Purple-Grain Wheat Regulation of Blood Lipids and Blood Glucose in Diet-Induced Hyperlipidemic Mice and Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Agricultural Paste/Juice Preparation
2.2. Bacteria and Culture
2.3. Viable Bacteria Count
2.4. Hyperlipidemic Mouse Model and Experimental Design
2.4.1. Body Weight
2.4.2. Physiological Indicators
2.5. Type 2 Diabetes Mouse Model and Experimental Design
2.5.1. Body Weight
2.5.2. Blood Glucose
2.6. Statistical Methods
3. Results
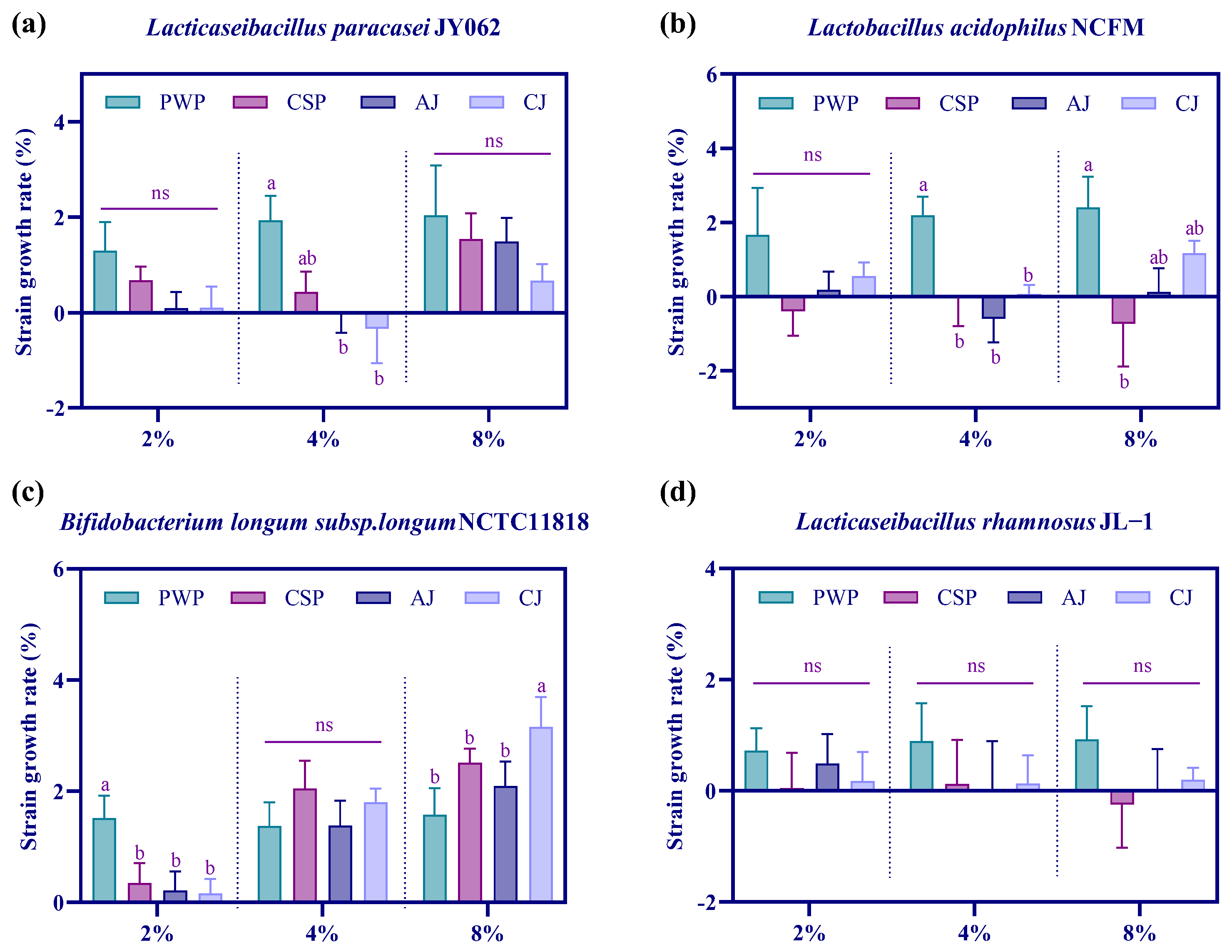
3.1. Effect of Agricultural Products on the Growth of Probiotics
3.2. Effects of PWP on Body Weight in Hyperlipidemic Mice
3.3. Effects of PWP on Blood Lipids in Hyperlipidemic Mice
3.4. Effects of PWP on Body Weight in Type 2 Diabetes Mice
3.5. Effects of PWP on Blood Glucose in Type 2 Diabetes Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Johnston, L.J.; Wu, C.; Ma, X. Gut microbiota and its metabolites: Bridge of dietary nutrients and obesity-related diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 3236–3253. [Google Scholar] [CrossRef]
- Zhong, Y.; Marungruang, N.; Fak, F.; Nyman, M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br. J. Nutr. 2015, 113, 1558–1570. [Google Scholar] [CrossRef]
- Borychowski, M.; Sapa, A.; Czyzewski, B.; Stepien, S.; Poczta-Wajda, A. Interactions between food and nutrition security and the socio-economic and environmental dimensions of sustainability in small-scale farms: Evidence from a simultaneous confirmatory factor analysis in Poland. Int. J. Agr. Sustain. 2022, 20, 998–1014. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Benedetti, A.; Di Paolo, A.; Giannese, D.; Cupisti, A. Interactions between Food and Drugs, and Nutritional Status in Renal Patients: A Narrative Review. Nutrients 2022, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Triticale: Nutritional composition and food uses. Food Chem. 2018, 241, 468–479. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Lan, S.; Zhang, Y.; Li, X. Breeding and Quality Analysis of a New Purple-grain wheat Germplasm Jizimai No.14 with High Nutrition. J. Hebei Agric. Sci. 2019, 23, 61–64. (In Chinese) [Google Scholar]
- Dhua, S.; Kumar, K.; Kumar, Y.; Singh, L.; Sharanagat, V.S. Composition, characteristics and health promising prospects of black wheat: A review. Trends Food Sci. Tech. 2021, 112, 780–794. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, J.; Yue, Y.; Li, K.; Ren, G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y. A potential synbiotic strategy for the prevention of type 2 diabetes: Lactobacillus paracasei JY062 and exopolysaccharide isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive effect and molecular mechanism of Lactobacillus rhamnosus JL1 on food-borne obesity in mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef]
- Koca Bozalan, N.; Karadeniz, F. Carotenoid profile, total phenolic content, and antioxidant activity of carrots. Int. J. Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Fujihara, K.; Nogawa, S.; Saito, K.; Horikawa, C.; Takeda, Y.; Cho, K.; Ishiguro, H.; Kodama, S.; Nakagawa, Y.; Matsuzaka, T.; et al. Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711. Nutrients 2021, 13, 3478. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.; Joye, I.J. Prebiotic Potential of Cereal Components. Foods 2021, 10, 2338. [Google Scholar] [CrossRef]
- Liu, P.; Yu, S.; Liu, J.; Zhou, Y.; Cao, R.; Zhou, Y.; Shi, L.; Du, J. Effects of Lactobacillus on hyperlipidemia in high-fat diet-induced mouse model. Arch. Med. Sci. 2023, 19, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Wang, D.; Zuo, Z.; Wang, Y.; Sun, W.; Zhang, N.; Zhang, D. Kidney Bean Fermented Broth Alleviates Hyperlipidemic by Regulating Serum Metabolites and Gut Microbiota Composition. Nutrients 2022, 14, 3202. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Yue, Y.; Rong, C.; Huang, W.; Xue, P.; Li, T.; Zhang, J. Hypolipidemic Activity of Seed Oil and Procyanidins of Rosa davurica Pall. Food Sci. 2017, 38, 213–218. [Google Scholar]
- Lan, S.; Meng, Y.; Li, G.; Wang, M.; Zhang, Y.; Li, X. Effects of Purple-grain wheat Flour on Lipid Metabolism in Hyperlipidemia Rats. J. Chin. Inst. Food Sci. Technol. 2022, 22, 161–167. (In Chinese) [Google Scholar]
- Dembowski, E.; Freedman, I.; Grundy, S.M.; Stone, N.J. Guidelines for the management of hyperlipidemia: How can clinicians effectively implement them? Prog. Cardiovasc. Dis. 2022, 75, 4–11. [Google Scholar] [CrossRef]
- Junejo, S.A.; Zhang, L.; Yang, L.; Wang, N.; Zhou, Y.; Xia, Y.; Wang, H. Anti-hyperlipidemic and hepatoprotective properties of wheat bran with different particle sizes. J. Sci. Food Agric. 2019, 99, 1990–1996. [Google Scholar] [CrossRef]
- Zhang, W.; Jiao, J.; Qin, L. Effects of cereal dietary fiber on lipid metabolism and lipotoxicity in mice fed with high fat/cholesterol diet. Sci. Rep. 2015, 31, 1038–1040. [Google Scholar] [CrossRef]
- Perez-Gallardo, L.; Gomez, M.; Parra, P.; Sanchez, J.; Palou, A.; Serra, F. Effect of calcium-enriched high-fat diet on calcium, magnesium and zinc retention in mice. Br. J. Nutr. 2009, 101, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Yokoyama, Y.; Taoka, H.; Nagano, U.; Hosoda, S.; Taworntawat, T.; Nakamura, A.; Ogawa, Y.; Tsubota, K.; Watanabe, M. Iron supplementation regulates the progression of high fat diet induced obesity and hepatic steatosis via mitochondrial signaling pathways. Sci. Rep. 2021, 11, 10753. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Wang, Y.; Wang, K.; Ji, B.-P.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B 2018, 19, 559–569. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Huo, Y.; Mijiti, A.; Cai, R.N.; Gao, Z.H.; Aini, M.; Mijiti, A.; Wang, Z.L.; Qie, R. Scutellarin alleviates type 2 diabetes (HFD/low dose STZ)-induced cardiac injury through modulation of oxidative stress, inflammation, apoptosis and fibrosis in mice. Hum. Exp. Toxicol. 2021, 40, S460–S474. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A.; Mahmoud, A.M. Simvastatin Ameliorates Diabetic Cardiomyopathy by Attenuating Oxidative Stress and Inflammation in Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1092015. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des. Dev. Ther. 2016, 10, 2095–2107. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Tu, J.; Liu, G.H.; Cao, X.T.; Zhu, S.Y.; Li, Q.; Ji, G.S.; Han, Y.H.; Xiao, H. Hypoglycemic effects of wheat bran alkyresorcinols in high-fat/high-sucrose diet and low-dose streptozotocin-induced type 2 diabetic male mice and protection of pancreatic β cells. Food Funct. 2019, 10, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, Z.; Liu, S.; Aluo, Z.; Zhang, L.; Yu, L.; Li, Y.; Song, Z.; Zhou, L. Zinc Supplementation Alleviates Lipid and Glucose Metabolic Disorders Induced by a High-Fat Diet. J. Agric. Food Chem. 2020, 68, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Capetini, V.; Alves de Vasconcelos, D.A.; Martins, A.R.; Hirabara, S.M.; Donato, J., Jr.; Carpinelli, A.R.; Abdulkader, F. Zinc Supplementation Improves Glucose Homeostasis in High Fat-Fed Mice by Enhancing Pancreatic β-Cell Function. Nutrients 2017, 9, 1150. [Google Scholar] [CrossRef]
- Groeber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Hruby, A.; Meigs, J.B.; O’Donnell, C.J.; Jacques, P.F.; McKeown, N.M. Higher Magnesium Intake Reduces Risk of Impaired Glucose and Insulin Metabolism and Progression From Prediabetes to Diabetes in Middle-Aged Americans. Diabetes Care 2014, 37, 419–427. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]



| Category | Ingredient | Content |
|---|---|---|
| Macronutrients (g/kg) | Protein | 155 |
| Fat | 19 | |
| Starch | 562 | |
| Reducing sugar | 14 | |
| Dietary fiber | 144 | |
| Minerals (mg/kg) | Zinc | 29 |
| Iron | 36 | |
| Calcium | 453 | |
| Magnesium | 1340 | |
| Selenium | 0.105 | |
| Essential amino acids (g/kg) | Lysine | 4.7 |
| Threonine | 3.3 | |
| Methionine | 3.2 | |
| Phenylalanine | 7.4 | |
| Leucine | 18.2 | |
| Isoleucine | 4.1 | |
| Valine | 11.1 |
| Groups | TC (mmol·L−1) | TG (mmol·L−1) | HDL-C (mmol·L−1) | LDL-C (mmol·L−1) |
|---|---|---|---|---|
| N | 2.81 ± 0.17 a | 1.14 ± 0.09 a | 1.59 ± 0.14 a | 1.01 ± 0.15 a |
| HFD | 3.96 ± 0.79 b | 1.93 ± 0.23 b | 1.20 ± 0.11 b | 2.37 ± 0.23 b |
| H | 3.47 ± 0.19 b | 1.27 ± 0.31 c | 1.41 ± 0.09 c | 1.67 ± 0.14 c |
| M | 3.35 ± 0.45 b | 1.31 ± 0.17 c | 1.37 ± 0.07 c | 1.51 ± 0.22 c |
| L | 3.61 ± 0.27 b | 1.27 ± 0.21 c | 1.36 ± 0.11 c | 1.45 ± 0.08 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Cheng, S.; Wei, X.; Man, C. Purple-Grain Wheat Regulation of Blood Lipids and Blood Glucose in Diet-Induced Hyperlipidemic Mice and Type 2 Diabetic Mice. Nutrients 2025, 17, 1310. https://doi.org/10.3390/nu17081310
Hu D, Cheng S, Wei X, Man C. Purple-Grain Wheat Regulation of Blood Lipids and Blood Glucose in Diet-Induced Hyperlipidemic Mice and Type 2 Diabetic Mice. Nutrients. 2025; 17(8):1310. https://doi.org/10.3390/nu17081310
Chicago/Turabian StyleHu, Dong, Shasha Cheng, Xiaoyan Wei, and Chaoxin Man. 2025. "Purple-Grain Wheat Regulation of Blood Lipids and Blood Glucose in Diet-Induced Hyperlipidemic Mice and Type 2 Diabetic Mice" Nutrients 17, no. 8: 1310. https://doi.org/10.3390/nu17081310
APA StyleHu, D., Cheng, S., Wei, X., & Man, C. (2025). Purple-Grain Wheat Regulation of Blood Lipids and Blood Glucose in Diet-Induced Hyperlipidemic Mice and Type 2 Diabetic Mice. Nutrients, 17(8), 1310. https://doi.org/10.3390/nu17081310







