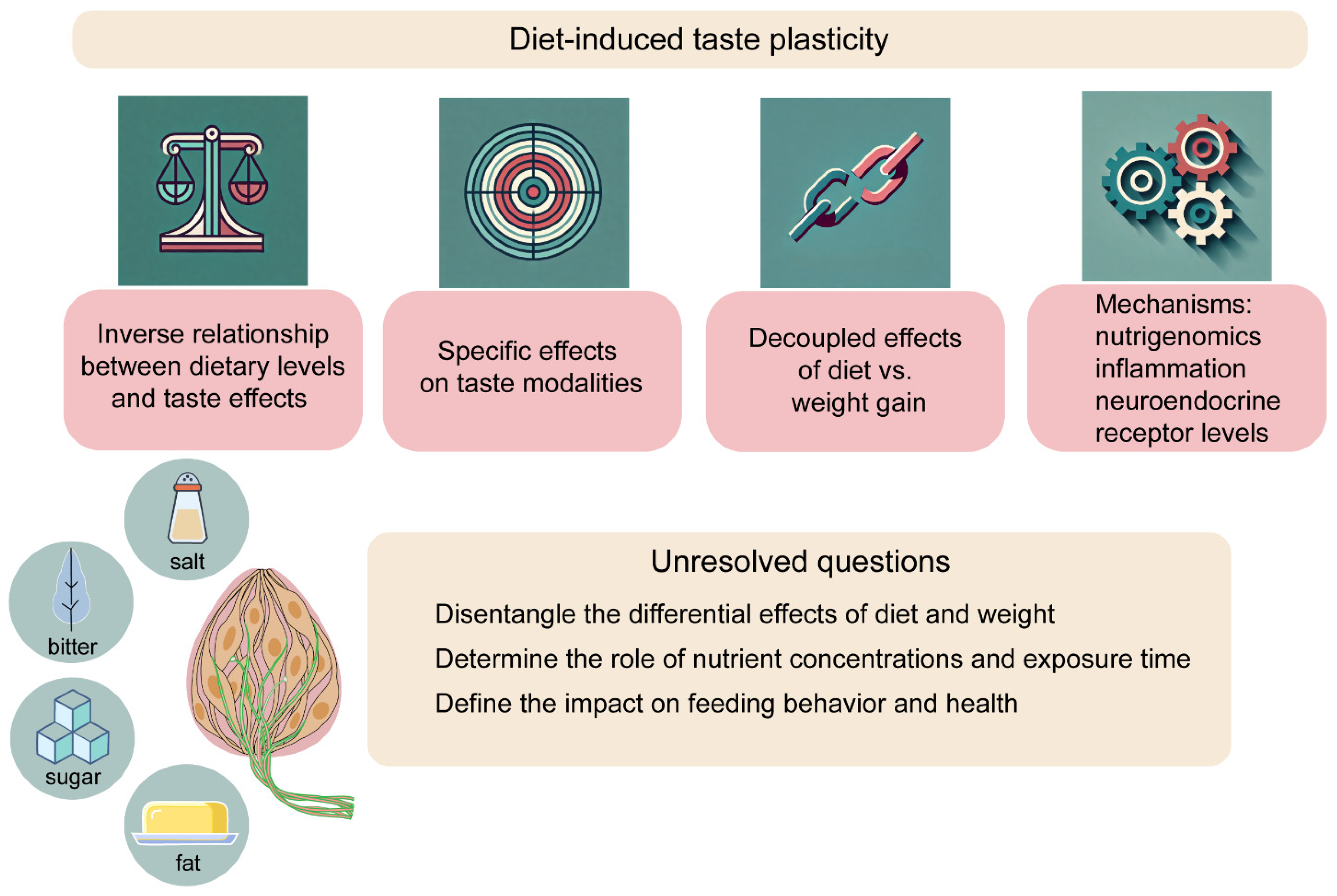
3. Diet-Induced Taste Plasticity from Insects to Humans
4. Uncovering the Mechanisms of Diet-Induced Taste Plasticity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fukunaga, A.; Uematsu, H.; Sugimoto, K. Influences of Aging on Taste Perception and Oral Somatic Sensation. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sensory Science and Chronic Diseases, 1st ed.; Joseph, P.V., Duffy, V.B., Eds.; Springer Nature: Cham, Switzerland, 2021; ISBN 9783030862817. [Google Scholar]
- May, C.E.; Dus, M. Confection Confusion: Interplay between Diet, Taste, and Nutrition. Trends Endocrinol. Metab. 2021, 32, 95–105. [Google Scholar] [CrossRef]
- Micarelli, A.; Malacrida, S.; Strapazzon, G.; Mrakic-Sposta, S.; Micarelli, B.; Alessandrini, N.; Carbini, V.; Caputo, S.; Falla, M.; Alessandrini, M. Impact of Nutritional Intervention on Taste Perception—A Scoping Review. Foods 2021, 10, 2747. [Google Scholar] [CrossRef] [PubMed]
- Peinado, B.R.R.; Frazão, D.R.; Bittencourt, L.O.; de Souza-Rodrigues, R.D.; Vidigal, M.T.C.; da Silva, D.T.; Paranhos, L.R.; Magno, M.B.; Fagundes, N.C.F.; Maia, L.C.; et al. Is Obesity Associated with Taste Alterations? A Systematic Review. Front. Endocrinol. 2023, 14, 1167119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-S.; Wang, C.-Z.; van Loon, J.J.A. Habituation to a Deterrent Plant Alkaloid Develops Faster in the Specialist Herbivore Helicoverpa Assulta than in Its Generalist Congener Helicoverpa Armigera and Coincides with Taste Neuron Desensitisation. Insects 2021, 13, 21. [Google Scholar] [CrossRef]
- Zhou, D.-S.; Wang, C.-Z.; van Loon, J.J.A. Chemosensory Basis of Behavioural Plasticity in Response to Deterrent Plant Chemicals in the Larva of the Small Cabbage White Butterfly Pieris Rapae. J. Insect Physiol. 2009, 55, 788–792. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Raghuwanshi, R.P.; Shen, W.L.; Montell, C. Food Experience-Induced Taste Desensitization Modulated by the Drosophila TRPL Channel. Nat. Neurosci. 2013, 16, 1468–1476. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Brown, H.; Capoor, M.; Davis, A.; Gbedemah, A.; Long, E. A Peripheral Mechanism for Behavioral Adaptation to Specific “Bitter” Taste Stimuli in an Insect. J. Neurosci. 2001, 21, 3688–3696. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Ensslen, S.; Eisenberg, M.E.; Weiskopf, P. Diet-Induced Plasticity in the Taste System of an Insect: Localization to a Single Transduction Pathway in an Identified Taste Cell. J. Exp. Biol. 1999, 202, 2091–2102. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Q.; Wang, B.-J.; Li, K.; Lövy, M.; Nevo, E.; Li, Q.; Su, W.; Jiang, P.; Zhao, H. Local Adaptation of Bitter Taste and Ecological Speciation in a Wild Mammal. Mol. Biol. Evol. 2021, 38, 4562–4572. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Zhou, L.-H.; Ban, X.; Liu, D.-X.; Jiang, W.; Liu, X.-M. Decreased Expression of CD36 in Circumvallate Taste Buds of High-Fat Diet Induced Obese Rats. Acta Histochem. 2011, 113, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher Sensitivity to Sweet and Salty Taste in Obese Compared to Lean Individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef]
- Weiss, M.S.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M. Taste Responses in the Nucleus of the Solitary Tract of Awake Obese Rats Are Blunted Compared with Those in Lean Rats. Front. Integr. Neurosci. 2019, 13, 35. [Google Scholar] [CrossRef]
- Harnischfeger, F.; O’Connell, F.; Weiss, M.; Axelrod, B.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M.; Dando, R. Sprague Dawley Rats Gaining Weight on a High Energy Diet Exhibit Damage to Taste Tissue Even after Return to a Healthy Diet. Nutrients 2021, 13, 3062. [Google Scholar] [CrossRef] [PubMed]
- Maliphol, A.B.; Garth, D.J.; Medler, K.F. Diet-Induced Obesity Reduces the Responsiveness of the Peripheral Taste Receptor Cells. PLoS ONE 2013, 8, e79403. [Google Scholar] [CrossRef]
- Brondel, L.; Quilliot, D.; Mouillot, T.; Khan, N.A.; Bastable, P.; Boggio, V.; Leloup, C.; Pénicaud, L. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients 2022, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of Fat Taste Perception: Association with Diet and Obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef]
- May, C.E.; Vaziri, A.; Lin, Y.Q.; Grushko, O.; Khabiri, M.; Wang, Q.-P.; Holme, K.J.; Pletcher, S.D.; Freddolino, P.L.; Neely, G.G.; et al. High Dietary Sugar Reshapes Sweet Taste to Promote Feeding Behavior in Drosophila Melanogaster. Cell Rep. 2019, 27, 1675–1685.e7. [Google Scholar] [CrossRef]
- Ahart, Z.C.; Martin, L.E.; Kemp, B.R.; Dutta Banik, D.; Roberts, S.G.E.; Torregrossa, A.-M.; Medler, K.F. Differential Effects of Diet and Weight on Taste Responses in Diet-Induced Obese Mice. Obesity 2020, 28, 284–292. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and Transduction in Taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common Sense about Taste: From Mammals to Insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C.; Finger, T.E. Recent Advances in Taste Transduction and Signaling. F1000Research 2019, 8, 2117. [Google Scholar] [CrossRef]
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Tränkner, D.; Ryba, N.J.P.; Zuker, C.S. The Cells and Logic for Mammalian Sour Taste Detection. Nature 2006, 442, 934–938. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.P.; Zuker, C.S. The Cells and Peripheral Representation of Sodium Taste in Mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Greater Reductions in Fat Preferences in CALHM1 than CD36 Knockout Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R576–R585. [Google Scholar] [CrossRef]
- Freeman, E.G.; Dahanukar, A. Molecular Neurobiology of Drosophila Taste. Curr. Opin. Neurobiol. 2015, 34, 140–148. [Google Scholar] [CrossRef]
- Joseph, R.M.; Carlson, J.R. Drosophila Chemoreceptors: A Molecular Interface between the Chemical World and the Brain. Trends Genet. 2015, 31, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. Drosophila Sensory Receptors-a Set of Molecular Swiss Army Knives. Genetics 2021, 217, 1–34. [Google Scholar] [CrossRef]
- Ma, D.; Hu, M.; Yang, X.; Liu, Q.; Ye, F.; Cai, W.; Wang, Y.; Xu, X.; Chang, S.; Wang, R.; et al. Structural Basis for Sugar Perception by Drosophila Gustatory Receptors. Science 2024, 383, eadj2609. [Google Scholar] [CrossRef]
- Frank, H.M.; Walujkar, S.; Walsh, R.M., Jr.; Laursen, W.J.; Theobald, D.L.; Garrity, P.A.; Gaudet, R. Structural Basis of Ligand Specificity and Channel Activation in an Insect Gustatory Receptor. Cell Rep. 2024, 43, 114035. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.D.; Dahanukar, A. Recent Advances in the Genetic Basis of Taste Detection in Drosophila. Cell. Mol. Life Sci. 2020, 77, 1087–1101. [Google Scholar] [CrossRef]
- Scott, K. Gustatory Processing in Drosophila Melanogaster. Annu. Rev. Entomol. 2018, 63, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.; Bleiser-Avivi, N.; Atidia, J. Labellar Taste Organs of Drosophila Melanogaster: Taste oragns of dorhophila. J. Morphol. 1976, 150, 327–341. [Google Scholar] [CrossRef]
- Benton, R.; Dahanukar, A. Chemosensory Coding in Drosophila Single Sensilla. Cold Spring Harb. Protoc. 2023, 2023, db.top107803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-P.; Lin, Y.Q.; Zhang, L.; Wilson, Y.A.; Oyston, L.J.; Cotterell, J.; Qi, Y.; Khuong, T.M.; Bakhshi, N.; Planchenault, Y.; et al. Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metab. 2016, 24, 75–90. [Google Scholar] [CrossRef]
- Wise, P.M.; Nattress, L.; Flammer, L.J.; Beauchamp, G.K. Reduced Dietary Intake of Simple Sugars Alters Perceived Sweet Taste Intensity but Not Perceived Pleasantness. Am. J. Clin. Nutr. 2016, 103, 50–60. [Google Scholar] [CrossRef]
- Sartor, F.; Donaldson, L.F.; Markland, D.A.; Loveday, H.; Jackson, M.J.; Kubis, H.-P. Taste Perception and Implicit Attitude toward Sweet Related to Body Mass Index and Soft Drink Supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef]
- Sung, H.; Vaziri, A.; Wilinski, D.; Woerner, R.K.R.; Freddolino, P.L.; Dus, M. Nutrigenomic Regulation of Sensory Plasticity. Elife 2023, 12, e83979. [Google Scholar] [CrossRef]
- Vaziri, A.; Khabiri, M.; Genaw, B.T.; May, C.E.; Freddolino, P.L.; Dus, M. Persistent Epigenetic Reprogramming of Sweet Taste by Diet. Sci. Adv. 2020, 6, eabc8492. [Google Scholar] [CrossRef]
- Wang, Q.-P.; Lin, Y.Q.; Lai, M.-L.; Su, Z.; Oyston, L.J.; Clark, T.; Park, S.J.; Khuong, T.M.; Lau, M.-T.; Shenton, V.; et al. PGC1α Controls Sucrose Taste Sensitization in Drosophila. Cell Rep. 2020, 31, 107480. [Google Scholar] [CrossRef]
- McCluskey, L.P.; He, L.; Dong, G.; Harris, R. Chronic Exposure to Liquid Sucrose and Dry Sucrose Diet Have Differential Effects on Peripheral Taste Responses in Female Rats. Appetite 2020, 145, 104499. [Google Scholar] [CrossRef]
- Sung, H.; Vesela, I.; Driks, H.; Ferrario, C.R.; Mistretta, C.M.; Bradley, R.M.; Dus, M. High-Sucrose Diet Exposure Is Associated with Selective and Reversible Alterations in the Rat Peripheral Taste System. Curr. Biol. 2022, 32, 4103–4113.e4. [Google Scholar] [CrossRef]
- Tsan, L.; Chometton, S.; Hayes, A.M.; Klug, M.E.; Zuo, Y.; Sun, S.; Bridi, L.; Lan, R.; Fodor, A.A.; Noble, E.E.; et al. Early-Life Low-Calorie Sweetener Consumption Disrupts Glucose Regulation, Sugar-Motivated Behavior, and Memory Function in Rats. JCI Insight 2022, 7, e157714. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Keast, R.S.J. Recent Fat Intake Modulates Fat Taste Sensitivity in Lean and Overweight Subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.-F.; Chevrot, M.; Besnard, P. The Lipid-Sensor Candidates CD36 and GPR120 Are Differentially Regulated by Dietary Lipids in Mouse Taste Buds: Impact on Spontaneous Fat Preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.P.; Bolhuis, D.P.; Torres, S.J.; Keast, R.S.J. Dietary Fat Restriction Increases Fat Taste Sensitivity in People with Obesity. Obesity 2016, 24, 328–334. [Google Scholar] [CrossRef]
- Costanzo, A.; Liu, D.; Nowson, C.; Duesing, K.; Archer, N.; Bowe, S.; Keast, R. A Low-Fat Diet up-Regulates Expression of Fatty Acid Taste Receptor Gene FFAR4 in Fungiform Papillae in Humans: A Co-Twin Randomised Controlled Trial. Br. J. Nutr. 2019, 122, 1212–1220. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Bertino, M.; Burke, D.; Engelman, K. Experimental Sodium Depletion and Salt Taste in Normal Human Volunteers. Am. J. Clin. Nutr. 1990, 51, 881–889. [Google Scholar] [CrossRef]
- Bertino, M.; Beauchamp, G.K.; Engelman, K. Long-Term Reduction in Dietary Sodium Alters the Taste of Salt. Am. J. Clin. Nutr. 1982, 36, 1134–1144. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Pecore, S.D. Taste Perception of Sodium Chloride in Relation to Dietary Intake of Salt. Am. J. Clin. Nutr. 1982, 35, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Noel, C.A.; Finlayson, G.; Dando, R. Prolonged Exposure to Monosodium Glutamate in Healthy Young Adults Decreases Perceived Umami Taste and Diminishes Appetite for Savory Foods. J. Nutr. 2018, 148, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Dey, M.; Scott, C.; Duong, V.-K.; Arun Dahanukar, A. Dietary Macronutrient Imbalances Lead to Compensatory Changes in Peripheral Taste via Independent Signaling Pathways. J. Neurosci. 2021, 41, 10222–10246. [Google Scholar] [CrossRef]
- Cattaneo, C.; Mambrini, S.P.; Gilardini, L.; Scacchi, M.; Pagliarini, E.; Bertoli, S. Impact of 4-Week of a Restricted Mediterranean Diet on Taste Perception, Anthropometric, and Blood Parameters in Subjects with Severe Obesity. Front. Nutr. 2023, 10, 1196157. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.A.; Choo, E.; Dando, R. Receptor Regulation in Taste: Can Diet Influence How We Perceive Foods? J 2018, 1, 106–115. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhou, D.-S.; Gao, S.-X.; Zhao, X.-C.; Tang, Q.-B.; Wang, C.-Z.; van Loon, J.J.A. Higher Plasticity in Feeding Preference of a Generalist than a Specialist: Experiments with Two Closely Related Helicoverpa Species. Sci. Rep. 2017, 7, 17876. [Google Scholar] [CrossRef]
- May, C.E.; Rosander, J.; Gottfried, J.; Dennis, E.; Dus, M. Dietary Sugar Inhibits Satiation by Decreasing the Central Processing of Sweet Taste. Elife 2020, 9, e54530. [Google Scholar] [CrossRef]
- Pardo-Garcia, T.R.; Gu, K.; Woerner, R.K.R.; Dus, M. Food Memory Circuits Regulate Eating and Energy Balance. Curr. Biol. 2023, 33, 215–227.e3. [Google Scholar] [CrossRef]
- Itoigawa, A.; Hayakawa, T.; Zhou, Y.; Manning, A.D.; Zhang, G.; Grutzner, F.; Imai, H. Functional Diversity and Evolution of Bitter Taste Receptors in Egg-Laying Mammals. Mol. Biol. Evol. 2022, 39, msac107. [Google Scholar] [CrossRef]
- Schiff, H.C.; Kogan, J.F.; Isaac, M.; Czarnecki, L.A.; Fontanini, A.; Maffei, A. Experience-Dependent Plasticity of Gustatory Insular Cortex Circuits and Taste Preferences. Sci. Adv. 2023, 9, eade6561. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Steltz, S.K.; Quinn, N.L.; Robinson, L.M.; Ludwig, D.S. Effects of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Beverages on Cardiometabolic Risk Factors, Body Composition, and Sweet Taste Preference: A Randomized Controlled Trial. J. Am. Heart Assoc. 2020, 9, e015668. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Chow, J.Y.L.; Martire, S.I.; Rooney, K.B.; Boakes, R.A. Switching from Sugar- to Artificially-Sweetened Beverages: A 12-Week Trial. Nutrients 2023, 15, 2191. [Google Scholar] [CrossRef]
- Noel, C.A.; Sugrue, M.; Dando, R. Participants with Pharmacologically Impaired Taste Function Seek out More Intense, Higher Calorie Stimuli. Appetite 2017, 117, 74–81. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Kamil, A.; Blonquist, T.M.; Rehm, C.D.; Qu, S.; Stern, P.; Wilson, A.R. Change in Liking Following Reduction in Sweetness Level of Carbonated Beverages: A Randomized Controlled Parallel Trial. Sci. Rep. 2024, 14, 26742. [Google Scholar] [CrossRef]
- Appleton, K.M.; Tuorila, H.; Bertenshaw, E.J.; de Graaf, C.; Mela, D.J. Sweet Taste Exposure and the Subsequent Acceptance and Preference for Sweet Taste in the Diet: Systematic Review of the Published Literature. Am. J. Clin. Nutr. 2018, 107, 405–419. [Google Scholar] [CrossRef]
- Noel, C.A.; Cassano, P.A.; Dando, R. College-Aged Males Experience Attenuated Sweet and Salty Taste with Modest Weight Gain. J. Nutr. 2017, 147, 1885–1891. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of Sweet and Fat Perception in Obesity: Problems, Solutions and New Perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chetrit, E.; Ben-Ya’acov, A.; Quitina, A.; Atia, O.; Regev, E.; Shteyer, E.; Nashef, R. Anosmia and Dysgeusia amongst COVID-19 Patients Are Associated with Low Levels of Serum Glucagon-like Peptide 1. Int. J. Clin. Pract. 2021, 75, e14996. [Google Scholar] [CrossRef]
- Martin, B.; Dotson, C.D.; Shin, Y.-K.; Ji, S.; Drucker, D.J.; Maudsley, S.; Munger, S.D. Modulation of Taste Sensitivity by GLP-1 Signaling in Taste Buds. Ann. N. Y. Acad. Sci. 2009, 1170, 98–101. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Martin, B.; Golden, E.; Dotson, C.D.; Maudsley, S.; Kim, W.; Jang, H.-J.; Mattson, M.P.; Drucker, D.J.; Egan, J.M.; et al. Modulation of Taste Sensitivity by GLP-1 Signaling. J. Neurochem. 2008, 106, 455–463. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Palayyan, S.R. Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms. Int. J. Mol. Sci. 2022, 23, 8225. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Zheng, H. Modulation of Taste Responsiveness and Food Preference by Obesity and Weight Loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Krieger, J.-P.; Langhans, W.; Lee, S.J. Vagal Mediation of GLP-1’s Effects on Food Intake and Glycemia. Physiol. Behav. 2015, 152, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. The Role of Glucagon-like Peptide-1 Impairment in Obesity and Potential Therapeutic Implications. Diabetes Obes. Metab. 2014, 16, 9–21. [Google Scholar] [CrossRef]
- Moran, T.H. Gut Peptides in the Control of Food Intake. Int. J. Obes. 2009, 33 (Suppl. 1), S7–S10. [Google Scholar] [CrossRef]
- Hengist, A.; Sciarrillo, C.M.; Guo, J.; Walter, M.; Hall, K.D. Gut-Derived Appetite Hormones Do Not Explain Energy Intake Differences in Humans Following Low-Carbohydrate versus Low-Fat Diets. Obesity 2024, 32, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, J.; Xia, P.; Li, S.; Wang, Q.; Li, K.; Li, B.; Li, J. Effects of Konjac Glucomannan Intake Patterns on Glucose and Lipid Metabolism of Obese Mice Induced by a High Fat Diet. Food Funct. 2024, 15, 9116–9135. [Google Scholar] [CrossRef]
- Anini, Y.; Brubaker, P.L. Role of Leptin in the Regulation of Glucagon-like Peptide-1 Secretion. Diabetes 2003, 52, 252–259. [Google Scholar] [CrossRef]
- Gniuli, D.; Calcagno, A.; Dalla Libera, L.; Calvani, R.; Leccesi, L.; Caristo, M.E.; Vettor, R.; Castagneto, M.; Ghirlanda, G.; Mingrone, G. High-Fat Feeding Stimulates Endocrine, Glucose-Dependent Insulinotropic Polypeptide (GIP)-Expressing Cell Hyperplasia in the Duodenum of Wistar Rats. Diabetologia 2010, 53, 2233–2240. [Google Scholar] [CrossRef]
- Feng, X.-H.; Liu, X.-M.; Zhou, L.-H.; Wang, J.; Liu, G.-D. Expression of Glucagon-like Peptide-1 in the Taste Buds of Rat Circumvallate Papillae. Acta Histochem. 2008, 110, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.-C.; Brondel, L.; Meillon, S.; Barthet, S.; Grall, S.; Fenech, C.; Liénard, F.; Schlich, P.; Astruc, K.; Mouillot, T.; et al. Proof of Concept: Effect of GLP-1 Agonist on Food Hedonic Responses and Taste Sensitivity in Poor Controlled Type 2 Diabetic Patients. Diabetes Metab. Syndr. 2019, 13, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation Arising from Obesity Reduces Taste Bud Abundance and Inhibits Renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste Loss with Obesity in Mice and Men. Int. J. Obes. 2020, 44, 739–743. [Google Scholar] [CrossRef]
- Chen, K.; Yan, J.; Suo, Y.; Li, J.; Wang, Q.; Lv, B. Nutritional Status Alters Saccharin Intake and Sweet Receptor mRNA Expression in Rat Taste Buds. Brain Res. 2010, 1325, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liman, E.R.; Kinnamon, S.C. Sour Taste: Receptors, Cells and Circuits. Curr. Opin. Physiol. 2021, 20, 8–15. [Google Scholar] [CrossRef]
- Chometton, S.; Tsan, L.; Hayes, A.M.R.; Kanoski, S.E.; Schier, L.A. Early-Life Influences of Low-Calorie Sweetener Consumption on Sugar Taste. Physiol. Behav. 2023, 264, 114133. [Google Scholar] [CrossRef]
| Diet | Organism [Ref] | Effect on Taste Sensation | Taste System Alteration/Underlying Mechanisms |
|---|---|---|---|
| Bitter | |||
| Strychnine | Caterpillars of the moth [6] | Diminished responses to strychnine | Unidentified |
| Strychnine | Larvae of white butterfly [7] | Diminished responses to bitter compounds | Unidentified |
| Caffeine | Caterpillars of the moth [9,10] | Diminished responses to caffeine | Unidentified |
| Camphor | Fruit flies [8] | Diminished responses to camphor | Downregulated TRPL mediated via Ube3a in sweet neurons |
| Sweet | |||
| Non-nutritive sweetener | Fruit flies [37] | Increased responses to low concentrations of sucrose | Unidentified |
| Low sugar | Humans [38] | Increased perceived sweet intensity | Unidentified |
| Soft drink supplementation | Humans [39] | Reduced perceived sweet intensity | Unidentified |
| High sugar | Fruit flies [19,40,41] | Desensitized responses to sucrose | Epigenetic modulation mediated by PRC2.1/OGT MAPK/ERK signaling pathway |
| Sorbitol | Fruit flies [42] | Increased sensitivity to sucrose | Dopamine signaling via DopR1 and PGC1α pathway |
| High sugar | Rats [43,44] | Attenuated responses to sucrose | Decreased PLCβ2+ type II taste cells in fungiform papillae |
| Low-calorie sweetener | Rats [45] | Unidentified | Reduction in Tas1R2 and Tas1R3 in circumvallate papilla |
| Fat | |||
| High fat | Humans [46] | Increased fat (C18:1) taste threshold | Unidentified |
| High fat | Mice [20] | Reduced responses of taste cells to sucralose and denatonium | Reduction in mRNA of α-gustducin and PLCβ2 in taste buds |
| High fat | Mice [47] | Unidentified | Reduced mRNA of CD36 in circumvallate papillae |
| Low fat | Humans [46,48,49] | Decreased fat (C18:1) taste threshold | Upregulated mRNA of FFAR4 in fungiform papillae |
| Salt | |||
| Low salt | Humans [50,51] | Reduced salty threshold | Unidentified |
| High salt | Humans [52] | Difficulty in discriminating salt concentration | Unidentified |
| Umami | |||
| Monosodium glutamate | Humans [53] | Reduced perceived umami taste intensity | Unidentified |
| Protein | |||
| Protein restricted | Fruit flies [42] | Increased sensitivity to sucrose | Dopamine signaling via DopR1 and PGC1α pathway |
| Other | |||
| Sugar-enriched/Protein-depleted | Fruit flies [54] | Decreased responses to sugars/ Increased responses to amino acids | Downregulation of Dilp5 |
| Sugar-reduced/Protein-enriched | Fruit flies [54] | Increased responses to sugars | Dopamine neuromodulation via Dop2R |
| Mediterranean | Human [55] | Reduced salty threshold | Unidentified |
| Appetitive taste solutions | Mice [56] | Unidentified | Decreased mRNA for Tas1R1, Tas1R2, or ENaC |
| Aversive taste solutions | Mice [56] | Unidentified | Increased mRNA for Tas2R5 or PKD2L1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, H.; Heaton, E.; Dus, M. Taste Plasticity in Nutrition and Health: A Scoping Review. Nutrients 2025, 17, 1336. https://doi.org/10.3390/nu17081336
Sung H, Heaton E, Dus M. Taste Plasticity in Nutrition and Health: A Scoping Review. Nutrients. 2025; 17(8):1336. https://doi.org/10.3390/nu17081336
Chicago/Turabian StyleSung, Hayeon, Elizabeth Heaton, and Monica Dus. 2025. "Taste Plasticity in Nutrition and Health: A Scoping Review" Nutrients 17, no. 8: 1336. https://doi.org/10.3390/nu17081336
APA StyleSung, H., Heaton, E., & Dus, M. (2025). Taste Plasticity in Nutrition and Health: A Scoping Review. Nutrients, 17(8), 1336. https://doi.org/10.3390/nu17081336







