Homocysteine, Nutrition, and Gut Microbiota: A Comprehensive Review of Current Evidence and Insights
Abstract
1. Introduction
Homocysteine and Gut Microbiota
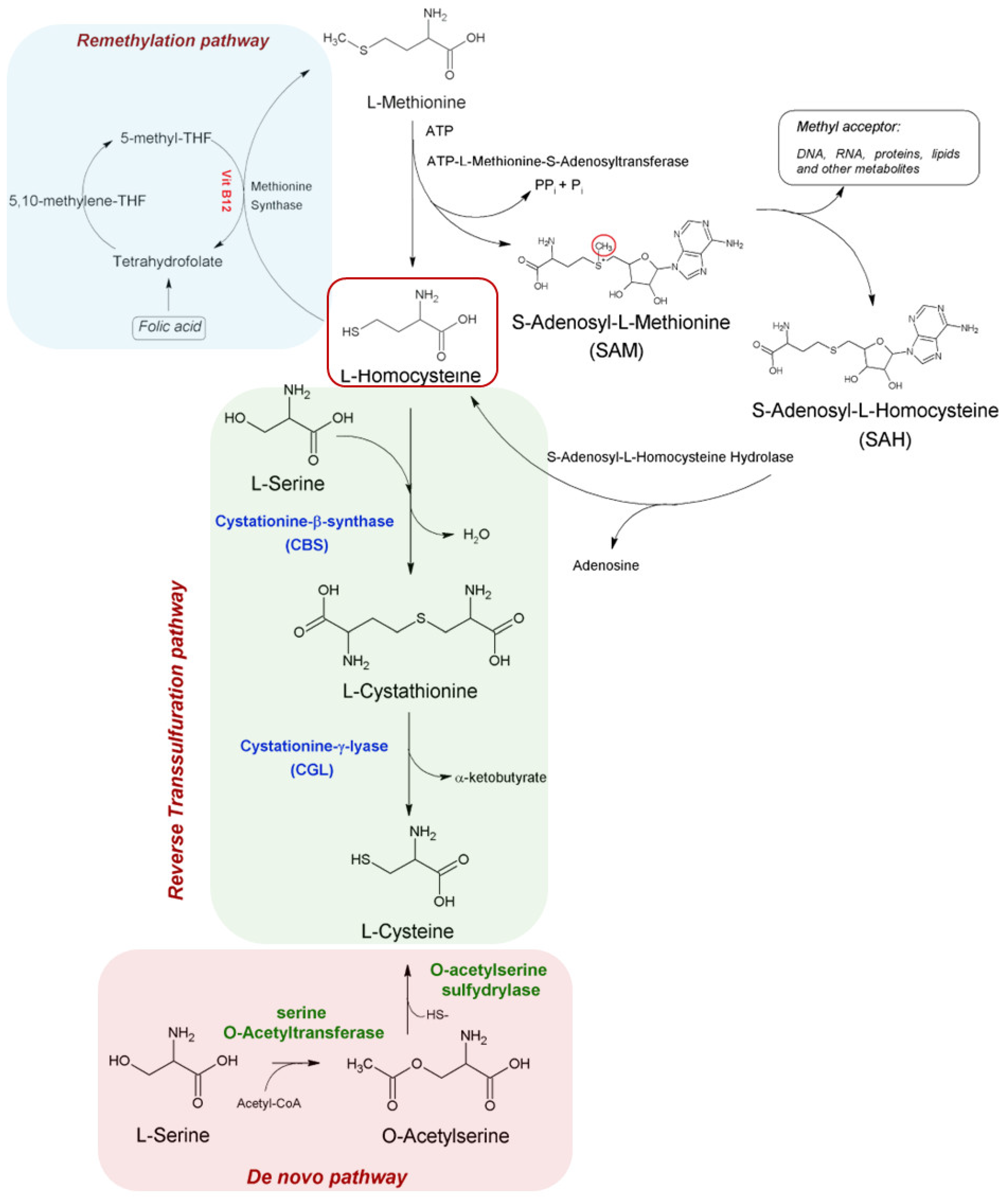
2. Homocysteine Metabolism
2.1. Biosynthesis and Metabolism of Homocysteine
2.2. Remethylation Pathway
2.3. Reverse Transsulfuration and De Novo Pathways
3. The Relationship Between Diet and Homocysteine
4. The Relationship Between Supplements and Homocysteine Level
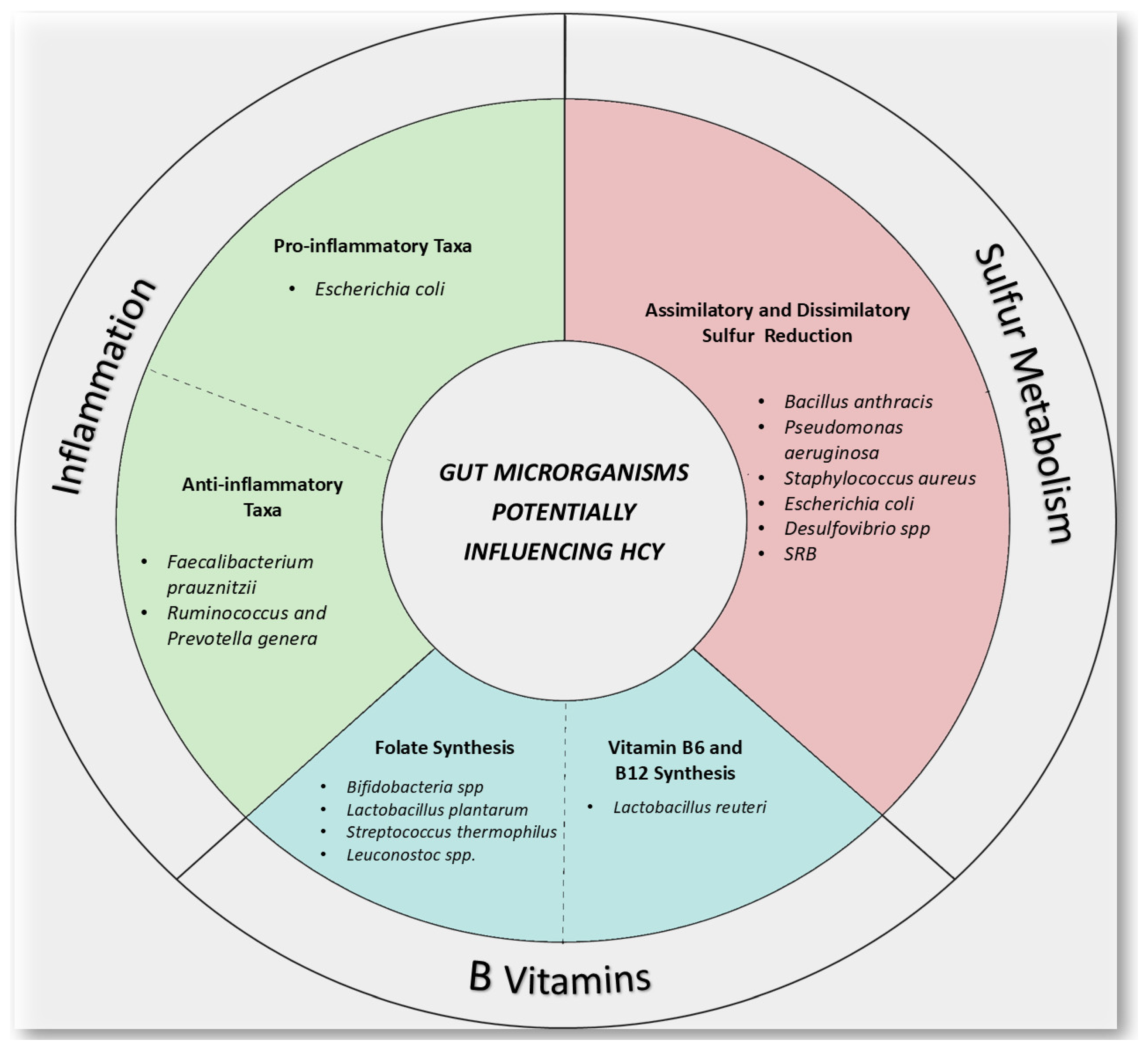
5. Gut Microbiota and Homocysteine
5.1. The Relationship Between Gut Microbiota, Diet and B Vitamins
5.2. How Gut Microbiota Can Influence Homocysteine Levels?
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Hcy | Homocysteine |
| Met | L-Methionine |
| PLP | pyridoxal-5′-phosphate |
| SAM | S-adenosylmethionine |
| MTHFR | methylenetetrahydrofolate reductase |
| CBS | cystathionine β-synthase |
| HHcy | hyperhomocysteinemia |
| CVDs | cardiovascular diseases |
| FA | folic acid |
| MS | methionine synthase |
| EC | endothelial cells |
| GIT | gastrointestinal tissue |
| MAT | ATP-L-Methionine S-Adenosyltranferase |
| SAH | S-Adenosyl homocysteine |
| AHCY | S-adenosyl homocysteine hydrolase |
| THF | tetrahydrofolate |
| 5-methyl-THF | 5-N-methyl tetrahydrofolate |
| BHMT | betaine-homocysteine methyltransferase |
| CGL | cystathione γ-lyase |
| SAT | O-acetyl transferase |
| OASS | O-acetylserine sulfhydrylase |
| OCBSs | O-acetylserine dependent CBSs |
| ACS | acute coronary syndrome |
| MD | Mediterranean Diet |
| MUFAs | monounsaturated fatty acids (MUFAs) |
| PUFAs | polyunsaturated fatty acids |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| LBM | Lean body mass |
| apoE | Apolipoprotein-E |
| HHD | hyperhomocysteinemic diet |
| CD | control diet |
| 5-MTHF | 5-methyltetrahydrofolate |
| DHFR | dihydrofolate reductase |
| UMFA | unmetabolized FA |
| RDA | recommended daily allowance |
| DFE | dietary folate equivalent |
| MetS | Metabolic Syndrome |
| IBD | inflammatory bowel disease |
| MDD | major depressive disorder |
References
- Selhub, J. HOMOCYSTEINE METABOLISM. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Uzunhan, T.A.; Aydinli, N.; Çalişkan, M.; Tatli, B.; Özmen, M. Short-term Neurological Outcomes in Ischemic and Hemorrhagic Pediatric Stroke. Pediatr. Int. 2019, 61, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.A.; Parish, S.; Verhoef, P.; Dötsch-Klerk, M.; Lathrop, M.; Xu, P.; Nordestgaard, B.G.; Holm, H.; Hopewell, J.C.; et al. Homocysteine and Coronary Heart Disease: Meta-Analysis of MTHFR Case-Control Studies, Avoiding Publication Bias. PLoS Med. 2012, 9, e1001177. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K.; Kostense, P.J.; Beks, P.J.; Mackaay, A.J.C.; Jakobs, C.; Bouter, L.M.; Heine, R.J.; Stehouwer, C.D.A. Hyper-homocysteinemia Is Associated with an Increased Risk of Cardiovascular Disease, Especially in Non–Insulin-Dependent Dia-betes Mellitus. Arter. Thromb. Vasc. Biol. 1998, 18, 133–138. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine—From Disease Biomarker to Disease Prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Djuric, D.; Jakovljevic, V.; Zivkovic, V.; Srejovic, I. Homocysteine and Homocysteine-Related Compounds: An Overview of the Roles in the Pathology of the Cardiovascular and Nervous Systems. Can. J. Physiol. Pharmacol. 2018, 96, 991–1003. [Google Scholar] [CrossRef]
- Lalouschek, W.; Aull, S.; Serles, W.; Schnider, P.; Mannhalter, C.; Lang, T.; Deecke, L.; Zeiler, K. Genetic and Nongenetic Factors Influencing Plasma Homocysteine Levels in Patients with Ischemic Cerebrovascular Disease and in Healthy Control Subjects. J. Lab. Clin. Med. 1999, 133, 575–582. [Google Scholar] [CrossRef]
- D’Souza, S.W.; Glazier, J.D. Homocysteine Metabolism in Pregnancy and Developmental Impacts. Front. Cell Dev. Biol. 2022, 10, 802285. [Google Scholar] [CrossRef]
- Riedijk, M.A.; Stoll, B.; Chacko, S.; Schierbeek, H.; Sunehag, A.L.; van Goudoever, J.B.; Burrin, D.G. Methionine Transmethyl-ation and Transsulfuration in the Piglet Gastrointestinal Tract. Proc. Natl. Acad. Sci. USA 2007, 104, 3408–3413. [Google Scholar] [CrossRef]
- Schalinske, K.L.; Smazal, A.L. Homocysteine Imbalance: A Pathological Metabolic Marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial Dysfunction: The Link Between Homocysteine and Hydrogen Sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Cueto, R.; Jiang, X.; Lu, L.; Sardy, J.; Xiong, X.; Yu, J.E.; Pham, H.; Khan, M.; Qin, X.; et al. Molecular Processes Me-diating Hyperhomocysteinemia-Induced Metabolic Reprogramming, Redox Regulation and Growth Inhibition in Endothelial Cells. Redox Biol. 2021, 45, 102018. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A Systematic Framework for Understanding the Microbiome in Human Health and Disease: From Basic Principles to Clinical Translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2022, 12, 34. [Google Scholar] [CrossRef]
- Dehghani, E.; Karimi, K.; Arekhi, S.; Ardeshir, M.; Rezapour, R.; Shayestehfar, M.; Memari, A.H. Effect of nutritional supplements on gut microbiome in individuals with neurodevelopmental disorders: A systematic review and narrative synthesis. BMC Nutr. 2025, 11, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Amatori, S.; Sisti, D.; Gervasi, M.; Agostini, D.; Piccoli, G.; Pazienza, V.; Gobbi, P.; Rocchi, M.B.L.; Sestili, P.; et al. Nine Weeks of High-Intensity Indoor Cycling Training Induced Changes in the Microbiota Composition in Non-Athlete Healthy Male College Students. J. Int. Soc. Sports Nutr. 2021, 18, 74. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S -Adenosylmethionine in Liver Health, Injury, and Cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Rivera, I.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Homocysteine Metabolism, Hyperhomocysteinaemia and Vascular Disease: An Overview. J. Inherit. Metab. Dis. 2006, 29, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Téllez, J.; Yamanaka, L.; Steindel, M.; Romanha, A.; Grisard, E. Transsulfuration Is an Active Pathway for Cysteine Biosynthesis in Trypanosoma Rangeli. Parasit. Vectors 2014, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. SULFUR AMINO ACID METABOLISM: Pathways for Production and Removal of Homocysteine and Cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Werge, M.P.; McCann, A.; Galsgaard, E.D.; Holst, D.; Bugge, A.; Albrechtsen, N.J.W.; Gluud, L.L. The Role of the Transsul-furation Pathway in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 1081. [Google Scholar] [CrossRef]
- Rabeh, W.M.; Cook, P.F. Structure and Mechanism of O-Acetylserine Sulfhydrylase. J. Biol. Chem. 2004, 279, 26803–26806. [Google Scholar] [CrossRef]
- Wirtz, M.; Droux, M. Synthesis of the Sulfur Amino Acids: Cysteine and Methionine. Photosynth. Res. 2005, 86, 345–362. [Google Scholar] [CrossRef]
- KOPRIVA, S. Regulation of Sulfate Assimilation in Arabidopsis and Beyond. Ann. Bot. 2006, 97, 479–495. [Google Scholar] [CrossRef]
- Hullo, M.-F.; Auger, S.; Soutourina, O.; Barzu, O.; Yvon, M.; Danchin, A.; Martin-Verstraete, I. Conversion of Methionine to Cysteine in Bacillus Subtilis and Its Regulation. J. Bacteriol. 2007, 189, 187–197. [Google Scholar] [CrossRef]
- Devi, S.; Abdul Rehman, S.A.; Tarique, K.F.; Gourinath, S. Structural Characterization and Functional Analysis of Cystathi-onine Β-synthase: An Enzyme Involved in the Reverse Transsulfuration Pathway of Bacillus Anthracis. FEBS J. 2017, 284, 3862–3880. [Google Scholar] [CrossRef]
- Matoba, Y.; Yoshida, T.; Izuhara-Kihara, H.; Noda, M.; Sugiyama, M. Crystallographic and Mutational Analyses of Cystathi-onine Β-synthase in the H 2 S-synthetic Gene Cluster in Lactobacillus Plantarum. Protein Sci. 2017, 26, 763–783. [Google Scholar] [CrossRef] [PubMed]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of Vitamin B6, B9, and B12 Supplemen-tation on Homocysteine Level and Cardiovascular Outcomes in Stroke Patients: A Meta-Analysis of Randomized Controlled Trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homo-cysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- De Bree, A.; Verschuren, W.M.; Kromhout, D.; Mennen, L.I.; Blom, H.J. Homocysteine and Coronary Heart Disease: The Im-portance of a Distinction between Low and High Risk Subjects. Int. J. Epidemiol. 2002, 31, 1268–1272. [Google Scholar] [CrossRef]
- Liew, S.-C.; Das Gupta, E. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism: Epidemiology, Metabolism and the Associated Diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Chan, C.Y.T.; Cheng, S.W.K. Elevated Homocysteine in Human Abdominal Aortic Aneurysmal Tissues. Vasc. Med. 2017, 22, 370–377. [Google Scholar] [CrossRef]
- Wyse, A.T.S.; Bobermin, L.D.; dos Santos, T.M.; Quincozes-Santos, A. Homocysteine and Gliotoxicity. Neurotox. Res. 2021, 39, 966–974. [Google Scholar] [CrossRef]
- Foscolou, A.; Rallidis, L.S.; Tsirebolos, G.; Critselis, E.; Katsimardos, A.; Drosatos, A.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B. The Association between Homocysteine Levels, Mediterranean Diet and Cardiovascular Disease: A Case-Control Study. Int. J. Food Sci. Nutr. 2019, 70, 603–611. [Google Scholar] [CrossRef]
- Tajima, A.; Kubo, Y.; Horiguchi, S.; Shoji, K.; Kawabata, T. Relationship between Serum Homocysteine Concentration and Dietary Factors in Young Japanese Women. Nutrients 2023, 15, 4740. [Google Scholar] [CrossRef]
- Czerwonogrodzka-Senczyna, A.; Jerzak, M.; Jeznach-Steinhagen, A.; Karzel, K.; Boniecka, I. Content of Fatty Acids in a Diet and the Homocysteine Levels in Women with Fertility Disorders. Neuro Endocrinol. Lett. 2018, 39, 56–64. [Google Scholar]
- Yun, K.U.; Ryu, C.S.; Oh, J.M.; Kim, C.H.; Lee, K.S.; Lee, C.-H.; Lee, H.-S.; Kim, B.-H.; Kim, S.K. Plasma Homocysteine Level and Hepatic Sulfur Amino Acid Metabolism in Mice Fed a High-Fat Diet. Eur. J. Nutr. 2013, 52, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; Portari, G.V.; Marchini, J.S.; Vannucchi, H.; Jordao, A.A. Effects of a Low-Protein Diet on Plasma Amino Acid and Homocysteine Levels and Oxidative Status in Rats. Ann. Nutr. Metab. 2009, 54, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Wang, M.; Li, X.; Xia, M.; Ling, W. Dietary Protein and Plasma Total Homocysteine, Cysteine Concentrations in Coronary Angiographic Subjects. Nutr. J. 2013, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Ligthart-Melis, G.C.; Engelen, M.P.; Simbo, S.Y.; Ten Have, G.A.; Thaden, J.J.; Cynober, L.; Deutz, N.E. Metabolic Consequences of Supplemented Methionine in a Clinical Context. J. Nutr. 2020, 150, 2538S–2547S. [Google Scholar] [CrossRef]
- Andrews, S.G.; Koehle, A.M.; Paudel, D.; Neuberger, T.; Ross, A.C.; Singh, V.; Bottiglieri, T.; Castro, R. Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipopro-tein-E-Deficient Mice. Nutrients 2024, 16, 330. [Google Scholar] [CrossRef]
- Levin, B.L.; Varga, E. MTHFR: Addressing Genetic Counseling Dilemmas Using Evidence-Based Literature. J. Genet. Couns. 2016, 25, 901–911. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic Acid versus 5- Methyl Tetrahydrofolate Supplementation in Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Akoglu, B.; Schrott, M.; Bolouri, H.; Jaffari, A.; Kutschera, E.; Caspary, W.F.; Faust, D. The Folic Acid Metabolite L-5-Methyltetrahydrofolate Effectively Reduces Total Serum Homocysteine Level in Orthotopic Liver Transplant Recipients: A Double-Blind Placebo-Controlled Study. Eur. J. Clin. Nutr. 2008, 62, 796–801. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Wang, C.; Gong, C. Comparison of the Antihypertensive Effects of Folic Acid and Resveratrol in Spontane-ously Hypertensive Rats Combined with Hyperhomocysteinemia. SAGE Open Med. 2023, 11, 20503121231220813. [Google Scholar] [CrossRef]
- Paniz, C.; Bertinato, J.F.; Lucena, M.R.; De Carli, E.; Amorim, P.M.d.S.; Gomes, G.W.; Palchetti, C.Z.; Figueiredo, M.S.; Pfeiffer, C.M.; Fazili, Z.; et al. A Daily Dose of 5 Mg Folic Acid for 90 Days Is Associated with Increased Serum Unmetabolized Folic Acid and Reduced Natural Killer Cell Cytotoxicity in Healthy Brazilian Adults. J. Nutr. 2017, 147, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mu-tations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. DACH-LIGA Homocystein (German, Austrian and Swiss Homocysteine Society): Consensus Paper on the Rational Clinical Use of Homocysteine, Folic Acid and B-Vitamins in Cardiovascular and Thrombotic Diseases: Guidelines and Recommendations. Clin. Chem. Lab. Med. 2003, 41, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Huang, X.; Bao, H.; Ding, C.; Li, J.; Cao, T.; Liu, L.; Wei, Y.; Zhou, Z.; Zhang, N.; Song, Y.; et al. Optimal Folic Acid Dosage in Lowering Homocysteine: Precision Folic Acid Trial to Lower Homocysteine (PFAT-Hcy). Eur. J. Nutr. 2024, 63, 1513–1528. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Al-kassab-Córdova, A.; Cabrera-Guzmán, J.C.; Herrera-Añazco, P.; Benites-Zapata, V.A. Vitamin B12, Folate, and Homocysteine in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1221259. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 Absorption: Mammalian Physiology and Acquired and Inherited Disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.A.M.; de Vries, J.H.M.; de Bree, A.; van der Put, N.; van Staveren, W.A.; de Groot, L.C.P.G.M. Dietary Intake and Status of Folate and Vitamin B12 and Their Association with Homocysteine and Cardiovascular Disease in European Populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef]
- Porter, K.; Hoey, L.; Hughes, C.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef]
- Hoey, L.; Strain, J.; McNulty, H. Studies of Biomarker Responses to Intervention with Vitamin B-12: A Systematic Review of Randomized Controlled Trials. Am. J. Clin. Nutr. 2009, 89, 1981S–1996S. [Google Scholar] [CrossRef]
- Fernandes, S.; Oliveira, L.; Pereira, A.; Costa, M.d.C.; Raposo, A.; Saraiva, A.; Magalhães, B. Exploring Vitamin B12 Sup-plementation in the Vegan Population: A Scoping Review of the Evidence. Nutrients 2024, 16, 1442. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, M.H.; Almuqayyid, F.; Alfardous Alazm, A.; Ziamanesh, F.; Izze da Silva Magalhães, E.; Bagheri, S.E.; Rodrigues de Oliveira, B.; Alfardous Alazm, M.; Adi, A.R.; Alomar, S.; et al. A Comprehensive Review and Meta-Regression Analysis of Randomized Controlled Trials Examining the Impact of Vitamin B12 Supplementation on Homocysteine Levels. Nutr. Rev. 2024, 82, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, H.L.T. Lowering Blood Homocysteine with Folic Acid Based Supplements: Meta-Analysis of Randomised Trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998, 316, 894–898. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Wang, F. Effect of Nutritional Supplements for Reducing Homocysteine Levels in Healthy Adults: A Systematic Review and Network Meta-Analysis of Randomized Trials. Nutr. Rev. 2025, nuae191. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Laganà, A.S. The Link between Homocysteine and Omega-3 Polyunsaturated Fatty Acid: Critical Appraisal and Future Directions. Biomolecules 2020, 10, 219. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Roshan, M.M.; Olusola, O.F.; Fatahi, S.; Omidi, H.R.; Sharifi, P.; Hekmatdoost, A.; Kutbi, E.; Abu-Zaid, A. Impact of Omega-3 Supplementation on Homocysteine Levels in Humans: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2013–2025. [Google Scholar] [CrossRef]
- Miao, Y.; Guo, Y.; Chen, Y.; Lin, Y.; Lu, Y.; Guo, Q. The Effect of B-Vitamins on the Prevention and Treatment of Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutr. Rev. 2024, 82, 1386–1401. [Google Scholar] [CrossRef]
- Kuroda, K.; Horikawa, T.; Gekka, Y.; Moriyama, A.; Nakao, K.; Juen, H.; Takamizawa, S.; Ojiro, Y.; Nakagawa, K.; Sugiyama, R. Effects of Periconceptional Multivitamin Supplementation on Folate and Homocysteine Levels Depending on Genetic Variants of Methyltetrahydrofolate Reductase in Infertile Japanese Women. Nutrients 2021, 13, 1381. [Google Scholar] [CrossRef]
- Son, P.; Lewis, L. Hyperhomocysteinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wald, D.S. Homocysteine and Cardiovascular Disease: Evidence on Causality from a Meta-Analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-Cause Mortality: A Dose-Response Me-ta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef]
- Esteghamati, A.; Hafezi-Nejad, N.; Zandieh, A.; Sheikhbahaei, S.; Ebadi, M.; Nakhjavani, M. Homocysteine and Metabolic Syndrome: From Clustering to Additional Utility in Prediction of Coronary Heart Disease. J. Cardiol. 2014, 64, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Pi, F.; Ding, Z.; Chen, W.; Pang, S.; Dong, W.; Zhang, Q. Efficacy of Supplementation with B Vitamins for Stroke Prevention: A Network Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0137533. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health Office of Dietary Supplements Folate. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 9 April 2025).
- Vezzoli, A.; Dellanoce, C.; Maria Caimi, T.; Vietti, D.; Montorsi, M.; Mrakic-Sposta, S.; Accinni, R. Influence of Dietary Sup-plementation for Hyperhomocysteinemia Treatments. Nutrients 2020, 12, 1957. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut Microbiota Contributes to the Methionine Metabolism in Host. Front. Microbiol. 2022, 13, 1065668. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Gong, Z.; Dong, Z.; Yu, F.; Fu, Y.; Jiang, C.; Kong, W. Ablation of the Gut Microbiota Alleviates High-Methionine Diet-Induced Hyperhomocysteinemia and Glucose Intolerance in Mice. NPJ Sci. Food 2023, 7, 36. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic Analysis of Gut Microbiome Reveals the Role of Bacterial Folate and Homocysteine Metabolism in Parkinson’s Disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Joseph, R.; Nath, S.G.; Joseraj, M.G. Elevated Plasma Homocysteine Levels in Chronic Periodontitis: A Hospital-Based Case-Control Study. J. Periodontol. 2011, 82, 439–444. [Google Scholar] [CrossRef]
- Lurz, E.; Horne, R.G.; Määttänen, P.; Wu, R.Y.; Botts, S.R.; Li, B.; Rossi, L.; Johnson-Henry, K.C.; Pierro, A.; Surette, M.G.; et al. Vitamin B12 Deficiency Alters the Gut Microbiota in a Murine Model of Colitis. Front. Nutr. 2020, 7, 83. [Google Scholar] [CrossRef]
- Rizowy, G.M.; Poloni, S.; Colonetti, K.; Donis, K.C.; Dobbler, P.T.; Leistner-Segal, S.; Roesch, L.F.W.; Schwartz, I.V.D. Is the Gut Microbiota Dysbiotic in Patients with Classical Homocystinuria? Biochimie 2020, 173, 3–11. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, S.; Wang, X.; Weng, Y.; Fan, X.; Sheng, H.; Zhu, X.; Lou, L.; Zhang, F. The Flavonoid-Rich Quzhou Fructus Aurantii Extract Modulates Gut Microbiota and Prevents Obesity in High-Fat Diet-Fed Mice. Nutr. Diabetes 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Lee, S.M.; Jung, J. Integrated Omics Analysis Unraveled the Microbiome-Mediated Effects of Yijin-Tang on Hepa-tosteatosis and Insulin Resistance in Obese Mouse. Phytomedicine 2020, 79, 153354. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, H.J.; Kim, Y.; Lee, M.S.; Lee, H.S. Properties of the Corynebacterium Glutamicum MetC Gene Encoding Cystathionine Beta-Lyase. Mol. Cells 2001, 11, 220–225. [Google Scholar] [CrossRef]
- Seiflein, T.A.; Lawrence, J.G. Two Transsulfurylation Pathways in Klebsiella Pneumoniae. J. Bacteriol. 2006, 188, 5762–5774. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite Interactions between Host and Microbiota during Health and Disease: Which Feeds the Other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J. Differences in Folate Production by Bifidobacteria of Different Origins. Biosci. Microbiota Food Health 2015, 34, 87–93. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Storelli, G.; Téfit, M.; Leulier, F. Metformin, Microbes, and Aging. Cell Metab. 2013, 17, 809–811. [Google Scholar] [CrossRef]
- Strozzi, G.P.; Mogna, L. Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. S3), S179–S184. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Majewska, K.; Kręgielska-Narożna, M.; Jakubowski, H.; Szulińska, M.; Bogdański, P. The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. J. Clin. Med. 2020, 9, 998. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients 2021, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Gurwara, S.; Ajami, N.J.; Jang, A.; Hessel, F.C.; Chen, L.; Plew, S.; Wang, Z.; Graham, D.Y.; Hair, C.; White, D.L.; et al. Dietary Nutrients Involved in One-Carbon Metabolism and Colonic Mucosa-Associated Gut Microbiome in Individuals with an En-doscopically Normal Colon. Nutrients 2019, 11, 613. [Google Scholar] [CrossRef]
- Zinno, P.; Motta, V.; Guantario, B.; Natella, F.; Roselli, M.; Bello, C.; Comitato, R.; Carminati, D.; Tidona, F.; Meucci, A.; et al. Supplementation with Dairy Matrices Impacts on Homocysteine Levels and Gut Microbiota Composition of Hyperhomocys-teinemic Mice. Eur. J. Nutr. 2020, 59, 345–358. [Google Scholar] [CrossRef]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Effects of Cultivation Conditions on Folate Production by Lactic Acid Bacteria. Appl. Env. Microbiol. 2003, 69, 4542–4548. [Google Scholar] [CrossRef]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus Reuteri CRL1098 Produces Cobalamin. J. Bac-Teriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef]
- Yang, Q.; He, G.-W. Imbalance of Homocysteine and H 2 S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury. Oxid. Med. Cell Longev. 2019, 2019, 7629673. [Google Scholar] [CrossRef]
- O, K.; Siow, Y.L. Metabolic Imbalance of Homocysteine and Hydrogen Sulfide in Kidney Disease. Curr. Med. Chem. 2018, 25, 367–377. [Google Scholar] [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy Within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H 2 S: A Universal Defense Against Antibiotics in Bacteria. Science (1979) 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial Pathways in Colonic Sulfur Metabolism and Links with Health and Disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Cowley, E.S.; Breister, A.; Matatov, S.; Lucio, L.; Polak, P.; Ridlon, J.M.; Gaskins, H.R.; Anantharaman, K. Diversity and Distribution of Sulfur Metabolic Genes in the Human Gut Microbiome and Their Association with Colorectal Cancer. Mi-Crobiome 2022, 10, 64. [Google Scholar] [CrossRef]
- Gori, A.M.; Corsi, A.M.; Fedi, S.; Gazzini, A.; Sofi, F.; Bartali, B.; Bandinelli, S.; Gensini, G.F.; Abbate, R.; Ferrucci, L. A Proin-flammatory State Is Associated with Hyperhomocysteinemia in the Elderly. Am. J. Clin. Nutr. 2005, 82, 335–341. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Mu, N.; Lou, X.; Li, W.; Chen, Y.; Fan, D.; Tan, H. Activation of NLRP3 Inflammasomes Contributes to Hyperhomocysteinemia-Aggravated Inflammation and Atherosclerosis in ApoE-Deficient Mice. Lab. Investig. 2017, 97, 922–934. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Kurilshikov, A.; ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of Gut Microbiota in Chronic Low-grade Inflammation as Potential Driver for Athero-sclerotic Cardiovascular Disease: A Systematic Review of Human Studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery–Induced Weight Loss. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminara-yanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Gofron, K.; Berezowski, A.; Gofron, M.; Borówka, M.; Dziedzic, M.; Kazimierczak, W.; Kwiatkowski, M.; Gofron, M.; Nowaczyk, Z.; Małgorzewicz, S. Akkermansia Muciniphila—Impact on the Cardiovascular Risk, the Intestine Inflammation and Obesity. Acta Biochim. Pol. 2024, 71, 13550. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A Metabolomics Pipeline for the Mechanistic Interrogation of the Gut Microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Preciado, A.; Henkin, T.M.; Grundy, F.J.; Yanofsky, C.; Merino, E. Biochemical Features and Functional Implications of the RNA-Based T-Box Regulatory Mechanism. Microbiol. Mol. Biol. Rev. 2009, 73, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Breaker, R.R. Prospects for Riboswitch Discovery and Analysis. Mol. Cell 2011, 43, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A.; Nudler, E. A Decade of Riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef]
- Henkin, T.M. The T Box Riboswitch: A Novel Regulatory RNA That Utilizes TRNA as Its Ligand. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2014, 1839, 959–963. [Google Scholar] [CrossRef]
- Sherwood, A.V.; Henkin, T.M. Riboswitch-Mediated Gene Regulation: Novel RNA Architectures Dictate Gene Expression Responses. Annu. Rev. Microbiol. 2016, 70, 361–374. [Google Scholar] [CrossRef]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch Diversity and Distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef]
- Winkler, W.C.; Nahvi, A.; Sudarsan, N.; Barrick, J.E.; Breaker, R.R. An MRNA Structure That Controls Gene Expression by Binding S-Adenosylmethionine. Nat. Struct. Mol. Biol. 2003, 10, 701–707. [Google Scholar] [CrossRef]
- Epshtein, V.; Mironov, A.S.; Nudler, E. The Riboswitch-Mediated Control of Sulfur Metabolism in Bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 5052–5056. [Google Scholar] [CrossRef]
- Wang, J.X.; Breaker, R.R. Riboswitches That Sense S -Adenosylmethionine and S -AdenosylhomocysteineThis Paper Is One of a Selection of Papers Published in This Special Issue, Entitled CSBMCB—Systems and Chemical Biology, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2008, 86, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Quarta, G.; Schlick, T. Riboswitch Distribution in the Human Gut Microbiome Reveals Common Metabolite Pathways. J. Phys. Chem. B 2024, 128, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.-J.; Du, X.; Shi, Q.; Zhang, J.-L.; He, Y.-P.; Chen, Y.-M.; Ming, Z.; Wang, D.; Zhong, W.-Y.; Liang, Y.-W.; et al. A SAM-I Riboswitch with the Ability to Sense and Respond to Uncharged Initiator TRNA. Nat. Commun. 2020, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Martinelli, S.; Menchetti, S.; Bombardiere, F.; Martelli, F.S. Hyperhomocysteinemia and Disease—Is 10 Μmol/L a Suitable New Threshold Limit? Int. J. Mol. Sci. 2024, 25, 12295. [Google Scholar] [CrossRef]
- Tian, R.; Liu, H.-H.; Feng, S.-Q.; Wang, Y.-F.; Wang, Y.-Y.; Chen, Y.-X.; Wang, H.; Zhang, S.-Y. Gut Microbiota Metabolic Characteristics in Coronary Artery Disease Patients with Hyperhomocysteine. J. Microbiol. 2022, 60, 419–428. [Google Scholar] [CrossRef]
- Ling, Y.; Gong, T.; Zhang, J.; Gu, Q.; Gao, X.; Weng, X.; Liu, J.; Sun, J. Gut Microbiome Signatures Are Biomarkers for Cognitive Impairment in Patients With Ischemic Stroke. Front. Aging Neurosci. 2020, 12, 511562. [Google Scholar] [CrossRef]
- Roth, W.; Mohamadzadeh, M. Vitamin B12 and Gut-Brain Homeostasis in the Pathophysiology of Ischemic Stroke. EBioMed-Icine 2021, 73, 103676. [Google Scholar] [CrossRef]
- Xu, C.-C.; Zhao, W.-X.; Sheng, Y.; Yun, Y.-J.; Ma, T.; Fan, N.; Song, J.-Q.; Wang, J.; Zhang, Q. Serum Homocysteine Showed Potential Association with Cognition and Abnormal Gut Microbiome in Major Depressive Disorder. World J. Psychiatry 2025, 15, 102567. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Zhu, Z.; Xiong, X.; Huang, Y.; Feng, Y.; Li, Z.; Wu, K.; Wu, F. Association of Serum Homocysteine Levels with Intestinal Flora and Cognitive Function in Schizophrenia. J. Psychiatr. Res. 2023, 159, 258–265. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Ferrini, F.; Agostini, D.; Amatori, S.; Barbieri, E.; Piccoli, G.; Sestili, P.; Stocchi, V. Nutraceuticals and Physical Activity as Antidepressants: The Central Role of the Gut Microbiota. Antioxidants 2022, 11, 236. [Google Scholar] [CrossRef]
| Clinical Implications | Gut Microbiota Taxa | Clinical Evidence | Reference |
|---|---|---|---|
| Major depressive disorder (MDD) vs. Healthy Controls |
| In patients with MDD, elevated blood Hcy levels have a negative correlation with cognitive performance. This association could be linked to modifications in the gut microbiomal community structure. | [130] |
| Post-stroke cognitive impairment (PSCI) vs. post-stroke non-cognitive impairment (PSNCI) |
| Gut microbiota was closely associated with Montral Cognitive Assessment (MoCA) scores and the risk factors for PSCI, including Hcy | [128] |
| Coronary artery disease (CAD) with and without HHcy vs. healthy controls. |
| Patients with HHcy had a higher atherosclerotic burden linked to specific metabolites (BHMT, S-methyltransferase and trimethylamine N-oxide related). Patients with CVD who have HHcy, exhibit a higher atherosclerotic burden, poor Hcy metabolism, and an increased risk of cardiovascular disease, due to certain metabolites and an imbalance in gut microbiota | [127] |
| Early-stage levodopa (L-DOPA)-naive PD patients vs. matched PD and Healthy controls |
| Personalized community-level metabolic modeling shows the microbial contribution to folate deficiency and HHcy | [78] |
| Schizophrenia (SZ) patients vs. healthy controls |
| Cognitive function and gut microbial species are associated with blood Hcy levels | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, D.; Bartolacci, A.; Rotondo, R.; De Pandis, M.F.; Battistelli, M.; Micucci, M.; Potenza, L.; Polidori, E.; Ferrini, F.; Sisti, D.; et al. Homocysteine, Nutrition, and Gut Microbiota: A Comprehensive Review of Current Evidence and Insights. Nutrients 2025, 17, 1325. https://doi.org/10.3390/nu17081325
Agostini D, Bartolacci A, Rotondo R, De Pandis MF, Battistelli M, Micucci M, Potenza L, Polidori E, Ferrini F, Sisti D, et al. Homocysteine, Nutrition, and Gut Microbiota: A Comprehensive Review of Current Evidence and Insights. Nutrients. 2025; 17(8):1325. https://doi.org/10.3390/nu17081325
Chicago/Turabian StyleAgostini, Deborah, Alessia Bartolacci, Rossella Rotondo, Maria Francesca De Pandis, Michela Battistelli, Matteo Micucci, Lucia Potenza, Emanuela Polidori, Fabio Ferrini, Davide Sisti, and et al. 2025. "Homocysteine, Nutrition, and Gut Microbiota: A Comprehensive Review of Current Evidence and Insights" Nutrients 17, no. 8: 1325. https://doi.org/10.3390/nu17081325
APA StyleAgostini, D., Bartolacci, A., Rotondo, R., De Pandis, M. F., Battistelli, M., Micucci, M., Potenza, L., Polidori, E., Ferrini, F., Sisti, D., Pegreffi, F., Pazienza, V., Virgili, E., Stocchi, V., & Donati Zeppa, S. (2025). Homocysteine, Nutrition, and Gut Microbiota: A Comprehensive Review of Current Evidence and Insights. Nutrients, 17(8), 1325. https://doi.org/10.3390/nu17081325










