Sn1,3 Regiospecificity of DHA (22:6ω-3) of Plant Origin (DHA-Canola®) Facilitates Its Preferential Tissue Incorporation in Rats Compared to sn2 DHA in Algal Oil at Low Dietary Inclusion Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Biochemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Animal Growth and Feed Intake
3.2. Body Composition
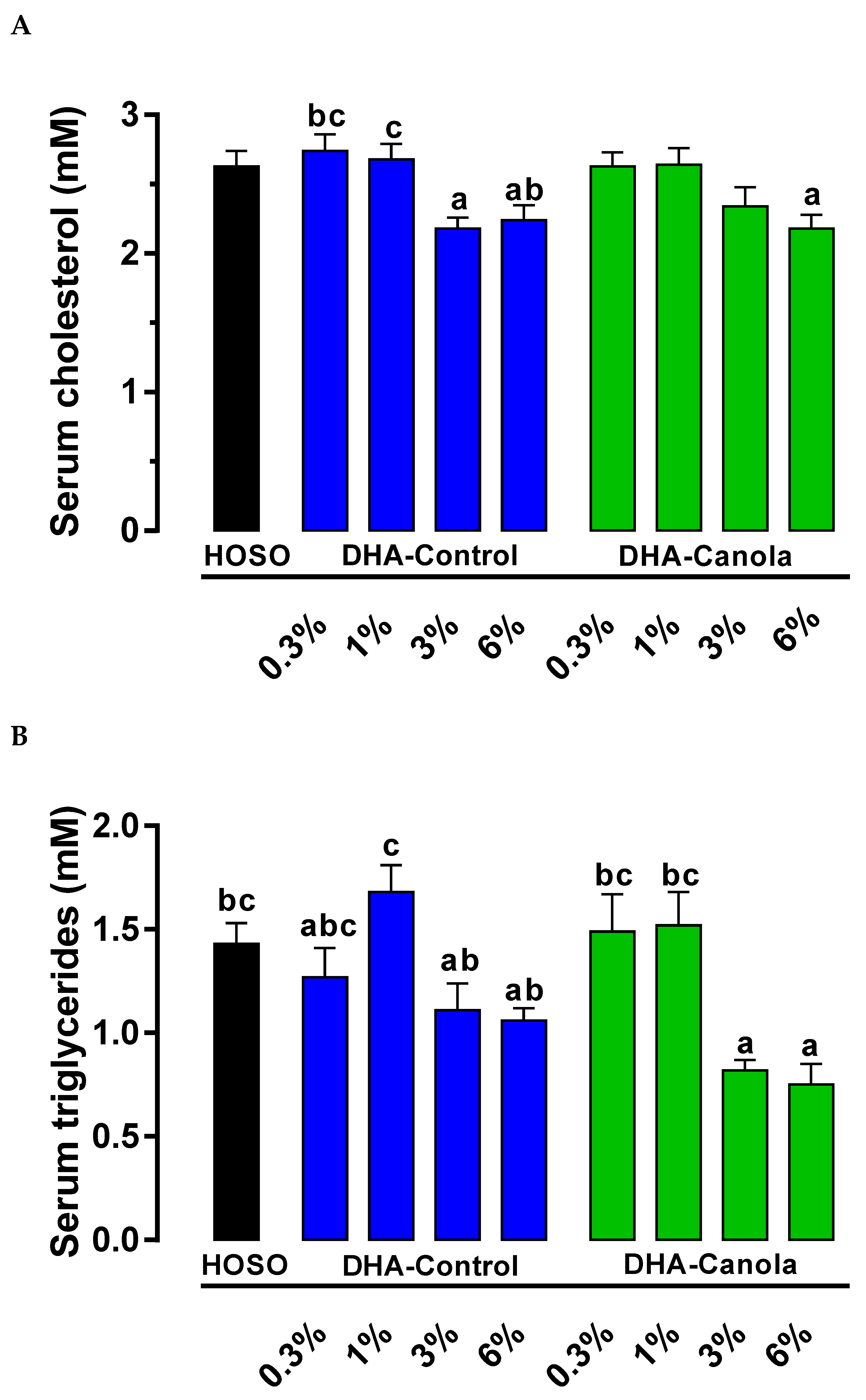
3.3. Serum Cholesterol and Lipids
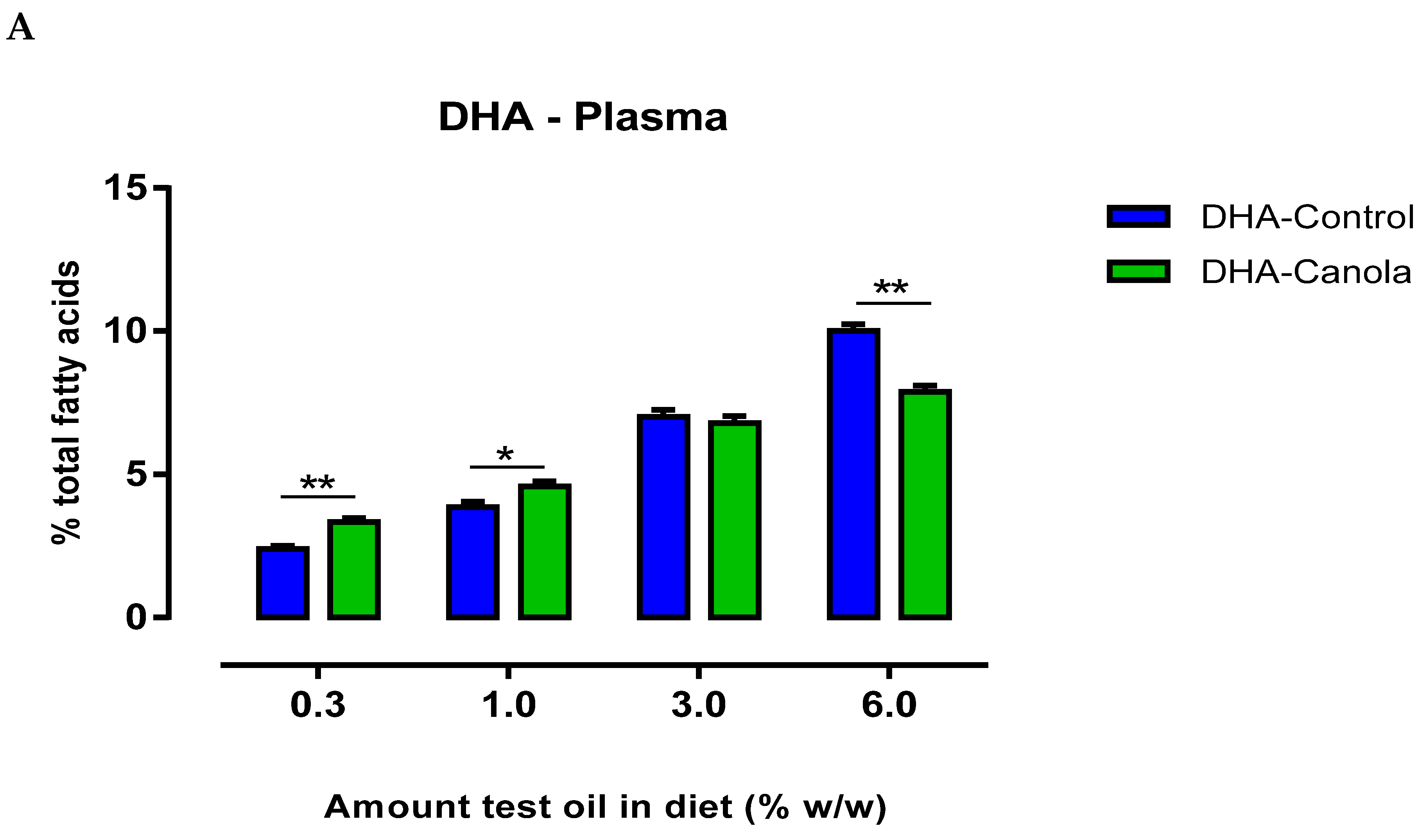
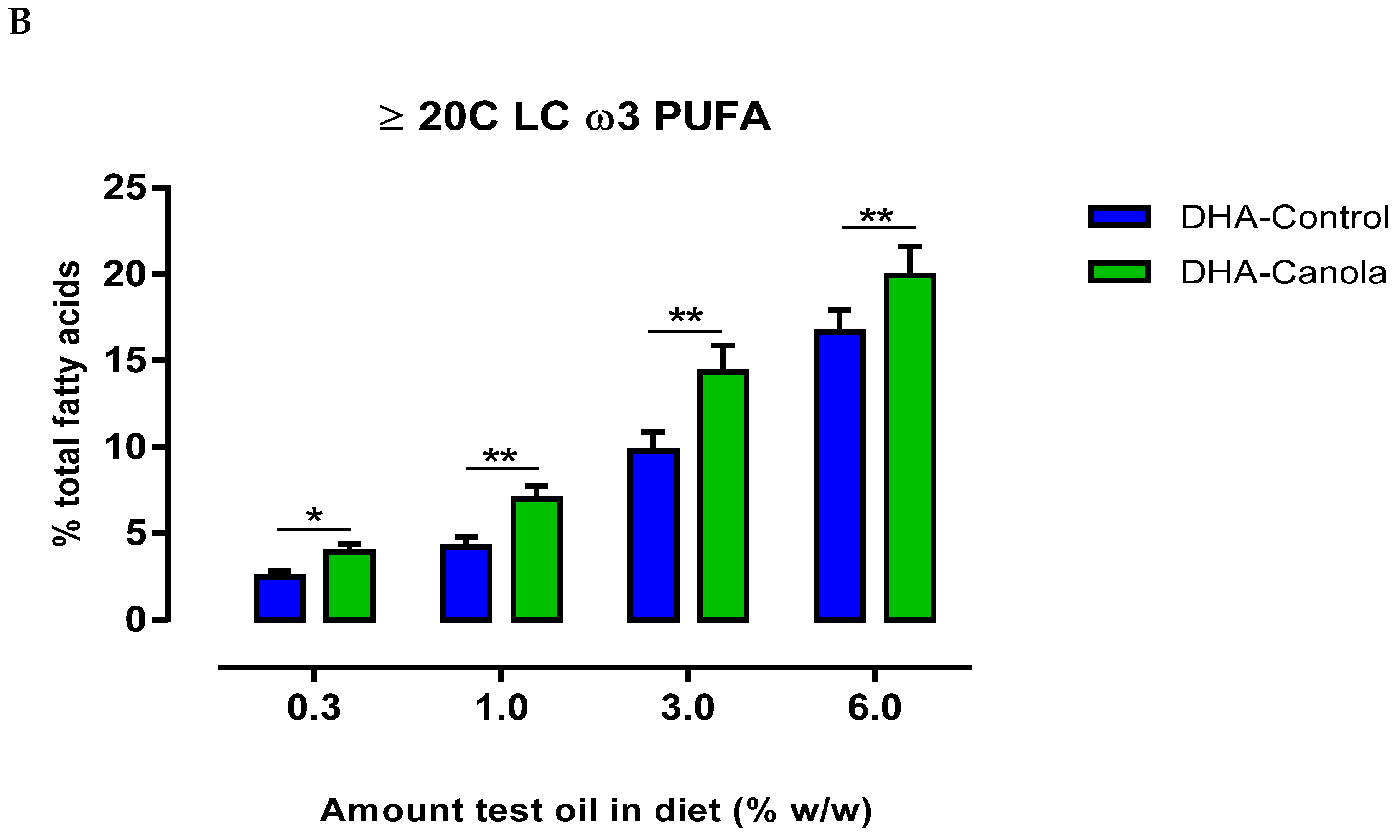
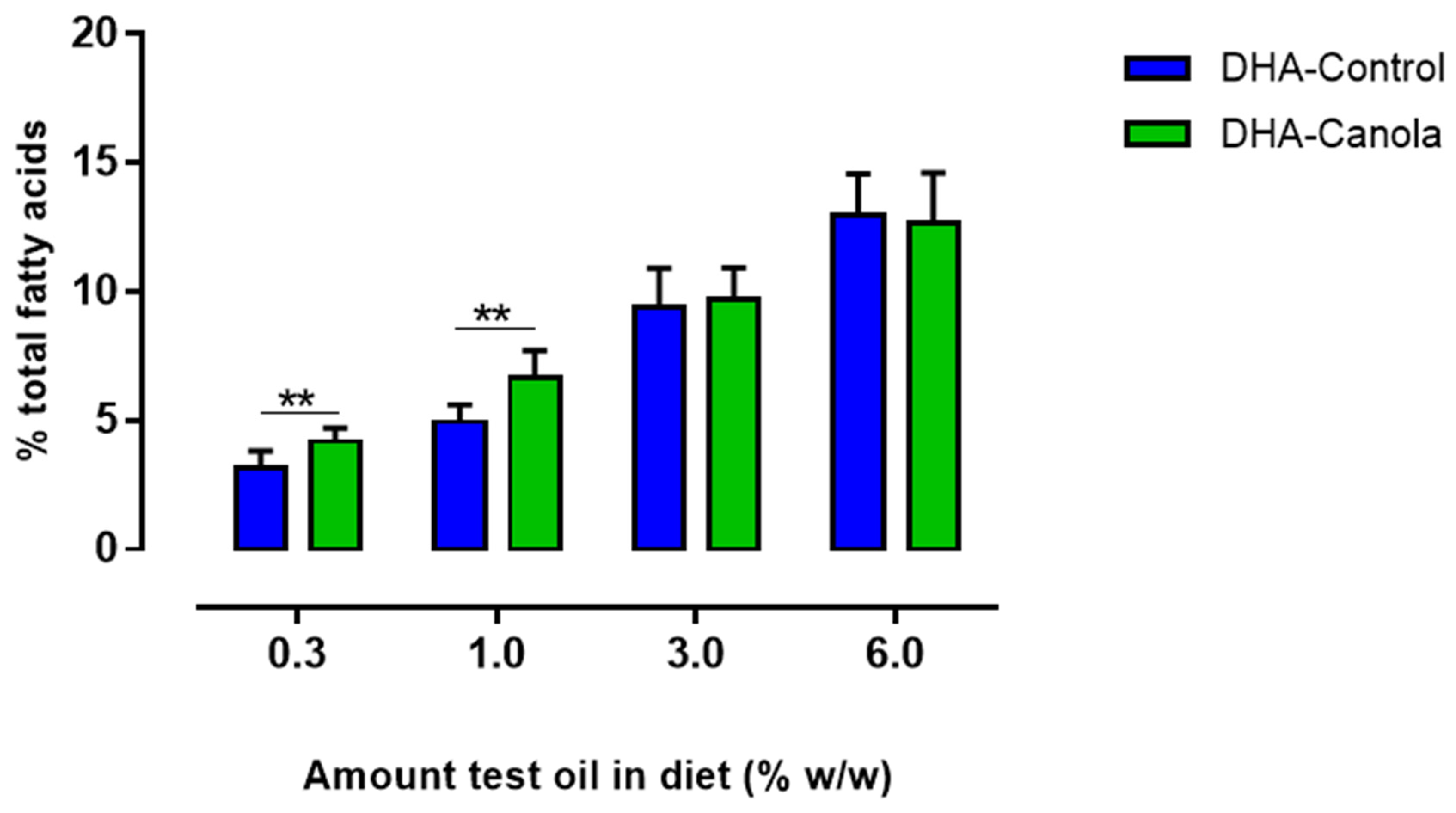
3.4. Plasma Fatty Acids
3.5. Red Blood Cell Fatty Acids
3.6. Heart Fatty Acids
3.7. Liver Fatty Acids
3.8. Kidney Fatty Acids
3.9. Brain Fatty Acids
3.10. Muscle Fatty Acids
3.11. Testes Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Harris, W.S.; Kris-Etherton, P.M. Long-chain omega-3 fatty acids: Time to establish a dietary reference intake. Nutr. Rev. 2013, 71, 692–707. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M. Ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S. Health benefits of plant-derived alpha-linolenic acid. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 443S–448S. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C.; for the International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Abeywardena, M.; Wijesundera, C.; Nichols, P.D. DHA-containing oilseed: A timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients 2014, 6, 2035–2058. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; for the Nutrition Committee. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L., 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef]
- Wijesundera, C.; Kitessa, S.; Abeywardena, M.; Binell, W.; Nichols, P. Long-chain omega-3 oils: Current and future supplies, food and feed applications, and stability. Lipid Technol. 2011, 23, 55–58. [Google Scholar]
- Abeywardena, M.Y.; Nicholls, P.; Singh, S. Future Omega-3s. In The World of Food Ingredients; CNS Media: Arnhem, The Netherlands, 2005; pp. 50–54. [Google Scholar]
- Ruiz-Lopez, N.; Haslam, R.P.; Venegas-Caleron, M.; Larson, T.R.; Graham, I.A.; Napier, J.A.; Sayanova, O. The synthesis and accumulation of stearidonic acid in transgenic plants: A novel source of ‘heart-healthy’ omega-3 fatty acids. Plant Biotechnol. J. 2009, 7, 704–716. [Google Scholar] [CrossRef]
- Napier, J.A.; Graham, I.A. Tailoring plant lipid composition: Designer oilseeds come of age. Curr. Opin. Plant Biol. 2010, 13, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Caleron, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lopez, N.; Haslam, R.P.; Venegas-Caleron, M.; Li, T.; Bauer, J.; Napier, J.A.; Sayanova, O. Enhancing the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Arabidopsis thaliana via iterative metabolic engineering and genetic crossing. Transgenic Res. 2012, 21, 1233–1243. [Google Scholar] [CrossRef]
- Robert, S.S. Production of eicosapentaenoic and docosahexaenoic acid-containing oils in transgenic land plants for human and aquaculture nutrition. Mar. Biotechnol. 2006, 8, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Caleron, M.; Beaudoin, F.; Garces, R.; Napier, J.A.; Martinez-Force, E. The sunflower plastidial omega3-fatty acid desaturase (HaFAD7) contains the signalling determinants required for targeting to, and retention in, the endoplasmic reticulum membrane in yeast but requires co-expressed ferredoxin for activity. Phytochemistry 2010, 71, 1050–1058. [Google Scholar] [CrossRef]
- Petrie, J.R.; Vanhercke, T.; Shrestha, P.; El Tahchy, A.; White, A.; Zhou, X.R.; Liu, Q.; Mansour, M.P.; Nichols, P.D.; Singh, S.P. Recruiting a new substrate for triacylglycerol synthesis in plants: The monoacylglycerol acyltransferase pathway. PLoS ONE 2012, 7, e35214. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Zhou, X.R.; Mansour, M.P.; Liu, Q.; Belide, S.; Nichols, P.D.; Singh, S.P. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE 2012, 7, e49165. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Mansour, M.P.; Liu, Q.; Horne, J.; Nichols, P.D.; Singh, S.P. Transgenic production of arachidonic acid in oilseeds. Transgenic Res. 2012, 21, 139–147. [Google Scholar] [CrossRef]
- Petrie, J.R.; Zhou, X.R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W.X.; et al. Development of a (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.Y.; Wijesundera, C.; Nichols, P.D. Fatty acid regiospecificity as a potential determinant of tissue uptake of dietary DHA. In Proceedings of the 103rd AOCS Annual Meeting and Expo, Long beach, CA, USA, 29 April–2 May 2012. [Google Scholar]
- Mu, H.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [PubMed]
- Mu, H.; Porsgaard, T. The metabolism of structured triacylglycerols. Prog. Lipid Res. 2005, 44, 430–448. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. Faseb J. 2008, 22, 659–661. [Google Scholar]
- Sanguansri, L.; Augustin, M.A.; Lockett, T.J.; Abeywardena, M.Y.; Royle, P.J.; Mano, M.T.; Patten, G.S. Bioequivalence of n-3 fatty acids from microencapsulated fish oil formulations in human subjects. Br. J. Nutr. 2015, 113, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Spiegelman, D.; Sacks, F.M.; Rimm, E.B.; Siscovick, D.S. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: The cardiovascular health study: A cohort study. Ann. Intern. Med. 2011, 155, 160–170. [Google Scholar] [CrossRef]
- Linderborg, K.M.; Kaur, G.; Miller, E.; Meikle, P.J.; Larsen, A.E.; Weir, J.M.; Nuora, A.; Barlow, C.K.; Kallio, H.P.; Cameron-Smith, D.; et al. Postprandial metabolism of docosapentaenoic acid (DPA, 22:5n-3) and eicosapentaenoic acid (EPA, 20:5n-3) in humans. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 313–319. [Google Scholar] [CrossRef]
- Mann, N.J.; O’Connell, S.L.; Baldwin, K.M.; Singh, I.; Meyer, B.J. Effects of seal oil and tuna-fish oil on platelet parameters and plasma lipid levels in healthy subjects. Lipids 2010, 45, 669–681. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Flock, M.R.; Richter, C.K.; Harris, W.S.; West, S.G.; Kris-Etherton, P.M. Red Blood Cell Docosapentaenoic Acid (DPA n-3) is Inversely Associated with Triglycerides and C-reactive Protein (CRP) in Healthy Adults and Dose-Dependently Increases Following n-3 Fatty Acid Supplementation. Nutrients 2015, 7, 6390–6404. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Sinclair, A.J.; Barrow, C.J. Pancreatic lipase selectively hydrolyses DPA over EPA and DHA due to location of double bonds in the fatty acid rather than regioselectivity. Food Chem. 2014, 160, 61–66. [Google Scholar] [PubMed]
- Kaur, G.; Molero, J.C.; Weisinger, H.S.; Sinclair, A.J. Orally administered [(1)(4)C]DPA and [(1)(4)C]DHA are metabolised differently to [(1)(4)C]EPA in rats. Br. J. Nutr. 2013, 109, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Kaur, G.; Miller, E.G.; Larsen, A.E.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 2016, 30, 3714. [Google Scholar] [CrossRef]
- Harris, W.S.; Lemke, S.L.; Hansen, S.N.; Goldstein, D.A.; DiRienzo, M.A.; Su, H.; Nemeth, M.A.; Taylor, M.L.; Ahmed, G.; George, C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 2008, 43, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: Randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Adams, M.; Dallimore, J.; Kitessa, S.M. Rise in DPA Following SDA-Rich Dietary Echium Oil Less Effective in Affording Anti-Arrhythmic Actions Compared to High DHA Levels Achieved with Fish Oil in Sprague-Dawley Rats. Nutrients 2016, 8, 14. [Google Scholar] [CrossRef]
- Tejera, N.; Vauzour, D.; Betancor, M.B.; Sayanova, O.; Usher, S.; Cochard, M.; Rigby, N.; Ruiz-Lopez, N.; Menoyo, D.; Tocher, D.R.; et al. A Transgenic Camelina sativa Seed Oil Effectively Replaces Fish Oil as a Dietary Source of Eicosapentaenoic Acid in Mice. J. Nutr. 2016, 146, 227–235. [Google Scholar] [CrossRef]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef]
- Itakura, H.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Kita, T.; et al. Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 2011, 18, 99–107. [Google Scholar]
- Ma, T.; Liaset, B.; Hao, Q.; Petersen, R.K.; Fjaere, E.; Ngo, H.T.; Lillefosse, H.H.; Ringholm, S.; Sonne, S.B.; Treebak, J.T.; et al. Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice. PLoS ONE 2011, 6, e21647. [Google Scholar] [CrossRef]
- Cunnane, S.C.; McAdoo, K.R.; Horrobin, D.F. n-3 Essential fatty acids decrease weight gain in genetically obese mice. Br. J. Nutr. 1986, 56, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Loffler, M.; Huber, J.; Bilban, M.; Reimers, M.; Kadl, A.; Zeyda, M.; Waldhausl, W.; Stulnig, T.M. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 2006, 49, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Alsina, E.; Macri, E.V.; Lifshitz, F.; Bozzini, C.; Rodriguez, P.N.; Boyer, P.M.; Friedman, S.M. Efficacy of phytosterols and fish-oil supplemented high-oleic-sunflower oil rich diets in hypercholesterolemic growing rats. Int. J. Food Sci. Nutr. 2016, 67, 441–453. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef]
- Hainault, I.; Carolotti, M.; Hajduch, E.; Guichard, C.; Lavau, M. Fish oil in a high lard diet prevents obesity, hyperlipemia, and adipocyte insulin resistance in rats. Ann. N. Y. Acad. Sci. 1993, 683, 98–101. [Google Scholar]
- Belzung, F.; Raclot, T.; Groscolas, R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am. J. Physiol. 1993, 264, R1111–R1118. [Google Scholar]
- Parrish, C.C.; Pathy, D.A.; Angel, A. Dietary fish oils limit adipose tissue hypertrophy in rats. Metabolism 1990, 39, 217–219. [Google Scholar] [CrossRef]
- Baillie, R.A.; Takada, R.; Nakamura, M.; Clarke, S.D. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: A mechanism for decreased body fat deposition. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 351–356. [Google Scholar]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential effects of high-fish oil and high-lard diets on cells and cytokines involved in the inflammatory process in rat insulin-sensitive tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef] [PubMed]
- Philp, L.K.; Heilbronn, L.K.; Janovska, A.; Wittert, G.A. Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice. PLoS ONE 2015, 10, e0117494. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Sasaki, K.; Watanabe, H.; Nagao, K.; Inoue, N.; Shirouchi, B.; Yanagita, T.; Nagai, T.; Mizobe, H.; Kojima, K.; et al. Differential effects of triacylglycerol positional isomers containing n-3 series highly unsaturated fatty acids on lipid metabolism in C57BL/6J mice. J. Nutr. Biochem. 2015, 26, 57–63. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | HOSO | DHA-Control | DHA-Canola |
|---|---|---|---|
| 12:0 | n.d | 0.3 ± 0.0 | n.d |
| 14:0 | n.d | 1.7 ± 0.1 | n.d |
| 16:0 | 4.3 ± 0.2 | 5.2 ± 0.0 | 5.0 ± 0.0 |
| 16:1 n9 | n.d | 0.6 ± 0.0 | 0.2 ± 0.0 |
| 18:0 | 3.6 ± 0.0 | 2.8 ± 0.0 | 2.5 ± 0.0 |
| 18:1 ω9c | 80.6 ± 0.2 | 72.0 ± 0.2 | 45.9 ± 0.4 |
| 18:2 ω6c | 8.7 ± 0.0 | 7.3 ± 0.0 | 9.4 ± 0.0 |
| 18:3 ω6 | n.d | n.d | 0.7 ± 0.0 |
| 18:3 ω3 | n.d | n.d | 20.7 ± 0.2 |
| 20:0 | 0.7 ± 0.0 | 0.3 ± 0.0 | 1.3 ± 0.0 |
| 20:1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 1.5 ± 0.0 |
| 20:4 ω6 | n.d | n.d | 0.7 ± 0.0 |
| 22:0 | 1.2 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.0 |
| 22:1 ω9 | n.d | n.d | 0.9 ± 0.0 |
| 20:5 ω3 | n.d | n.d | 0.3 ± 0.0 |
| 24:0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| 24:1 | n.d | n.d | 0.4 ± 0.0 |
| 22:5 ω3 | n.d | 0.1 ± 0.0 | 0.7 ± 0.0 |
| 22:6 ω3 | n.d | 8.4 ± 0.1 | 8.7 ± 0.2 |
| Final Body Weight (g) | Body Weight Gain (g/wk) | Visceral Fat Pad Weight (%) 3 | Fat Mass (%) | Lean Mass (%) | Bone Mass (%) | |
|---|---|---|---|---|---|---|
| HOSO | 496 ± 20.8 | 19 ± 1.0 | 4.4 ± 0.3 | 19.1 ± 1.2 | 75.9 ± 1.2 | 12.9 ± 0.6 |
| DHA-Control | ||||||
| 0.3% | 504 ± 10.8 | 19.4 ± 0.6 | 4.0 ± 0.3 | 18.6 ± 1.2 | 75.9 ± 1.1 | 13.2 ± 0.3 |
| 1.0% | 503 ± 13.8 | 19.4 ± 0.5 | 4.0 ± 0.2 | 17.6 ± 1.3 | 77.4 ± 1.4 | 13.2 ± 0.4 |
| 3.0% | 493 ± 12.1 | 18.4 ± 0.8 a | 3.7 ± 0.2 | 17.2 ± 0.7 | 78.6 ± 0.7 d | 12.9 ± 0.4 |
| 6.0% | 537 ± 13.3 | 21.8 ± 0.8 a | 4.5 ± 0.2 | 22.3 ± 1.2 abc | 72.8 ± 1.1 abcd | 14.4 ± 0.5 |
| DHA-Canola | ||||||
| 0.3% | 496 ± 7.9 | 18.7 ± 0.4 | 4.1 ± 0.3 | 17.9 ± 1.4 | 77.3 ± 1.4 | 12.9 ± 0.3 |
| 1.0% | 511 ± 18.4 | 19.9 ± 1.2 | 3.6 ± 0.3 | 16.4 ± 1.2 a | 78.5 ± 1.1 a | 13.4 ± 0.4 |
| 3.0% | 506 ± 10.7 | 19.7 ± 0.8 | 3.7 ± 0.2 | 16.1 ± 1.1 b | 79.7 ± 1.5 b | 13.5 ± 0.3 |
| 6.0% | 499 ± 15.9 | 18.9 ± 0.9 | 3.5 ± 0.1 | 15.5 ± 0.8 c | 79.0 ± 0.8 c | 13.0 ± 0.4 |
| Oil type | n.s. | n.s. | 0.04 | 0.004 | 0.005 | n.s. |
| Oil amount | n.s. | n.s. | n.s. | n.s. | 0.037 | n.s. |
| Type × amount | n.s. | 0.024 | n.s. | 0.030 | n.s. | n.s. |
| 0.3% | 1.0% | 3.0% | 6.0% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HOSO | Control | Canola | Control | Canola | Control | Canola | Control | Canola | |
| Plasma | |||||||||
| 20:4ω6 | 22.7 | 22.5 | 19.5 | 17.5 | 17.8 ** | 12.0 | 12.6 | 7.3 | 9.4 *** |
| 20:5ω3 | 0.03 | 0.02 | 0.4 *** | 0.2 | 1.9 *** | 2.3 | 6.5 *** | 5.8 | 10.5 *** |
| 22:5ω3 | 0.03 | 0.1 | 0.2 * | 0.1 | 0.5 *** | 0.4 | 1.1 *** | 0.8 | 1.5 *** |
| 22:6ω3 | 1.0 | 2.4 | 3.3 ** | 3.9 | 4.6 * | 7.0 | 6.8 | 10.0 | 7.9 *** |
| Σ ω3 PUFA | 1.0 | 2.5 | 4.1 *** | 4.3 | 7.6 *** | 10.0 | 15.7 *** | 16.7 | 22.9 *** |
| Red blood cell | |||||||||
| 20:4ω6 | 28.8 | 27.0 | 26.4 | 24.5 | 22.0 | 19.8 | 16.8 ** | 13.7 | 13.2 |
| 20:5ω3 | n.d. | 0.01 | 0.2 ** | 0.2 | 1.3 *** | 1.5 | 3.8 *** | 3.9 | 6.3 *** |
| 22:5ω3 | 0.3 | 0.4 | 1.1 ** | 0.9 | 2.0 *** | 1.6 | 2.8 *** | 1.8 | 3.4 *** |
| 22:6ω3 | 1.3 | 3.1 | 4.0 ** | 4.8 | 5.4 | 7.8 | 5.9 ** | 9.1 | 6.4 ** |
| Σ ω3 PUFA | 1.6 | 3.5 | 5.3 *** | 5.8 | 8.7 *** | 10.9 | 12.8 * | 14.8 | 16.6 |
| Heart | |||||||||
| 20:4ω6 | 30.0 | 26.3 | 23.9 ** | 20.9 | 18.1 ** | 13.7 | 13.7 | 9.6 | 10.2 |
| 20:5ω3 | n.d. | n.d. | 0.06 *** | 0.1 | 0.3 *** | 0.4 | 0.9 *** | 1.1 | 1.5 *** |
| 22:5ω3 | 0.1 | 0.4 | 0.9 *** | 0.6 | 1.6 *** | 1.0 | 2.3 *** | 1.1 | 2.6 ** |
| 22:6ω3 | 4.4 | 11.0 | 13.9 *** | 17.5 | 18.9 | 23.1 | 20.6 ** | 27.5 | 23.7 ** |
| Σ ω3 PUFA | 4.9 | 11.4 | 14.9 ** | 18.1 | 20.9 ** | 24.4 | 24.0 | 29.7 | 28.2 |
| Liver | |||||||||
| 20:4ω6 | 31.1 | 28.6 | 25.4 *** | 23.1 | 18.3 *** | 13.2 | 12.6 | 7.9 | 9.2 *** |
| 20:5ω3 | 0.1 | 0.1 | 0.5 *** | 0.3 | 2.6 *** | 2.9 | 6.9 *** | 6.8 | 10.5 *** |
| 22:5ω3 | 0.1 | n.d. | n.d. | n.d. | 0.8 *** | 0.7 | 1.7 *** | 1.1 | 2.0 *** |
| 22:6ω3 | 3.7 | 9.3 | 11.9 *** | 14.0 | 15.4 ** | 18.5 | 16.2 *** | 21.1 | 16.5 *** |
| Σ ω3 PUFA | 3.9 | 9.4 | 12.4 | 14.2 | 19.0 *** | 22.1 | 25.1 ** | 29.0 | 29.6 |
| Kidney | |||||||||
| 20:4ω6 | 41.9 | 43.5 | 39.0 | 39.7 | 36.4 | 27.0 | 25.4 | 20.4 | 19.1 |
| 20:5ω3 | 0.01 | 0.2 | 0.5 ** | 0.8 | 3.4 *** | 5.5 | 9.4 *** | 17.2 | 16.7 |
| 22:5ω3 | n.d. | n.d. | 0.03 *** | n.d. | 0.3 *** | 0.2 | 0.5 *** | 0.4 | 0.6 *** |
| 22:6ω3 | 1.4 | 2.7 | 2.9 | 3.7 | 3.9 | 4.7 | 4.2 | 6.5 | 4.5 *** |
| Σ ω3 PUFA | 1.4 | 2.9 | 3.4 * | 4.5 | 7.8 *** | 10.4 | 14.6 ** | 24.0 | 23.0 |
| Muscle | |||||||||
| 20:4ω6 | 22.6 | 16.3 | 16.4 | 11.1 | 11.8 *** | 7.3 | 7.3 | 4.8 | 7.1 |
| 20:5ω3 | 0.03 | 0.06 | 0.2 ** | 0.2 | 0.7 | 0.6 | 1.4 *** | 1.2 | 1.7 * |
| 22:5ω3 | 0.6 | 0.5 | 1.0 *** | 0.5 | 1.5 *** | 0.7 | 1.3 | 0.8 | 1.5 ** |
| 22:6ω3 | 5.3 | 13.4 | 14.5 | 18.6 | 18.6 | 23.6 | 22.1 | 27.7 | 21.5 ** |
| Σ ω3 PUFA | 5.9 | 14.0 | 15.7 | 19.6 | 20.9 * | 24.4 | 25.1 | 29.7 | 25.5 * |
| Testes | |||||||||
| 22:5ω6 | 23.7 | 22.3 | 21.5 | 21.5 | 22.6 | 21.5 | 20.4 * | 19.6 | 19.0 |
| 22:6ω3 | 0.5 | 1.1 | 1.4 ** | 1.7 | 1.9 ** | 2.6 | 2.67 | 3.5 | 3.1 |
| Σ ω3 PUFA | 0.5 | 1.1 | 1.4 ** | 1.7 | 2.1 ** | 2.7 | 3.0 ** | 3.8 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belobrajdic, D.P.; Dallimore, J.A.; Adams, M.J.; Singh, S.P.; Abeywardena, M.Y. Sn1,3 Regiospecificity of DHA (22:6ω-3) of Plant Origin (DHA-Canola®) Facilitates Its Preferential Tissue Incorporation in Rats Compared to sn2 DHA in Algal Oil at Low Dietary Inclusion Levels. Nutrients 2025, 17, 1306. https://doi.org/10.3390/nu17081306
Belobrajdic DP, Dallimore JA, Adams MJ, Singh SP, Abeywardena MY. Sn1,3 Regiospecificity of DHA (22:6ω-3) of Plant Origin (DHA-Canola®) Facilitates Its Preferential Tissue Incorporation in Rats Compared to sn2 DHA in Algal Oil at Low Dietary Inclusion Levels. Nutrients. 2025; 17(8):1306. https://doi.org/10.3390/nu17081306
Chicago/Turabian StyleBelobrajdic, Damien P., Julie A. Dallimore, Michael J. Adams, Surinder P. Singh, and Mahinda Y. Abeywardena. 2025. "Sn1,3 Regiospecificity of DHA (22:6ω-3) of Plant Origin (DHA-Canola®) Facilitates Its Preferential Tissue Incorporation in Rats Compared to sn2 DHA in Algal Oil at Low Dietary Inclusion Levels" Nutrients 17, no. 8: 1306. https://doi.org/10.3390/nu17081306
APA StyleBelobrajdic, D. P., Dallimore, J. A., Adams, M. J., Singh, S. P., & Abeywardena, M. Y. (2025). Sn1,3 Regiospecificity of DHA (22:6ω-3) of Plant Origin (DHA-Canola®) Facilitates Its Preferential Tissue Incorporation in Rats Compared to sn2 DHA in Algal Oil at Low Dietary Inclusion Levels. Nutrients, 17(8), 1306. https://doi.org/10.3390/nu17081306





