Vitamin D and Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Function Test and Diagnosis of MCI
2.3. Vitamin D and Blood Chemistry Measurements
2.4. Body Composition Measurements
2.5. Statistical Analysis
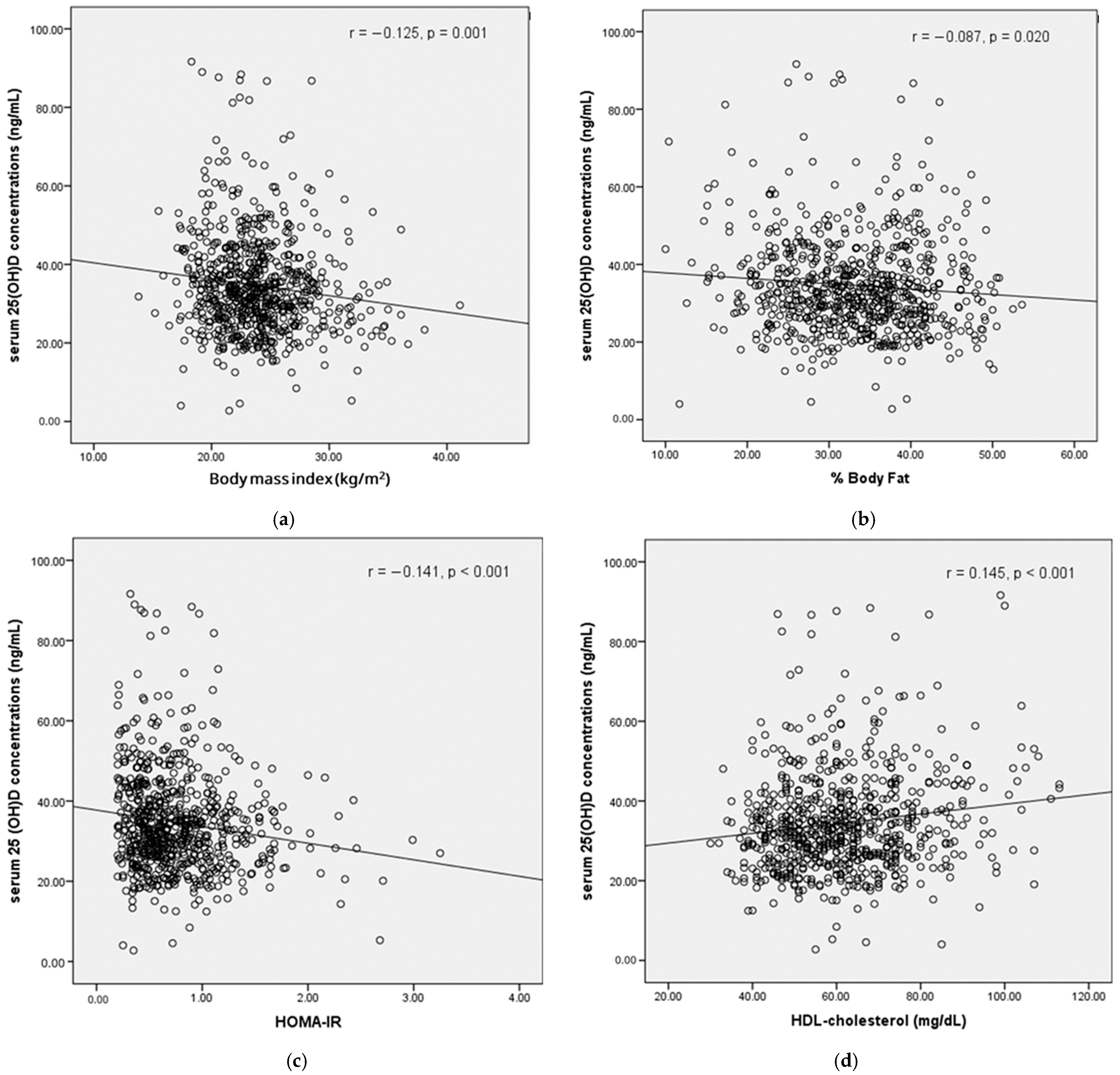
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soontrapa, S.; Soontrapa, S.; Bunyaratavej, N.; Rojanasthien, S.; Kittimanon, N.; Lektrakul, S. Vitamin D status of Thai premenopausal women. J. Med. Assoc. Thai 2009, 92 (Suppl. S5), S17–S20. [Google Scholar] [PubMed]
- Chailurkit, L.O.; Kruavit, A.; Rajatanavin, R. Vitamin D status and bone health in healthy Thai elderly women. Nutrition 2011, 27, 160–164. [Google Scholar] [CrossRef]
- Chailurkit, L.O.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Kruavit, A.; Chailurkit, L.O.; Thakkinstian, A.; Sriphrapradang, C.; Rajatanavin, R. Prevalence of vitamin D insufficiency and low bone mineral density in elderly Thai nursing home residents. BMC Geriatr. 2012, 12, 49. [Google Scholar] [CrossRef]
- Srinonprasert, V.; Chalermsri, C.; Chailurkit, L.O.; Ongphiphadhanakul, B.; Aekplakorn, W. Vitamin D insufficiency predicts mortality among older men, but not women: A nationwide retrospective cohort from Thailand. Geriatr. Gerontol. Int. 2018, 18, 1585–1590. [Google Scholar] [CrossRef]
- Jeenduang, N.; Sriprachan, C.; Plyduang, T.; Nuinoon, M.; Horpet, D.; Sangkaew, B.; Kaewboonlert, N. Vitamin D status and its associated factors in rural subjects in nakhon si thammarat province, Southern Thailand. J. Med. Assoc. Thail. 2018, 101, 397–404. [Google Scholar]
- Shantavasinkul, P.C.; Phanachet, P.; Puchaiwattananon, O.; Chailurkit, L.O.; Lepananon, T.; Chanprasertyotin, S.; Ongphiphadhanakul, B.; Warodomwichit, D. Vitamin D status is a determinant of skeletal muscle mass in obesity according to body fat percentage. Nutrition 2015, 31, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.-O.; Ongphiphadhanakul, B.; Aekplakorn, W. Update on vitamin D status in sunshine-abundant Thailand, 2019–2020. Nutrition 2023, 116, 112161. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678s–1688s. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.R.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Li, J.Q.; Tan, L.; Wang, H.F.; Tan, M.S.; Tan, L.; Xu, W.; Zhao, Q.F.; Wang, J.; Jiang, T.; Yu, J.T. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimers Dis. Other Dement. 2018, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Jones, A.; Ali, M.U.; Kenny, M.; Mayhew, A.; Mokashi, V.; He, H.; Lin, S.; Yavari, E.; Paik, K.; Subramanian, D.; et al. Potentially Modifiable Risk Factors for Dementia and Mild Cognitive Impairment: An Umbrella Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2024, 53, 91–106. [Google Scholar] [CrossRef]
- Nissou, M.F.; Guttin, A.; Zenga, C.; Berger, F.; Issartel, J.P.; Wion, D. Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J. Alzheimers Dis. 2014, 42, 789–799. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Menegaz, D.; Zhang, J.; Barrientos-Durán, A.; Tse, S.; Cashman, J.R.; Griffin, P.R.; Fiala, M. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimers Dis. 2012, 29, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ohtsuki, S.; Nezu, Y.; Koitabashi, Y.; Murata, S.; Terasaki, T. 1α,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1-40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS 2011, 8, 20. [Google Scholar] [CrossRef]
- Yu, J.; Gattoni-Celli, M.; Zhu, H.; Bhat, N.R.; Sambamurti, K.; Gattoni-Celli, S.; Kindy, M.S. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J. Alzheimers Dis. 2011, 25, 295–307. [Google Scholar] [CrossRef]
- Annweiler, C.; Fantino, B.; Schott, A.M.; Krolak-Salmon, P.; Allali, G.; Beauchet, O. Vitamin D insufficiency and mild cognitive impairment: Cross-sectional association. Eur. J. Neurol. 2012, 19, 1023–1029. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.M.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement. 2017, 13, 1207–1216. [Google Scholar] [CrossRef]
- Licher, S.; de Bruijn, R.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.; Ikram, M.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimers Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, C.S.; Talbot, D.; Nafti, M.; Giguère, Y.; Dodin, S.; Tourigny, A.; Carmichael, P.H.; Laurin, D. Vitamin D status, cognitive decline and incident dementia: The Canadian Study of Health and Aging. Can. J. Public Health 2020, 111, 312–321. [Google Scholar] [CrossRef]
- Olsson, E.; Byberg, L.; Karlström, B.; Cederholm, T.; Melhus, H.; Sjögren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Muniz-Terrera, G.; Phillips, C.L.; Cherubini, A.; Ferrucci, L.; Melzer, D. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010, 170, 1135–1141. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Melzer, D. Vitamin D and cognitive impairment in the elderly U.S. population. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 59–65. [Google Scholar] [CrossRef]
- McGrath, J.; Scragg, R.; Chant, D.; Eyles, D.; Burne, T.; Obradovic, D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology 2007, 29, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, A.M.; Williams, D.M.; Lawlor, D.A. The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology 2011, 22, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Mathiesen, E.B.; Rogne, S.; Wilsgaard, T.; Kjærgaard, M.; Grimnes, G.; Schirmer, H. Vitamin D and cognitive function: The Tromsø Study. J. Neurol. Sci. 2015, 355, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.; Houston, D.K.; Wilson, V.K.; Lovato, J.; Ayonayon, H.N.; Cauley, J.A.; Harris, T.; Simonsick, E.M.; Yaffe, K.; Kritchevsky, S.B.; et al. Low 25-Hydroxyvitamin D Concentrations and Risk of Incident Cognitive Impairment in Black and White Older Adults: The Health ABC Study. J. Nutr. Gerontol. Geriatr. 2018, 37, 1–13. [Google Scholar] [CrossRef]
- Graf, C.E.; Rossi, C.; Giannelli, S.V.; Nobari, B.H.; Gold, G.; Herrmann, F.R.; Zekry, D. Vitamin D is not associated with cognitive status in a cohort of very old hospitalized patients. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S53–S61. [Google Scholar] [CrossRef]
- Hemrungrojn, S.; Tangwongchai, S.; Charoenboon, T.; Panasawat, M.; Supasitthumrong, T.; Chaipresertsud, P.; Maleevach, P.; Likitjaroen, Y.; Phanthumchinda, K.; Maes, M. Use of the Montreal Cognitive Assessment Thai Version to Discriminate Amnestic Mild Cognitive Impairment from Alzheimer’s Disease and Healthy Controls: Machine Learning Results. Dement. Geriatr. Cogn. Disord. 2021, 50, 183–194. [Google Scholar] [CrossRef]
- Julayanont, P.; Tangwongchai, S.; Hemrungrojn, S.; Tunvirachaisakul, C.; Phanthumchinda, K.; Hongsawat, J.; Suwichanarakul, P.; Thanasirorat, S.; Nasreddine, Z.S. The Montreal Cognitive Assessment—Basic: A Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 2550–2554. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Thongtang, O. Thai Geriatric Depression Scale-TGDS. Siriraj Med. J. 1994, 46, 1–9. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- The Oxford Centre for Diabetes. Endocrinology & Metabolism. Diabetes Trial Unit. HOMA Calculator. Available online: http://www.dtu.ox.ac.uk (accessed on 28 September 2023).
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.M.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Intern. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F. Vitamin D status and sun exposure in southeast Asia. Dermatoendocrinology 2013, 5, 34–37. [Google Scholar] [CrossRef]
- Harinarayan, C.V.; Joshi, S.R. Vitamin D status in India—Its implications and remedial measures. J. Assoc. Physicians India 2009, 57, 40–48. [Google Scholar] [PubMed]
- Lin, L.; Ou, Q.; Lin, L.; Zhang, H.; Chen, K.; Chen, D.; Quan, H.; He, Y.; Fang, T. Low prevalence of vitamin D deficiency in adult residents in Hainan, the tropical island province of China. Ann. Palliat. Med. 2021, 10, 5580–5589. [Google Scholar] [CrossRef]
- Chailurkit, L.-O.; Thongmung, N.; Vathesatogkit, P.; Sritara, P.; Ongphiphadhanakul, B. Longitudinal study of vitamin D status among Thai individuals in a sun-abundant country. Public Health Pract. 2023, 6, 100439. [Google Scholar] [CrossRef]
- Chattranukulchai Shantavasinkul, P.; Nimitphong, H. Vitamin D and Visceral Obesity in Humans: What Should Clinicians Know? Nutrients 2022, 14, 3075. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163, Erratum in Lancet 2004, 363, 902. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Park, E.; Lee, M.J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pract. 2020, 14, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Gouranton, E.; Romier, B.; Tourniaire, F.; Astier, J.; Malezet, C.; Amiot, M.J.; Landrier, J.F. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 2012, 56, 1771–1782. [Google Scholar] [CrossRef]
- Granic, A.; Hill, T.R.; Kirkwood, T.B.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Saxby, B.K.; Wesnes, K.A.; Collerton, D.; et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: The Newcastle 85+ Study. Eur. J. Neurol. 2015, 22, 106-e7. [Google Scholar] [CrossRef]
| Variables | Total | MCI | Normal | MD (95%CI) | p-Value |
|---|---|---|---|---|---|
| n = 718 | n = 248 | n = 470 | |||
| Age, years | 65.7 (5.8) | 67.8 (6.0) | 64.6 (5.4) | −3.12 (−3.98, −2.25) | <0.001 |
| Age groups ‡, n (%) | |||||
| ≥65 years | 403 (56.1) | 172 (69.4) | 231 (49.1) | 2.34 (1.69, 3.24) | <0.001 |
| <65 years | 315 (43.9) | 76 (30.6) | 239 (50.9) | 1 | |
| Male ‡, n (%) | 202 (28.1) | 77 (31.0) | 125 (26.6) | 1.24 (0.89, 1.74) | 0.207 |
| Education, years | 14.3 (4.7) | 12.3 (5.2) | 15.4 (4.0) | 3.08 (2.40, 3.77) | <0.001 |
| Education, years ‡, n (%) | |||||
| ≤6 | 62 (8.6) | 38 (15.3) | 24 (5.1) | 3.36 (1.97, 5.75) | <0.001 |
| >6 | 656 (91.4) | 210 (84.7) | 446 (94.9) | 1 | |
| Systolic blood pressure, mmHg | 131.7 (17.8) | 134.1 (18.7) | 130.3 (17.2) | −3.78 (−6.64, −0.92) | 0.010 |
| Diastolic blood pressure, mmHg | 75.6 (11.0) | 76.4 (9.7) | 75.2 (11.5) | −1.30 (−3.05, 0.47) | 0.150 |
| Alcohol consumption ‡, n (%) | 241 (33.6) | 80 (32.3) | 161 (34.3) | 0.91 (0.66, 1.27) | 0.590 |
| Smoking ‡, n (%) | 31 (4.3) | 8 (3.2) | 23 (4.9) | 0.65 (0.29, 1.47) | 0.296 |
| Comorbid diseases | |||||
| Type 2 diabetes mellitus ‡, n (%) | 43 (6.0) | 16 (6.5) | 27 (5.7) | 1.13 (0.60, 2.14) | 0.704 |
| Hypertension ‡, n (%) | 200 (27.9) | 77 (31.0) | 123 (26.2) | 1.27 (0.91, 1.78) | 0.166 |
| Dyslipidemia ‡, n (%) | 265 (36.9) | 99 (39.9) | 166 (35.3) | 1.22 (0.89, 1.67) | 0.225 |
| Cardiovascular disease ‡, n (%) | 22 (3.1) | 12 (4.8) | 10 (2.1) | 2.34 (1.00, 5.49) | 0.045 |
| Vitamin D supplementation ‡, n (%) | 199 (27.7) | 62 (25.0) | 137 (29.1) | 0.81 (0.57, 1.15) | 0.238 |
| Cognitive tests | |||||
| MMSE scores | 27.3 (2.0) | 26.1 (2.3) | 27.9 (1.5) | 1.83 (1.55, 2.10) | <0.001 |
| MoCA scores | 25.2 (3.2) | 21.8 (2.4) | 27.1 (1.6) | 5.29 (5.00, 5.58) | <0.001 |
| Variables | Total | MCI | Normal | MD (95%CI) | p-Value |
|---|---|---|---|---|---|
| n = 718 | n = 248 | n = 470 | |||
| Weight, kg | 60.6 (11.6) | 61.7 (12.1) | 60.1 (11.2) | −1.59 (−3.37, 0.20) | 0.082 |
| BMI, kg/m2 | 23.9 (3.7) | 24.6 (4.2) | 23.5 (3.4) | −1.12 (−1.69, −0.54) | <0.001 |
| BMI groups ‡, n (%) | |||||
| <18.5 kg/m2 | 33 (4.6) | 9 (3.7) | 24 (5.1) | 1 | 0.001 |
| 18.5–22.9 kg/m2 | 272 (38.2) | 88 (35.9) | 184 (39.4) | 1.27 (0.57, 2.85) | |
| 23.0–24.9 kg/m2 | 172 (24.2) | 52 (21.2) | 120 (25.7) | 1.12 (0.50, 2.66) | |
| 25.0–29.9 kg/m2 | 186 (26.1) | 66 (26.9) | 120 (25.7) | 1.47 (0.64, 3.34) | |
| ≥30.0 kg/m2 | 49 (6.9) | 30 (12.2) | 19 (4.1) | 4.21 (1.62, 10.97) | |
| BMI groups ‡, n (%) | |||||
| <25.0 kg/m2 | 477 (67.0) | 149 (60.8) | 328 (70.2) | 1 | 0.011 |
| ≥25.0 kg/m2 | 235 (33.0) | 96 (39.2) | 139 (29.8) | 1.52 (1.10, 2.1) | |
| Waist circumference, cm | 81.9 (9.4) | 83.2 (10.4) | 81.3 (8.8) | −1.90 (−3.36, −0.45) | 0.011 |
| Hip circumference, cm | 93.6 (6.1) | 94.4 (6.5) | 93.2 (5.8) | −1.15 (−2.09, −0.21) | 0.017 |
| Waist–hip Ratio | 0.87 (0.05) | 0.87 (0.06) | 0.86 (0.5) | −0.01 (−0.02, −0.003) | 0.041 |
| Body fat, kg | 20.3 (7.0) | 21.5 (7.8) | 19.7 (6.6) | −1.74 (−2.82, −0.65) | 0.002 |
| % Body fat | 33.2 (7.9) | 34.3 (8.3) | 32.6 (7.6) | −1.74 (−2.96, −0.51) | 0.005 |
| Visceral fat area, cm2 | 101.6 (40.7) | 108.7 (44.3) | 97.8 (38.2) | −10.92 (−17.18, −4.66) | <0.001 |
| Skeletal muscle mass, kg | 21.6 (4.9) | 21.5 (4.9) | 21.7 (5.0) | 0.12 (−0.64, 0.89) | 0.750 |
| % Skeletal muscle mass | 35.7 (4.7) | 35.1 (4.8) | 36.1 (4.5) | 0.99 (0.28, 1.71) | 0.007 |
| Variables | Total | MCI | Normal | MD (95%CI) | p-Value |
|---|---|---|---|---|---|
| n = 718 | n = 248 | n = 470 | |||
| Biochemical parameters | |||||
| Total 25(OH)D levels, ng/mL | 34.5 (12.6) | 34.1 (12.0) | 34.7 (12.9) | 0.69 (−1.25, 2.62) | 0.488 |
| 25(OH)D2, ng/mL | 8.4 (15.5) | 7.4 (14.6) | 9.0 (15.9) | 1.60 (−0.79, 3.98) | 0.189 |
| 25(OH)D3, ng/mL | 24.9 (10.6) | 25.4 (10.8) | 24.6 (10.5) | −0.88 (−2.52, 0.76) | 0.290 |
| Vitamin D status | |||||
| Vitamin D deficiency ‡ (< 20 ng/mL) | 47 (6.5) | 15 (6.1) | 32 (6.8) | 0.88 (0.47, 1.66) | 0.695 |
| Vitamin D insufficiency ‡ (20–30 ng/mL) | 240 (33.4) | 86 (34.7) | 154 (32.8) | 1.09 (0.79, 1.54) | 0.606 |
| Vitamin D inadequacy ‡ (< 30 ng/mL) | 287 (40.0) | 101 (40.7) | 186 (39.6) | 1.05 (0.77, 1.44) | 0.765 |
| Insulin, µIU/mL | 5.8 (3.4) | 6.1 (3.8) | 5.6 (3.1) | −0.41 (−0.93, 0.11) | 0.122 |
| HOMA-IR | 0.77 (0.43) | 0.81 (0.49) | 0.74 (0.40) | −0.06 (−0.13, 0.003) | 0.062 |
| Cholesterol, mg/dL | 208.6 (42.7) | 204.3 (42.0) | 210.9 (43.0) | 6.64 (0.06, 13.22) | 0.048 |
| TG, mg/dL | 106.9 (52.4) | 107.3 (49.7) | 106.7 (53.9) | −0.65 (−8.74, 7.45) | 0.876 |
| LDL-C, mg/dL | 132.8 (40.7) | 129.1 (39.5) | 134.7 (41.1) | 5.60 (−0.66, 11.87) | 0.080 |
| HDL-C, mg/dL | 61.8 (15.0) | 60.3 (15.2) | 62.6 (14.9) | 2.32 (0.01, 4.64) | 0.049 |
| Fasting plasma glucose, mg/dL | 91.2 (11.7) | 92.5 (12.7) | 90.6 (11.0) | −1.96 (−3.75, −0.16) | 0.033 |
| HbA1C, % | 5.7 (0.4) | 5.7 (0.5) | 5.6 (0.4) | −0.12 (−0.18, −0.05) | <0.001 |
| HbA1C group ‡, n (%) | |||||
| <5.7% | 386 (54.0) | 115 (46.6) | 271 (57.9) | 1 | 0.004 |
| ≥5.7% | 329 (46.0) | 132 (53.4) | 197 (42.1) | 1.58 (1.16, 2.15) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Total 25(OH)D levels, ng/mL | 0.996 (0.983, 1.008) | 0.487 | ||
| Age, years | 1.102 (1.071, 1.133) | <0.001 | 1.073 (1.041, 1.106) | <0.001 |
| Education, years | 0.864 (0.833, 0.896) | <0.001 | 0.882 (0.849, 0.916) | <0.001 |
| Sex, male | 1.243 (0.886, 1.743) | 0.207 | ||
| Systolic blood pressure, mmHg | 1.012 (1.003, 1.021) | 0.010 | ||
| BMI, kg/m2 | 1.082 (1.038, 1.128) | <0.001 | 1.072 (1.025, 1.121) | 0.002 |
| HDL-C, mg/dL | 0.990 (0.979, 1.000) | 0.050 | ||
| Cardiovascular disease | 2.339 (0.996, 5.493) | 0.051 | ||
| HbA1C, mg/dL | 1.809 (1.269, 2.578) | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imerbsin, N.; Shantavasinkul, P.C.; Witoonpanich, P.; Sirivarasai, J.; Taonam, N.; Phanachet, P.; Warodomwichit, D.; Jayanama, K.; Boonyawat, K.; Somlaw, N.; et al. Vitamin D and Cognitive Impairment. Nutrients 2025, 17, 1301. https://doi.org/10.3390/nu17081301
Imerbsin N, Shantavasinkul PC, Witoonpanich P, Sirivarasai J, Taonam N, Phanachet P, Warodomwichit D, Jayanama K, Boonyawat K, Somlaw N, et al. Vitamin D and Cognitive Impairment. Nutrients. 2025; 17(8):1301. https://doi.org/10.3390/nu17081301
Chicago/Turabian StyleImerbsin, Nalinee, Prapimporn Chattranukulchai Shantavasinkul, Pirada Witoonpanich, Jintana Sirivarasai, Naphat Taonam, Pariya Phanachet, Daruneewan Warodomwichit, Kulapong Jayanama, Kochawan Boonyawat, Nicha Somlaw, and et al. 2025. "Vitamin D and Cognitive Impairment" Nutrients 17, no. 8: 1301. https://doi.org/10.3390/nu17081301
APA StyleImerbsin, N., Shantavasinkul, P. C., Witoonpanich, P., Sirivarasai, J., Taonam, N., Phanachet, P., Warodomwichit, D., Jayanama, K., Boonyawat, K., Somlaw, N., Ongphiphadhanakul, B., Nakawiro, D., & Tangwongchai, S. (2025). Vitamin D and Cognitive Impairment. Nutrients, 17(8), 1301. https://doi.org/10.3390/nu17081301






