Abstract
Background/Objectives: Infant functional disorders, including colic, irritability, and sleep disturbances, often overlap with symptoms of cow’s milk protein allergy (CMPA). While extensively hydrolyzed formulas are commonly used to address these issues, the short-term efficacy of amino acid formulas (AAF) for managing colic remains understudied. This secondary analysis of a previously published prospective cohort, the largest of its kind in the United States, evaluated the short-term impact of AAF in improving colic and associated symptoms in infants under six months of age with suspected CMPA. Methods: This real-world prospective cohort study analyzed data from 138 infants with suspected CMPA initiated on AAF at Visit 1. After excluding 34 infants due to incomplete data or treatment changes, 104 infants were included in the final analysis. Symptom severity and resolution were assessed through outcomes across two visits, with care provided by general pediatricians (82%) and pediatric gastroenterologists (18%). Results: At baseline, the most common symptoms were colic (n = 83), gassiness (n = 72), fussiness (n = 66), and sleep disturbances (n = 58). By the next follow-up visit (Visit 2), parents reported symptom improvements in colic (94%), gassiness (81%), fussiness (83%), and sleep disturbances (86%). Complete symptom resolution was observed in 73% of infants with colic, 68% with gassiness, 65% with fussiness, and 81% with sleep difficulties. By Visit 2, no infants had severe symptoms, with only mild residual symptoms reported. Conclusions: AAF was associated with significant short-term improvements in colic and related symptoms in infants with suspected CMPA. These preliminary findings highlight AAF as a promising dietary intervention during early infancy. Larger controlled studies are necessary to confirm these results and explore long-term outcomes.
1. Introduction
An estimated 20% to 30% of infants experience infant colic within the first 6 months of life, and though it is relatively common, it is a generally self-limiting condition [1]. Infants with colic often exhibit prolonged periods of inconsolable crying, along with raising of the legs, clenching of fists, and facial flushing [2]. Despite the prevalence of this condition, researchers have yet to determine its pathogenesis, and there is little consensus on its underlying mechanisms or overall etiology [3,4,5,6]. Clinicians rely on Rome IV criteria for diagnosing infant colic, which requires infants to be < 5 months old at onset, show no signs of failure to thrive, fever, or illness, and have unexplained recurrent periods of crying that caregivers cannot abate [3]. Symptoms of colic, including crying, irritability, flatulence, and sleeping difficulties, can lead to anxiety and depression in parents and have been linked to instances of child abuse [7,8,9,10]. According to the National Health Service, the healthcare system bears a significant cost of USD 104 million annually for treating infant colic [11]. These emotional and substantial economic costs underscore the need for an improved understanding of the pathogenesis of this condition and for healthcare providers to have better colic management strategies [12].
Treatment options for infant colic are limited, with most showing only moderate evidence of effectiveness [13]. Certain probiotics have demonstrated short-term efficacy in reducing colic symptoms [14]. Functional disorders in infancy, such as colic, irritability, and sleep disturbances, are often associated with cow’s milk protein allergy (CMPA). This prevalent food allergy shares symptoms with colic, including crying, fussiness, flatulence, and sleep difficulties [8,15].
Evidence suggests that eliminating certain allergenic foods (e.g., cow’s milk, eggs, fish, peanuts, soy, tree nuts, wheat) from breastfeeding mothers’ diets may reduce colic symptoms [7,16]. In formula-fed infants, first-line treatment typically involves extensively hydrolyzed formula (eHF), with amino acid formulas recommended for severe CMPA or cases unresponsive to eHF. While extensively hydrolyzed formulas have shown promise in alleviating symptoms like crying and irritability [17,18], there is a lack of studies examining the short-term use of amino acid formulas for managing infant colic. Additionally, the importance of achieving short-term symptom relief cannot be overstated, as it is a priority for parents and pediatric physicians seeking to quickly restore infant health and well-being [19,20,21].
This secondary analysis of a prospectively collected cohort aimed to evaluate changes in colic symptom severity in symptomatic infants under 6 months of age managed with a commercial amino acid formula (AAF) in infants with suspected CMPA. The primary objective of this real-world study was to assess short-term improvements in colic and related symptoms after initiating an AAF as prescribed by general pediatricians and pediatric gastroenterologists. We hypothesized that colic and associated symptoms would decrease in severity by the next follow-up visit (Visit 2) compared to the initial clinical diagnosis and enrollment (Visit 1).
2. Materials and Methods
2.1. Study Population
This study is a secondary analysis of a prospective cohort in the United States, the largest of its kind, evaluating the short-term symptom improvement of AAF in symptomatic infants under six months of age with suspected CMPA in a real-world clinical setting [22,23]. Pediatric HCPs evaluated infants included in the analysis for colic symptoms, gassiness, fussiness, and sleep difficulties from July 2021 to October 2021. They were prescribed a commercially available amino acid formula (PurAmino™, Mead Johnson Nutrition, Evansville, Indiana) as clinically indicated at Visit 1. They continued the formula use through the next outpatient follow-up visit (Visit 2). Infants were excluded from the analysis if they did not receive AAF at Visit 1, were over six months old at the time of formula initiation, had incomplete survey data at Visits 1 or 2, or discontinued or switched formulas between visits. This observational cohort study was approved by the Johns Hopkins All Children’s Institutional Review Board (IRB00279920) on 28 April 2021.
2.2. Data Collection
As previously described, all patient data were de-identified at Visit 1 by assigning each patient a unique animal name identifier, ensuring no personally identifiable information (e.g., name or birth date) was recorded [22,23]. Pediatric HCPs documented demographic information and baseline symptoms and assigned a study formula. Data were collected using ZSMoments™, a secure, mobile-based platform developed by ZS Associates (Bellevue, WA, USA). This mobile health application, installed on the HCP’s mobile device, enabled real-time data capture in outpatient clinical settings through an efficient interface that facilitated seamless data entry and patient de-identification, ensuring compliance with privacy standards.
For this secondary analysis, symptoms of interest included colic, gassiness, fussiness, and sleeping difficulties. Symptom severity was rated on a 0–3 scale: 0 (Not Present), 1 (Low), 2 (Moderate), 3 (Severe), or Not Assessed. An aggregate symptom score, combining individual symptom ratings, was calculated at both visits to assess changes in severity over the short term. Improvement was defined as a decrease in the aggregate symptom score from Visit 1 to Visit 2, while “no improvement” indicated no change in scores (e.g., an aggregate score of 4 at both visits). Sub-cohort analyses focused on patients presenting with specific symptoms at Visit 1 to evaluate symptom improvement and resolution. No post hoc analyses were performed.
2.3. Statistical Analyses
Continuous study variables were summarized with medians and ranges (minimum/maximum), and categorical study variables were reported as counts and percentages [24]. Data were inspected for completeness and outliers before analyses, and a complete case analysis was used to analyze variables with missing data. Statistical significance was evaluated using t-tests to calculate p-values between proportions, with alpha < 0.05 considered significant. All analyses were conducted with an SPSS-based analytics tool (IBM, Armonk, NY, USA).
3. Results
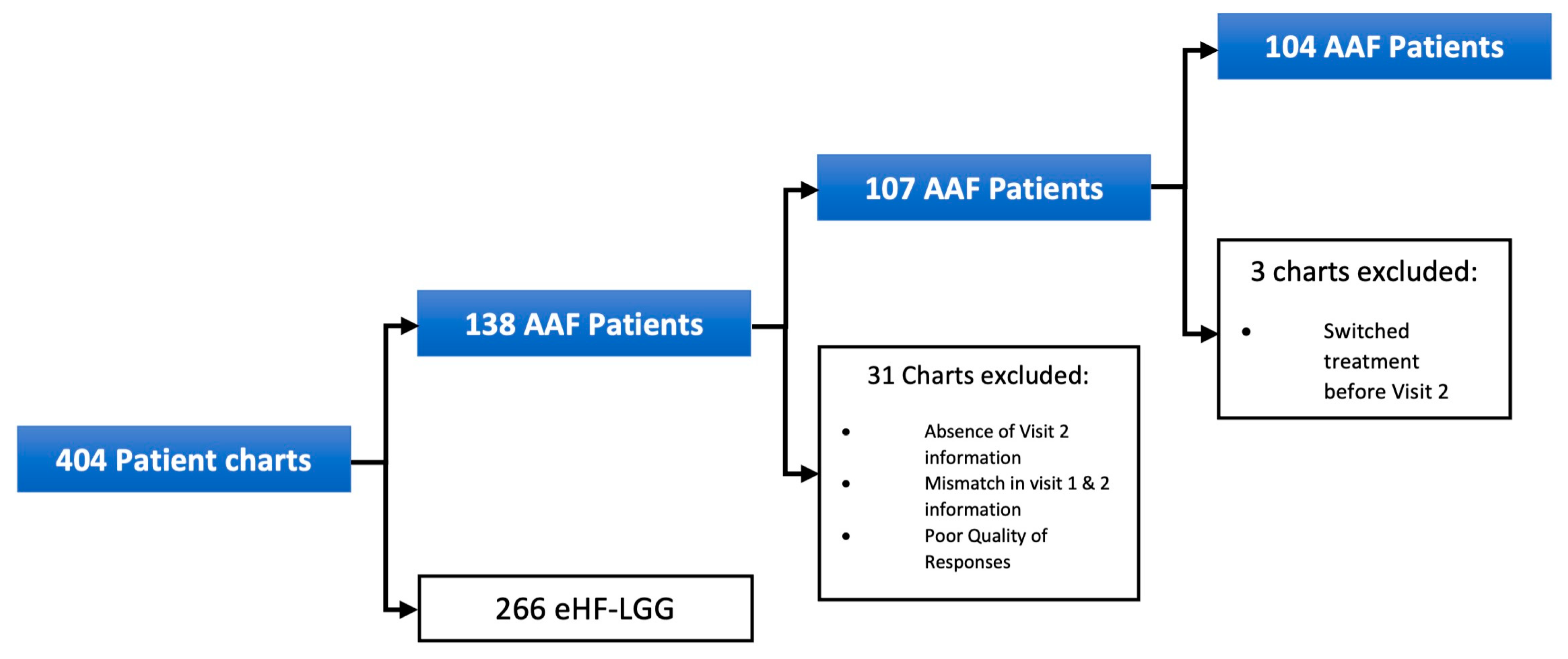
Pediatric HCPs started 138 symptomatic infants with clinically suspected CMPA on AAF at Visit 1. However, 31 patients were excluded from the final sample due to incomplete data. Among the 107 patients in the final sample, 88 (82%) were managed by general pediatricians, while 19 (18%) were seen by pediatric gastroenterologists. Three additional patients were excluded from the symptom analysis due to treatment switching before Visit 2. Further exclusions for patients not assessed for specific infant colic symptoms are detailed in Figure 1.
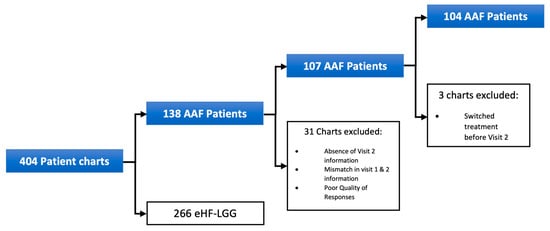
Figure 1.
Patient exclusion for initial AAF cohort.
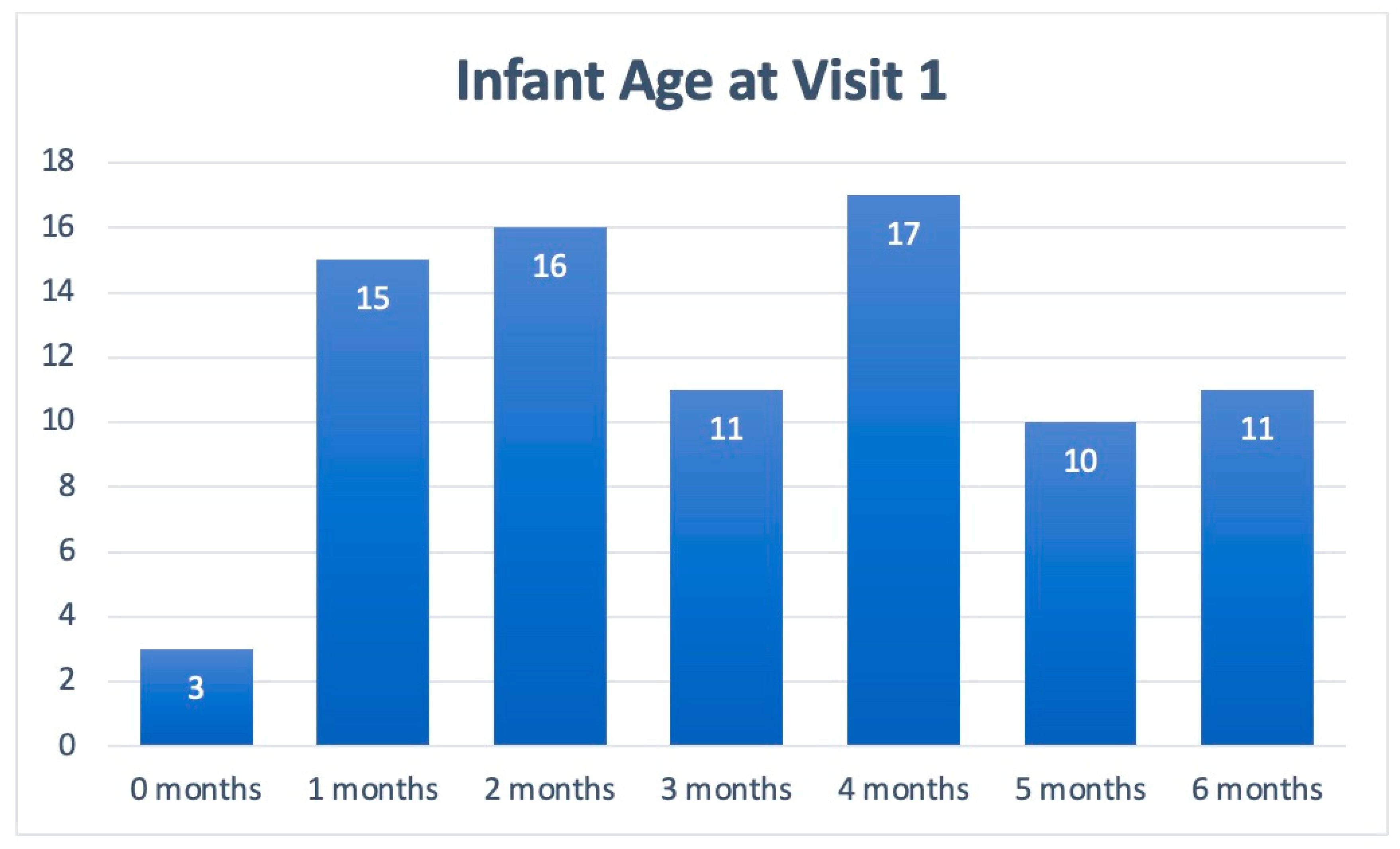
Table 1 includes patient demographics collected from Visits 1 and 2, while Figure 2 depicts the cohort’s age stratification. Of the patients included in the symptom analysis, 75% were under 5 months of age, and the majority were male (60%; n = 64). Across visits, the symptoms of all 104 infants in the cohort were assessed. Due to the natural progression of each clinical course, some patients presented with new symptoms at Visit 2.

Table 1.
Patient demographics.
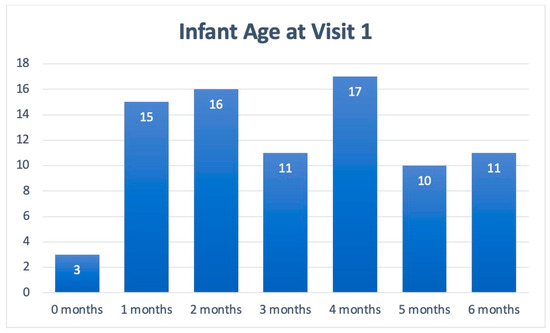
Figure 2.
Age stratification of patients presenting with infant colic symptoms at Visit 1.
As shown in Table 2, most symptomatic infants with colic and related symptoms experienced improvement or resolution of symptoms at Visit 2. Additional analyses focused on infants presenting specific symptoms at Visit 1, including colic (n = 83), gassiness (n = 72), fussiness (n = 66), and sleep difficulties (n = 58). By Visit 2, 94% of infants with colic and 81% with gassiness improved, 86% experienced improved sleep, and 83% reported reduced fussiness.

Table 2.
Symptom burden of the cohort at each visit.
Parents of the 83 symptomatic infants with infant colic at Visit 1 reported that 61 (73%) experienced complete resolution of symptoms by Visit 2. Similarly, among the 66 infants with fussiness, 46 (70%) were reported to have total resolution of their symptoms. Complete resolution was also reported for 50 of the 72 infants with gassiness (69%) and 48 of the 58 infants with sleep difficulties (83%). Table 3 provides further details on symptom resolution by initial severity. For instance, among the 18 infants presenting with severe colic at Visit 1, 14 (78%) were reported to have complete resolution by Visit 2.

Table 3.
Rate of total symptom resolution at first follow-up visit by severity at initial presentation.
This analysis showed that amino acid formula-fed infants experienced a significant reduction in reported colic and related symptom severity between Visit 1 and Visit 2. Most patients showed statistically significant symptom improvement, with nearly all symptomatic infants at Visit 2 presenting with only mild symptoms. Notably, no infants exhibited severe symptoms at Visit 2.
4. Discussion
This real-world prospective cohort study analyzed data from 104 symptomatic infants who were started on an amino acid formula (AAF) at their initial clinical presentation (Visit 1) and followed up at the next outpatient visit (Visit 2). Symptom severity and resolution were evaluated through outcomes across these two visits, with care provided by general pediatricians (82%) and pediatric gastroenterologists (18%).
This secondary analysis focused on colic and associated symptoms and found that the vast majority of infants experienced improvements across all symptoms assessed by clinicians at Visit 2. Notably, no infants had severe symptoms at follow-up, with only mild residual symptoms remaining. Additionally, the majority of patients who presented with a given symptom at Visit 1 experienced total resolution of that symptom at Visit 2 as seen in Figure 2. These findings highlight the potential of AAF to provide rapid relief for infant colic-related symptoms—often by the next follow-up visit—addressing the need to develop effective short-term colic management strategies [1,2,3,4,25,26].
While extensively hydrolyzed formulas are commonly used to address CMPA-related symptoms and, more recently, infant colic symptoms [20], the short-term efficacy of AAF for managing colic in infants remains understudied [26]. One previous six-patient case series observed exclusively breastfed infants and measured baseline crying/fussing time over 3–5 days before transitioning to an amino acid formula for 4–8 days [26]. Infants experienced significantly reduced crying times within 1 day of AAF initiation. This secondary analysis expands on the original cohort study—the largest of its kind in the United States—by focusing on the short-term impact of AAF on colic and numerous associated symptoms in infants during the first six months of life [22].
Infant colic is a common yet poorly understood condition characterized by episodes of excessive, inconsolable crying in an otherwise healthy infant [1,2,3,4,5,6]. It typically begins in the first few weeks of life, peaks around six weeks, and often resolves naturally by six months of age, with prevalence rates ranging from 10% to 40% [6,7,8,9,10]. While crying is a normal form of communication for infants, colic is distinguished by facial flushing, fist clenching, and prolonged, intense episodes of crying that are resistant to soothing efforts [2,3,4].
The etiology of colic remains elusive, with proposed mechanisms including increased intraluminal gas, gut dysmotility, visceral pain, food sensitivities, and environmental or psychosocial factors [5,10]. However, evidence supporting gastrointestinal dysfunction as the primary cause is inconclusive. Some studies, such as those by De Weerth et al., suggest that gut dysbiosis—reduced microbiota diversity and stability—may play a role in infant colic [27].
Though colic is generally self-limiting, its unpredictable, disruptive, and unexplained nature places significant emotional and physical strain on caregivers [1,2,3,4,5]. Feelings of inadequacy, anxiety, and depression are common among parents, compounding the challenges of managing the condition and prompting frequent healthcare visits [6,7,8,9,10,11].
Management strategies for colic remain varied, with limited robust evidence supporting specific interventions [1,2,3,4,25]. Recent research highlights the potential benefits of probiotics, such as Lactobacillus rhamnosus GG (LGG), shown in prospective controlled trials to reduce crying time and fecal calprotectin levels in infants with colic [28]. Similarly, Lactobacillus reuteri and certain Bifidobacterium animalis subspecies have helped reduce crying and fussing time in exclusively breastfed infants [29,30,31,32,33]. A meta-analysis of randomized controlled trials found that L. reuteri nearly doubled the likelihood of treatment success compared to placebo in breastfed infants [14]. However, its benefits in formula-fed infants remain uncertain.
For formula-fed infants with CMPA and symptoms resembling colic, extensively hydrolyzed formulas, including those containing LGG, are often first-line treatments. In severe cases or when extensively hydrolyzed formulas are not tolerated, amino acid formulas (AAF) may be used. However, there is a notable gap in the literature regarding the efficacy of AAF in managing colic symptoms [26]. Despite this limitation, AAF remains an important option for infants with co-occurring CMPA, highlighting the need for further research to better understand its role in colic management [25,26].
Despite ongoing research, the pathogenesis of colic remains unclear, necessitating further exploration of its underlying mechanisms and the effectiveness of current management strategies [12]. Addressing this knowledge gap is essential for alleviating the burden of colic on infants and their families [6,7,8,9,10,11].
When considering the management of infant colic with amino acid formula (AAF), it is important to weigh its clinical benefits against its higher cost compared to standard formulas. While AAF has shown promise as a potential management strategy [23], the financial impact on families and healthcare systems raises the need for a thorough cost–benefit analysis. Future research should focus on comparing these costs to help guide informed decisions about using AAF in managing colic.
We acknowledge several limitations in our study. Voluntary clinician participation may have introduced selection and response biases, and the focus on the U.S. may limit generalizability to other patient populations. Reliance on clinician assessment and judgment of infant colic, without strict adherence to Rome IV criteria, may have affected case identification. The real-world study design and absence of a control group limits comparisons to other treatments or no treatment. Including symptomatic infants over 4 months at the time of presentation raises the possibility of spontaneous symptom resolution before the next follow-up visit [34,35]. Despite efforts to standardize data collection, the natural progression of colic and potential placebo effects cannot be ruled out. Future research should include control groups, assessing long-term outcomes, and addressing these limitations.
5. Conclusions
This study highlights the potential of AAF to provide short-term improvement in colic symptoms, such as gassiness, fussiness, and sleep difficulties, in symptomatic infants. By addressing symptoms, our findings underscore the importance of short-term relief—a priority for both parents and pediatric providers. As the largest prospective study in this area, this secondary analysis helps address the knowledge gap in exploring the role of AAF in managing colic, particularly in infants with suspected CMPA. While these initial results may suggest potential benefits, further controlled studies are needed to confirm these preliminary results and to explore the broader implications of AAF use in this context.
Author Contributions
Conceptualization, M.J.W.; data curation, J.M.B., J.V.B., L.L., J.B., J.F., L.V.O., A.L.F. and A.A.F.; formal analysis, J.M.B., J.V.B., L.L., J.B., J.F., L.V.O., A.L.F. and A.A.F.; investigation, M.J.W.; methodology, M.J.W.; supervision, M.J.W.; writing—original draft, J.M.B. and J.V.B.; writing—review and editing, J.M.B., J.V.B., L.L., J.B., J.F., L.V.O., A.L.F., A.A.F. and M.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
A grant from Reckitt (RB2166) supported article processing and presentation fees for this research. Additionally, Reckitt provided funding for data collection through ZS Associates.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was determined to be exempt by the Johns Hopkins All Children’s Institutional Review Board (IRB00279920) on 28 April 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to subject confidentiality, but are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Heather Evans, Jennifer Wampler, and Claire Levine for their critical review of the manuscript.
Conflicts of Interest
Study funders had no role in study protocol development; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. M.J.W has served as a consultant for Reckitt; The remaining authors have no conflicts of interest to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| CMPA | Cow’s milk protein allergy |
| eHF | Extensively hydrolyzed formula |
| AAF | Amino acid formula |
| HCP | Healthcare provider |
| GI | Gastroenterology |
| LGG | Lactobacillus rhamnosus GG |
References
- Nocerino, R.; Pezzella, V.; Cosenza, L.; Amoroso, A.; Di Scala, C.; Amato, F.; Iacono, G.; Canani, R.B. The controversial role of food allergy in infantile colic: Evidence and clinical management. Nutrients 2015, 7, 2015–2025. [Google Scholar] [CrossRef]
- Zeevenhooven, J.; Browne, P.D.; L’Hoir, M.P.; de Weerth, C.; Benninga, M.A. Infant colic: Mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 479–496. [Google Scholar] [CrossRef]
- Koppen, I.J.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Benninga, M.A. The pediatric Rome IV criteria: What’s new? Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zeevenhooven, J.; Koppen, I.J.; Benninga, M.A. The New Rome IV Criteria for Functional Gastrointestinal Disorders in Infants and Toddlers. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.E.; Milla, P.J.; Benninga, M.A.; Davidson, G.P.; Fleisher, D.F.; Taminiau, J. Childhood functional gastrointestinal disorders: Neonate/toddler. Gastroenterology 2006, 130, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology 2016, 150, 1443–1455.e2. [Google Scholar] [CrossRef]
- Johnson, J.D.; Cocker, K.; Chang, E. Infantile Colic: Recognition and Treatment. Am. Fam. Physician 2015, 92, 577–582. [Google Scholar]
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van. Leeuwenhoek 2015, 107, 1547–1553. [Google Scholar] [CrossRef]
- Ellwood, J.; Draper-Rodi, J.; Carnes, D. Comparison of common interventions for the treatment of infantile colic: A systematic review of reviews and guidelines. BMJ Open 2020, 10, e035405. [Google Scholar] [CrossRef]
- Sung, V.; Hiscock, H.; Tang, M.; Mensah, F.K.; Heine, R.G.; Stock, A.; York, E.; Barr, R.G.; Wake, M. Probiotics to improve outcomes of colic in the community: Protocol for the Baby Biotics randomised controlled trial. BMC Pediatr. 2012, 12, 135. [Google Scholar] [CrossRef]
- Morris, S.; James-Roberts, I.S.; Sleep, J.; Gillham, P. Economic evaluation of strategies for managing crying and sleeping problems. Arch. Dis. Child. 2001, 84, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Savino, F.; Hill, S.; Kivelä, L.; De Koning, B.; Kӧglmeier, J.; Luque, V.; Moltu, S.J.; Norsa, L.; De Pipaon, M.S.; et al. Infant formulas for the treatment of functional gastrointestinal disorders: A position paper of the ESPGHAN Nutrition Committee. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 168–180. [Google Scholar] [CrossRef]
- Arikan, D.; Alp, H.; Gözüm, S.; Orbak, Z.; Cifçi, E.K. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. J. Clin. Nurs. 2008, 17, 1754–1761. [Google Scholar] [CrossRef]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C. Diagnosis of cow’s milk allergy in children: Determining the gold standard? Expert. Rev. Clin. Immunol. 2014, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Roy, N.; Heine, R.G.; Hosking, C.S.; Francis, D.E.; Brown, J.; Speirs, B.; Sadowsky, J.; Carlin, J.B. Effect of a low-allergen maternal diet on colic among breastfed infants: A randomized, controlled trial. Pediatrics 2005, 116, e709–e715. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Gutierrez-Castrellon, P.; Velasco-Benitez, C.; Palacios, J.; Jaen, D.; Ribeiro, H.; Shek, L.P.; Lee, B.W.; Alarcon, P. Practical algorithms for managing common gastrointestinal symptoms in infants. Nutrition 2013, 29, 184–194. [Google Scholar] [CrossRef]
- Berseth, C.L.; Johnston, W.H.; Stolz, S.I.; Harris, C.L.; Mitmesser, S.H. Clinical response to 2 commonly used switch formulas occurs within 1 day. Clin Pediatr 2009, 48, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Beacker, J.; Brown, J.M.; Florio, J.; Baran, J.V.; Lamos, L.; Oliveros, L.; Vanderhoof, J.A.; Sriaroon, P.; Wilsey, M.J. Clinician Experience with Using Hypoallergenic Formulas to Treat Infants with Suspected Cow’s Milk Protein Allergy: A Secondary Analysis of a Prospective Survey Cohort. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 277–283. [Google Scholar] [CrossRef]
- Brown, J.M.; Baran, J.V.; Lamos, L.; Beacker, J.; Florio, J.; Oliveros, L.V.; Fabbrini, A.L.; Farrar, A.A.; Vanderhoof, J.A.; Wilsey, M.J. Extensively Hydrolyzed Formula and Infant Colic Symptoms: Secondary Analysis of a Prospective Cohort Study. Clin. Pediatr. 2024, 63, 1579–1584. [Google Scholar] [CrossRef]
- Baran, J.V.; Brown, J.M.; Farrar, A.A.; Oliveros, L.V.; Beacker, J.; Lamos, L.; Florio, J.; Fabbrini, A.L.; Wilsey, M.J. Impact of the 2022 national formula shortage on clinical decision-making of healthcare providers in switching amino acid formulas for infants with cow’s milk protein allergy: A survey-based study. Front Pediatr. 2024, 12, 1328506. [Google Scholar] [CrossRef]
- Wilsey, M.J.; Florio, J.; Beacker, J.; Lamos, L.; Baran, J.V.; Oliveros, L.; Sriaroon, P.; Brown, J.M.; Vanderhoof, J.A. Extensively Hydrolyzed Formula Improves Allergic Symptoms in the Short Term in Infants with Suspected Cow’s Milk Protein Allergy. Nutrients 2023, 15, 1677. [Google Scholar] [CrossRef]
- Wilsey, M.J.; Baran, J.V.; Lamos, L.; Beacker, J.; Florio, J.; Oliveros, L.; Sriaroon, P.; Brown, J.M.; Vanderhoof, J.A. Short-term symptom improvement in infants with suspected cow’s milk protein allergy using amino acid formula: A prospective cohort analysis. Front. Nutr. 2023, 10, e1208334. [Google Scholar] [CrossRef]
- Lee, S.W. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e1. [Google Scholar] [CrossRef]
- Gordon, M.; Biagioli, E.; Sorrenti, M.; Lingua, C.; Moja, L.; Banks, S.S.; Ceratto, S.; Savino, F. Dietary modifications for infantile colic. Cochrane Database Syst. Rev. 2018, 10, CD011029. [Google Scholar] [CrossRef]
- Estep, D.C.; Kulczycki, A., Jr. Treatment of infant colic with amino acid-based infant formula: A preliminary study. Acta Paediatr. 2000, 89, 22–27. [Google Scholar] [CrossRef] [PubMed]
- de Weerth, C.; Fuentes, S.; de Vos, W.M. Crying in infants: On the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Montanari, P.; Galliano, I.; Daprà, V.; Bergallo, M. Lactobacillus rhamnosus GG (ATCC 53103) for the Management of Infantile Colic: A Randomized Controlled Trial. Nutrients 2020, 12, e1693. [Google Scholar] [CrossRef]
- Chau, K.; Lau, E.; Greenberg, S.; Jacobson, S.; Yazdani-Brojeni, P.; Verma, N.; Koren, G. Probiotics for infantile colic: A randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J. Pediatr. 2015, 166, 74–78. [Google Scholar] [CrossRef]
- Kianifar, H.; Ahanchian, H.; Grover, Z.; Jafari, S.; Noorbakhsh, Z.; Khakshour, A.; Sedaghat, M.; Kiani, M. Synbiotic in the management of infantile colic: A randomised controlled trial. J. Paediatr. Child. Health 2014, 50, 801–805. [Google Scholar] [CrossRef]
- Fatheree, N.Y.; Liu, Y.; Taylor, C.M.; Hoang, T.K.; Cai, C.; Rahbar, M.H.; Hessabi, M.; Ferris, M.; McMurtry, V.; Wong, C.; et al. Lactobacillus reuteri for Infants with Colic: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Pediatr. 2017, 191, 170–178.e2. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, R.; De Filippis, F.; Cecere, G.; Marino, A.; Micillo, M.; Di Scala, C.; De Caro, C.; Calignano, A.; Bruno, C.; Paparo, L.; et al. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12® in infant colic: A randomised, double blind, placebo-controlled trial. Aliment. Pharmacol. Ther. 2020, 51, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, G.; Xie, H.; You, L.; Li, H.; Zhang, Y.; Du, C.; Xu, S.; Melsaether, C.; Yuan, S. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant colic—A randomised, double-blinded, placebo-controlled study. Benef. Microbes. 2021, 12, 531–540. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Çokura, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Bilgin, A.; Samara, M. Systematic Review and Meta-Analysis: Fussing and Crying Durations and Prevalence of Colic in Infants. J. Pediatr 2017, 185, 55–61.e4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).