TOTUM-854 Human Circulating Bioactives Preserve Endothelial Cell Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Product
2.2. Ethics Preclinical Study
2.3. Ethics Clinical Trial
2.4. In Vitro ACE Inhibition Assays
2.5. Preclinical Protocol
2.6. Human Study Design and Pharmacokinetic of Absorption
2.7. Phenolic Compounds Extraction from Serum
2.8. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS/MS)
2.9. Human Umbilical Vein Endothelial Cell Cultures
2.10. Preparation of Palmitate Solution
2.11. Cell Viability
2.12. Angiotensin-Converting Enzyme 1 Assays
2.13. Dihydroethidium (DHE) Staining
2.14. Measurement of Nitric Oxide (NO)
2.15. IL-1β Release
2.16. Real-Time RT-qPCR
2.17. Statistics
3. Results
3.1. In Vitro and In Vivo First Evidences of the TOTUM-854 Potential in Regulating Blood Pressure


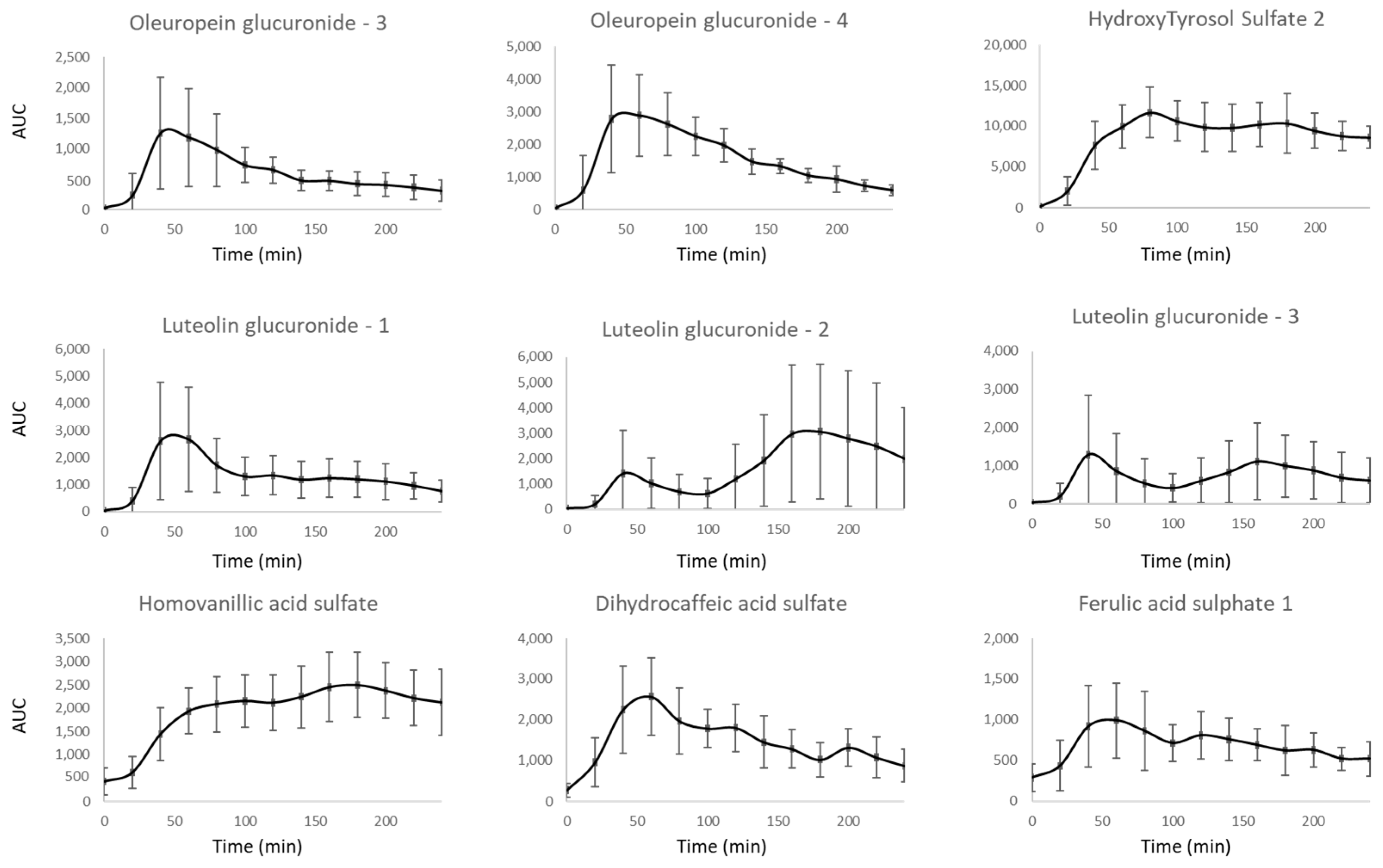
3.2. Kinetic of Apparition of Circulating Metabolites Resulting from TOTUM-854 Ingestion in Humans
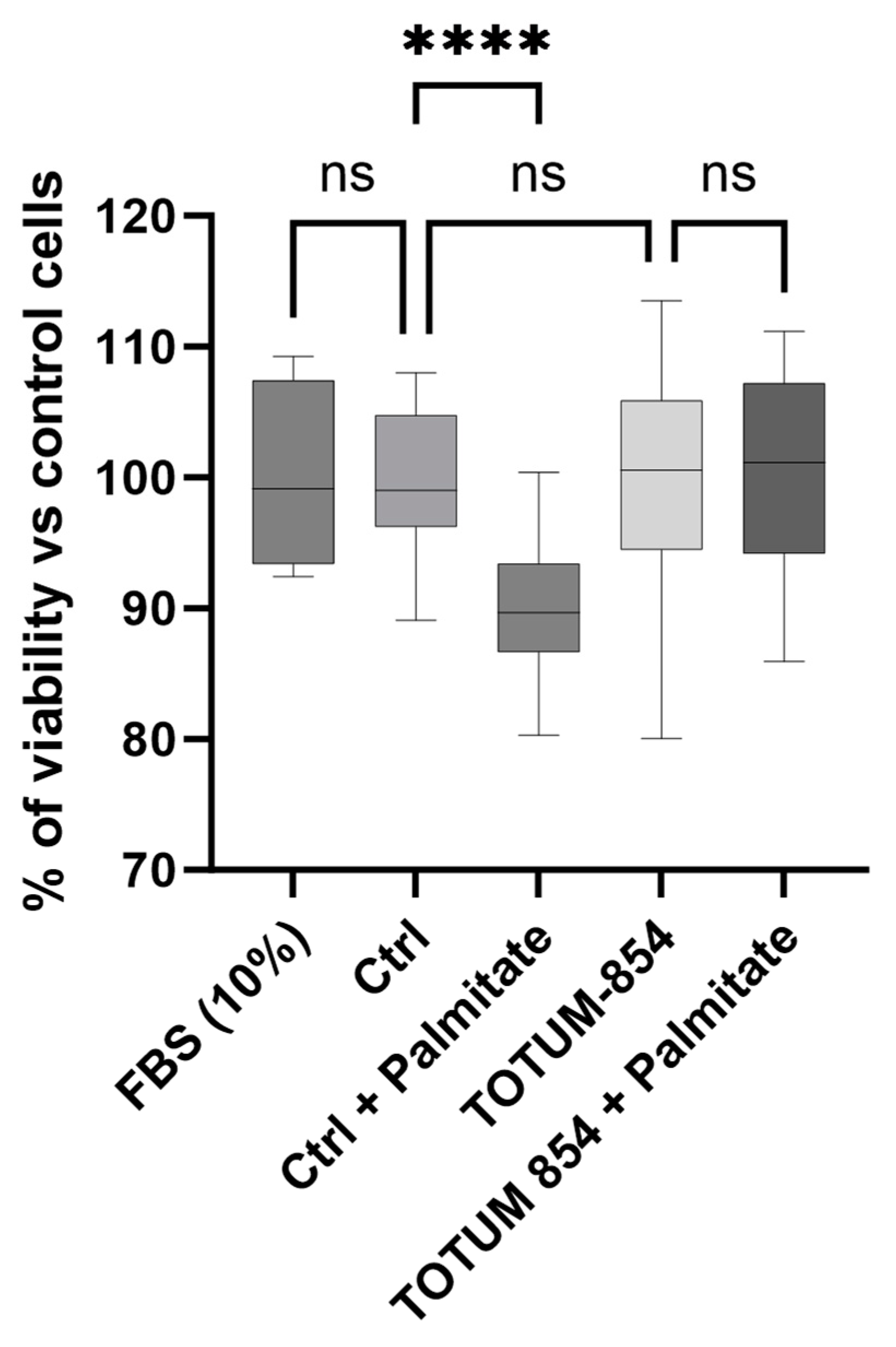
3.3. Absence of Impact on Cell Culture Growth Following Incubation of HUVECs with Either Naïve or Metabolite-Enriched Human Serum
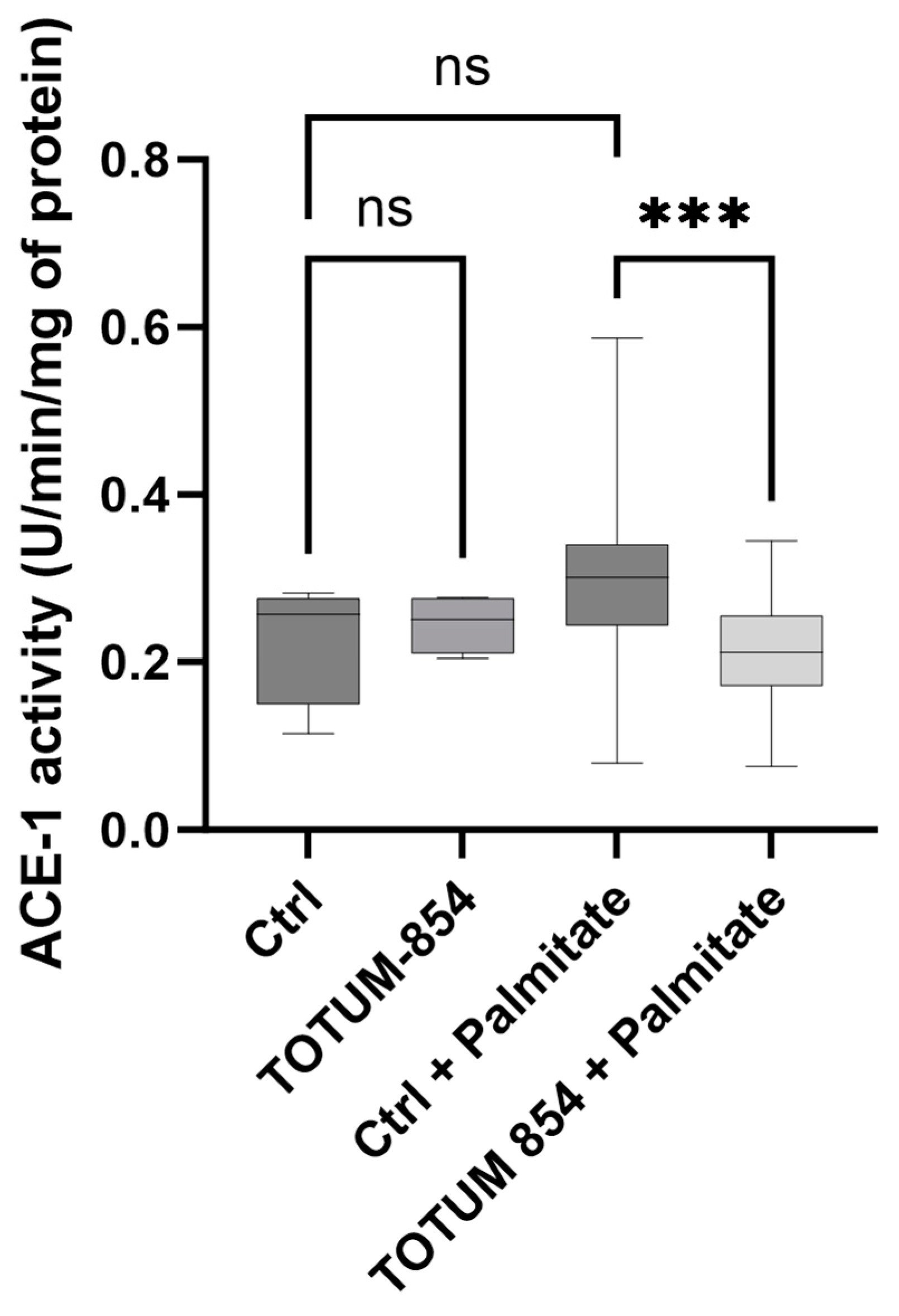
3.4. TOTUM-854 Human Metabolites Decrease Angiotensin-Converting Enzyme 1 Activity Under Palmitate Stress
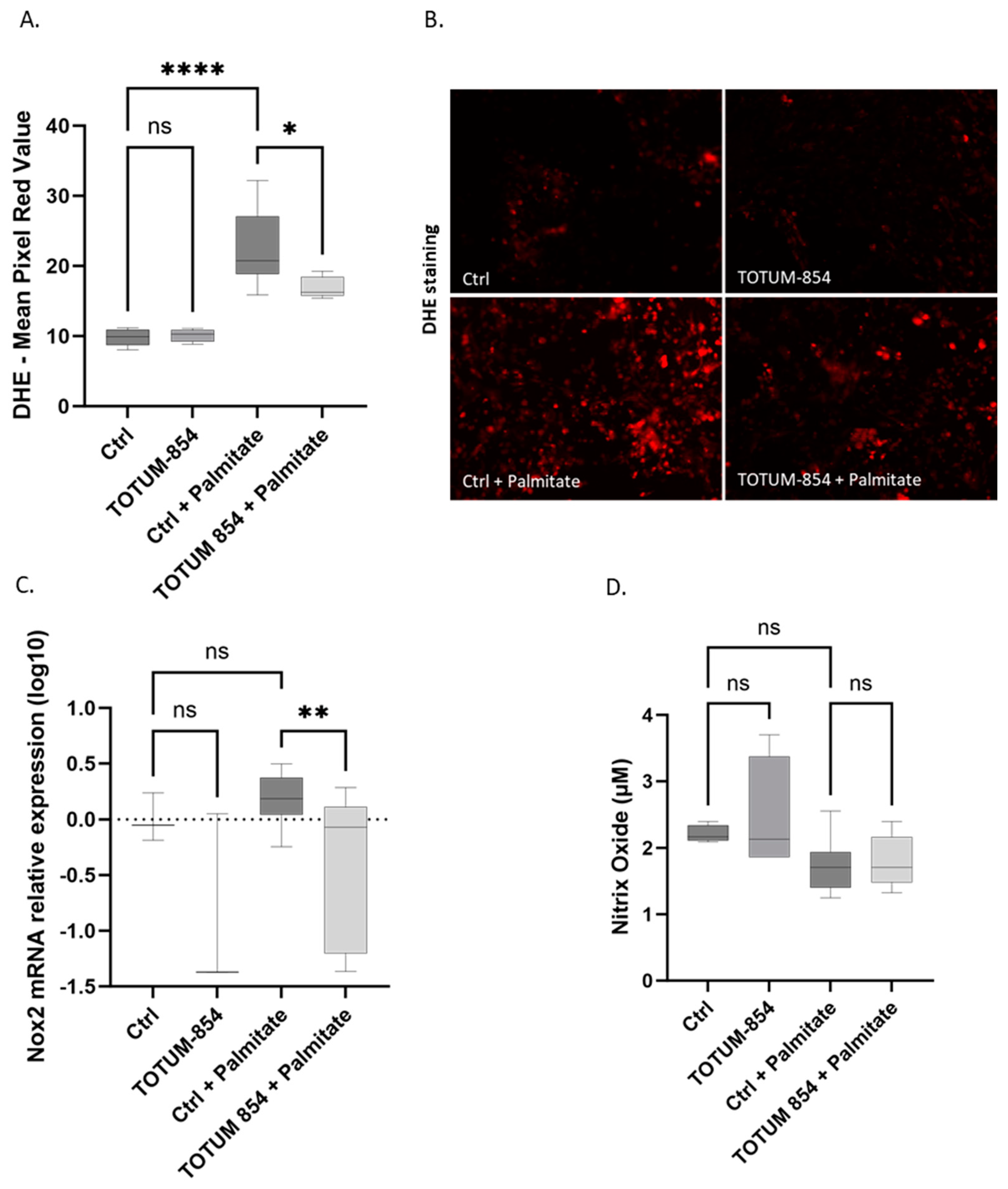
3.5. TOTUM-854 Metabolites Limit Oxidative Stress in a Lipotoxic Environment
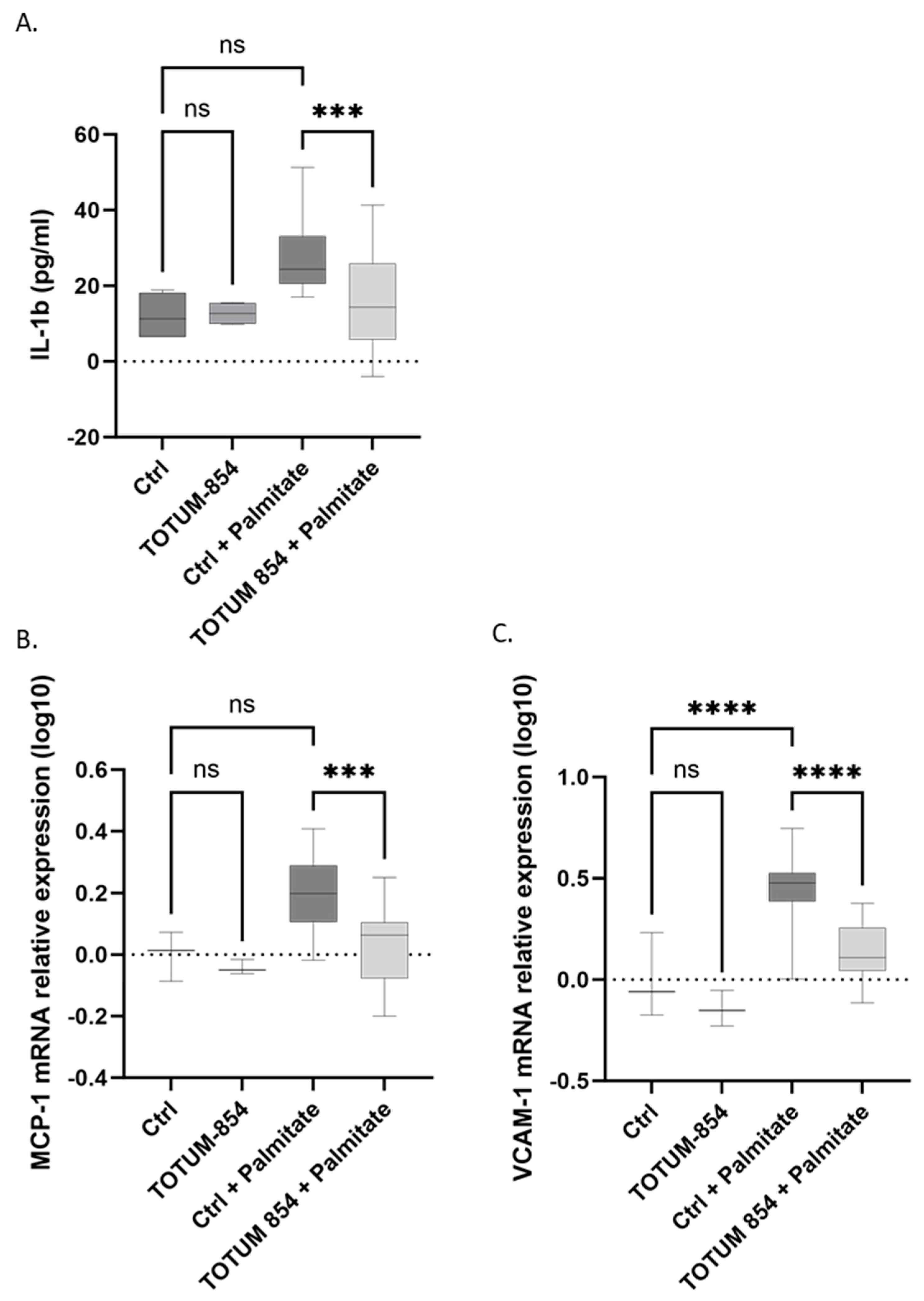
3.6. Inhibition of the Inflammatory Response
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies, C. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Emdin, C.A.; MacMahon, S. The epidemiology of blood pressure and its worldwide management. Circ. Res. 2015, 116, 925–936. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef]
- Alwan, H.; Ehret, G.; Ponte, B.; Pruijm, M.; Ackermann, D.; Guessous, I.; Staessen, J.A.; Asayama, K.; Kutalik, Z.; Vuistiner, P.; et al. Heritability of ambulatory and office blood pressure in the Swiss population. J. Hypertens. 2015, 33, 2061–2067. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Caulfield, M.; Dominiczak, A.F. Genetic and molecular aspects of hypertension. Circ. Res. 2015, 116, 937–959. [Google Scholar] [CrossRef] [PubMed]
- Khurana, V.; Goswami, B. Angiotensin converting enzyme (ACE). Clin. Chim. Acta 2022, 524, 113–122. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Mullick, A.E. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol. Res. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Coffman, T.M. Under pressure: The search for the essential mechanisms of hypertension. Nat. Med. 2011, 17, 1402–1409. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Krisa, S.; Valls, J.; Langhi, C.; Otero, Y.F.; Sirvent, P.; Peltier, S.; Bargetto, M.; Cazaubiel, M.; et al. Circulating Human Metabolites Resulting from TOTUM-070 Absorption (a Plant-Based, Polyphenol-Rich Ingredient) Improve Lipid Metabolism in Human Hepatocytes: Lessons from an Original Ex Vivo Clinical Trial. Nutrients 2023, 15, 1903. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, P.; Brambilla, L.; Brambilla, V.; Gualerzi, M.; Rossi, M.; Parati, G.; Di Rienzo, M.; Tadonio, J.; Novarini, A. Potassium depletion and salt sensitivity in essential hypertension. J. Clin. Endocrinol. Metab. 2001, 86, 2857–2862. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Matute, A.; Tabart, J.; Cheramy-Bien, J.P.; Pirotte, B.; Kevers, C.; Auger, C.; Schini-Kerth, V.; Dommes, J.; Defraigne, J.O.; Pincemail, J. Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to ex-vivo Vascular Reactivity and Potential in vivo Projection. Antioxidants 2020, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverrí, J.; Tapia, E.; Medina-Campos, O.N.; de los Angeles Granados, M.; Franco, M. Garlic prevents hypertension induced by chronic inhibition of nitric oxide synthesis. Life Sci. 1998, 62, 71–77. [Google Scholar] [CrossRef]
- Türck, P.; Fraga, S.; Salvador, I.; Campos-Carraro, C.; Lacerda, D.; Bahr, A.; Ortiz, V.; Hickmann, A.; Koetz, M.; Belló-Klein, A.; et al. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition 2020, 70, 110579. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Bello, G.T.; Oyagbemi, A.A. Caffeic and chlorogenic acids modulate altered activity of key enzymes linked to hypertension in cyclosporine-induced hypertensive rats. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 169–177. [Google Scholar] [CrossRef]
- Kleinnijenhuis, A.J.; van Holthoon, F.L.; Maathuis, A.J.H.; Vanhoecke, B.; Prawitt, J.; Wauquier, F.; Wittrant, Y. Non-targeted and targeted analysis of collagen hydrolysates during the course of digestion and absorption. Anal. Bioanal. Chem. 2020, 412, 973–982. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’Inside(TM)) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef]
- Wauquier, F.; Daneault, A.; Granel, H.; Prawitt, J.; Fabien Soule, V.; Berger, J.; Pereira, B.; Guicheux, J.; Rochefort, G.Y.; Meunier, N.; et al. Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: From Bedside to Bench and Vice Versa. Nutrients 2019, 11, 1249. [Google Scholar] [CrossRef]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef]
- Yves, H.; Herman, J.; Uebelhoer, M.; Wauquier, F.; Boutin-Wittrant, L.; Donneau, A.F.; Monseur, J.; Fotso, V.M.; Duquenne, M.; Wagner, M.; et al. Oral supplementation with fish cartilage hydrolysate in an adult population suffering from knee pain and function discomfort: Results from an innovative approach combining an exploratory clinical study and an ex vivo clinical investigation. BMC Musculoskelet. Disord. 2023, 24, 748. [Google Scholar] [CrossRef]
- Martin-Reyes, F.; Bernal, M.; Rodriguez-Diaz, C.; Rodriguez-de Los Reyes, D.; Ho-Plagaro, A.; Rodriguez-Pacheco, F.; Camacho-Martel, L.; Camargo-Camero, R.; Rodriguez-Gonzalez, F.J.; Alcain-Martinez, G.; et al. Mitochondrial Stress Links Environmental Triggers with Pro-Inflammatory Signaling in Crohn’s Disease. Antioxidants 2023, 12, 2105. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99 (Suppl. S3), S55–S58. [Google Scholar] [CrossRef]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Roscini, A.R.; Trinca, F.; Gemelli, F.; Vaudo, G.; Mannarino, E. Artichoke juice improves endothelial function in hyperlipemia. Life Sci. 2004, 76, 775–782. [Google Scholar] [CrossRef]
- Rangno, R.E.; Kaufmann, J.S.; Cavanaugh, J.H.; Island, D.; Watson, J.T.; Oates, J. Effects of a false neurotransmitter, p-hydroxynorephedrine, on the function of adrenergic neurons in hypertensive patients. J. Clin. Investig. 1973, 52, 952–960. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Hlavackova, L.; Urbanova, A.; Ulicna, O.; Janega, P.; Cerna, A.; Babal, P. Piperine, active substance of black pepper, alleviates hypertension induced by NO synthase inhibition. Bratisl. Lek. Listy 2010, 111, 426–431. [Google Scholar]
- Szulinska, M.; Kregielska-Narozna, M.; Swiatek, J.; Stys, P.; Kuznar-Kaminska, B.; Jakubowski, H.; Walkowiak, J.; Bogdanski, P. Garlic extract favorably modifies markers of endothelial function in obese patients -randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef]
- Atkin, M.; Laight, D.; Cummings, M.H. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J. Diabetes Its Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. PTR 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium sativum L.) Cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef]
- Shaughnessy, K.S.; Boswall, I.A.; Scanlan, A.P.; Gottschall-Pass, K.T.; Sweeney, M.I. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr. Res. 2009, 29, 130–138. [Google Scholar] [CrossRef]
- Wiseman, W.; Egan, J.M.; Slemmer, J.E.; Shaughnessy, K.S.; Ballem, K.; Gottschall-Pass, K.T.; Sweeney, M.I. Feeding blueberry diets inhibits angiotensin II-converting enzyme (ACE) activity in spontaneously hypertensive stroke-prone rats. Can. J. Physiol. Pharmacol. 2011, 89, 67–71. [Google Scholar] [CrossRef]
- Gao, X.; Xue, Z.; Ma, Q.; Guo, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism. J. Food Biochem. 2020, 44, e13126. [Google Scholar] [CrossRef]
- Drobiova, H.; Thomson, M.; Al-Qattan, K.; Peltonen-Shalaby, R.; Al-Amin, Z.; Ali, M. Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase method. Evid.-Based Complement. Altern. Med. 2011, 2011, 703049. [Google Scholar] [CrossRef]
- Ali, M.; Al-Qattan, K.K.; Al-Enezi, F.; Khanafer, R.M.; Mustafa, T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 253–259. [Google Scholar] [CrossRef]
- Iwashima, T.; Kudome, Y.; Kishimoto, Y.; Saita, E.; Tanaka, M.; Taguchi, C.; Hirakawa, S.; Mitani, N.; Kondo, K.; Iida, K. Aronia berry extract inhibits TNF-alpha-induced vascular endothelial inflammation through the regulation of STAT3. Food Nutr. Res. 2019, 63, 3361. [Google Scholar] [CrossRef]
- Castineiras-Landeira, M.I.; Rodino-Janeiro, B.K.; Paradela-Dobarro, B.; Batista-Oliveira, A.L.; Raposeiras-Roubin, S.; Gonzalez-Peteiro, M.; Gonzalez-Juanatey, J.R.; Alvarez, E. Change of concept about the regulation of angiotensin II-induced monocyte chemoattractant protein-1 production in human endothelial cells. Vasc. Pharmacol. 2016, 80, 20–34. [Google Scholar] [CrossRef]
- Karanovic, D.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Ivanov, M.; Vajic, U.-J.; Grujic-Milanovic, J.; Markovic-Lipkovski, J.; Dekanski, D.; Jovovic, D. Olive leaf extract attenuates adriamycin-induced focal segmental glomerulosclerosis in spontaneously hypertensive rats via suppression of oxidative stress, hyperlipidemia, and fibrosis. Phytother. Res. 2021, 35, 1534–1545. [Google Scholar] [CrossRef]
- Loffredo, L.; Baratta, F.; Ludovica, P.; Battaglia, S.; Carnevale, R.; Nocella, C.; Novo, M.; Pannitteri, G.; Ceci, F.; Angelico, F.; et al. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr. Metab. Cardiovasc. Dis. 2017, 28, 143–149. [Google Scholar] [CrossRef]
- Loffredo, L.; Zicari, A.M.; Occasi, F.; Perri, L.; Carnevale, R.; Angelico, F.; Del Ben, M.; Martino, F.; Nocella, C.; Savastano, V.; et al. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: Role of NADPH oxidase. Atherosclerosis 2015, 240, 222–227. [Google Scholar] [CrossRef]
- Del Ben, M.; Fabiani, M.; Loffredo, L.; Polimeni, L.; Carnevale, R.; Baratta, F.; Brunori, M.; Albanese, F.; Augelletti, T.; Violi, F.; et al. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm. Med. 2012, 12, 36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wauquier, F.; Ripoche, D.; Boutin-Wittrant, L.; Otero, Y.F.; Krisa, S.; Valls, J.; Maura, M.; Le Joubioux, F.; Maugard, T.; Bolea, G.; et al. TOTUM-854 Human Circulating Bioactives Preserve Endothelial Cell Function. Nutrients 2025, 17, 1331. https://doi.org/10.3390/nu17081331
Wauquier F, Ripoche D, Boutin-Wittrant L, Otero YF, Krisa S, Valls J, Maura M, Le Joubioux F, Maugard T, Bolea G, et al. TOTUM-854 Human Circulating Bioactives Preserve Endothelial Cell Function. Nutrients. 2025; 17(8):1331. https://doi.org/10.3390/nu17081331
Chicago/Turabian StyleWauquier, Fabien, Doriane Ripoche, Line Boutin-Wittrant, Yolanda F. Otero, Stéphanie Krisa, Josep Valls, Mahéva Maura, Florian Le Joubioux, Thierry Maugard, Gaëtan Bolea, and et al. 2025. "TOTUM-854 Human Circulating Bioactives Preserve Endothelial Cell Function" Nutrients 17, no. 8: 1331. https://doi.org/10.3390/nu17081331
APA StyleWauquier, F., Ripoche, D., Boutin-Wittrant, L., Otero, Y. F., Krisa, S., Valls, J., Maura, M., Le Joubioux, F., Maugard, T., Bolea, G., Meyer, G., Reboul, C., Roux, V., Macian, N., Pickering, G., Pereira, B., Bargetto, M., Sapone, V., Cazaubiel, M., ... Wittrant, Y. (2025). TOTUM-854 Human Circulating Bioactives Preserve Endothelial Cell Function. Nutrients, 17(8), 1331. https://doi.org/10.3390/nu17081331









