Longitudinal Association Between the Consumption of Vegetables, Fruits, and Red Meat and Diabetes Disease Burden: An Analysis of Multiple Global Datasets
Highlights
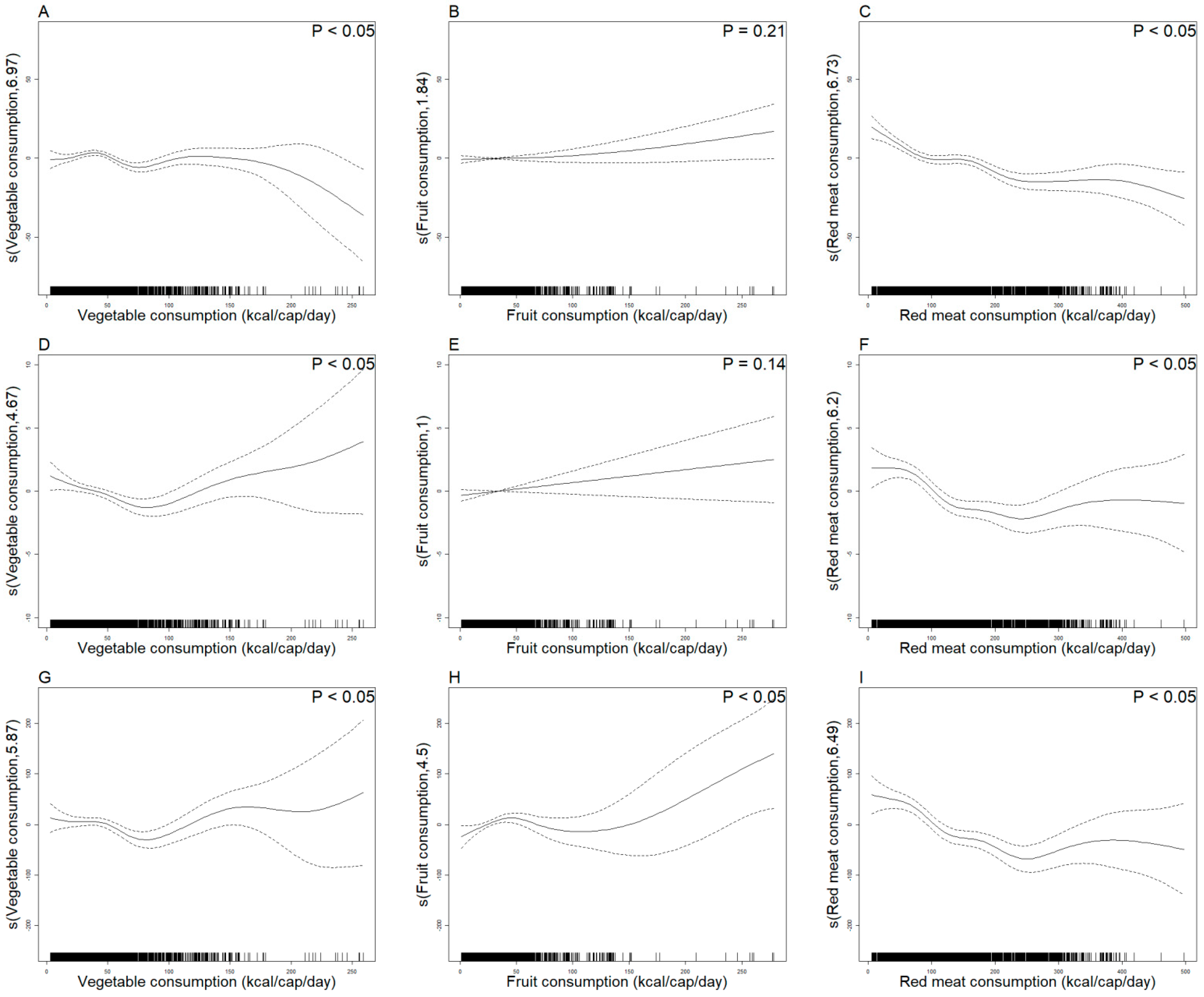
- A inverse correlation was found between vegetable consumption and ASIR, with a "J-shaped" curve between ASMR and ASDR.
- Fruit consumption exhibited a "U-shaped" curve with ASDR.
- Red meat consumption exhibited a negative correlation with ASIR and a "U-shaped" curve with ASMR and ASDR.
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Method
3. Results
3.1. Geographic Distribution of Global Diabetes Disease Burden and Consumption of Vegetables, Fruits, and Red Meat
3.2. Changes in Diabetes Disease Burden and per Capita Vegetable, Fruit, Red Meat Consumption
3.3. Diabetes Disease Burden to per Capita Vegetable, Fruit, Red Meat Consumption by 21 GBD Regions
3.4. GAMM Analyzes the Trend Between Diabetes Disease Burden and Per Capita Vegetable, Fruit, and Red Meat Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AIC | Akaike information criterion |
| ASDR | Age-standardized DALY rate |
| ASIR | Age-standardized incident rate |
| ASMR | Age-standardized mortality rate |
| BIC | Bayesian information criterion |
| BMI | Body mass index |
| DALY | Disability-adjusted life years |
| FAO | Food and Agriculture Organization |
| FBS | Food balance sheet |
| GAM | Generalized additive modeling |
| GAMM | Generalized additive mixed model |
| GBD | Global Burden of Disease Study |
| GI | Glycemic index |
| REML | Restricted maximum likelihood |
| SDI | Socio-demographic index |
References
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Gregg, E.W.; Buckley, J.; Ali, M.K.; Davies, J.; Flood, D.; Mehta, R.; Griffiths, B.; Lim, L.L.; Manne-Goehler, J.; Pearson-Stuttard, J.; et al. Improving health outcomes of people with diabetes: Target setting for the WHO Global Diabetes Compact. Lancet 2023, 401, 1302–1312. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Ying, X.; Li, Z.; Ma, Y.; Army of the Remaining Guards; Wang, D.; Zheng, L.; Zhao, R.; Yuan, Q.; Guo, Y. Disease Burden of Type 2 Diabetes Mellitus Attributable to Dietary Factors in Chinese Residents Aged 15and Above From 1990 to 20. Chin. Circ. J. 2022, 37, 1016–1022. [Google Scholar]
- Lennerz, B.S.; Mey, J.T.; Henn, O.H.; Ludwig, D.S. Behavioral Characteristics and Self-Reported Health Status among 2029 Adults Consuming a “Carnivore Diet”. Curr. Dev. Nutr. 2021, 5, nzab133. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 1 November 2024).
- Jiang, Z.; Sun, T.Y.; He, Y.; Gou, W.; Zuo, L.S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality—A systematic review and meta-analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef]
- Pokharel, P.; Kyrø, C.; Olsen, A.; Tjønneland, A.; Murray, K.; Blekkenhorst, L.C.; Bondonno, C.P.; Hodgson, J.M.; Bondonno, N.P. Vegetable, but Not Potato, Intake Is Associated With a Lower Risk of Type 2 Diabetes in the Danish Diet, Cancer and Health Cohort. Diabetes Care 2023, 46, 286–296. [Google Scholar] [CrossRef]
- Sanders, L.M.; Wilcox, M.L.; Maki, K.C. Red meat consumption and risk factors for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2023, 77, 156–165. [Google Scholar] [CrossRef]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red meat consumption, cardiovascular diseases, and diabetes: A systematic review and meta-analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.Y.; Momenabadi, V.; Faramarzi, A.; Kiani, A. Trends in burden of chronic obstructive pulmonary disease in Iran, 1995–2015: Findings from the global burden of disease study. Arch. Public Health 2020, 78, 45. [Google Scholar] [CrossRef]
- Thar, C.M.; Jackson, R.; Swinburn, B.; Mhurchu, C.N. A review of the uses and reliability of food balance sheets in health research. Nutr. Rev. 2020, 78, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Dutch Food Composition Database (NEVO). (NEVO-Tabel), RIVM/Voedingscentrum. Available online: https://nevo-online.rivm.nl/Home/En (accessed on 1 November 2024).
- Wang, J.; Lin, X.; Bloomgarden, Z.T.; Ning, G. The Jiangnan diet, a healthy diet pattern for Chinese. J. Diabetes 2020, 12, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Z.; Ruan, Y.; Yan, J.; Mukherjee, B.; Yang, F.; Duan, F.; Sun, L.; Liang, R.; Lian, H.; et al. Personal black carbon exposure influences ambulatory blood pressure: Air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension 2014, 63, 871–877. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Li, M.; Wang, T.; Wang, K.; Cao, Q.; Ding, Y.; Xiang, Y.; Wang, S.; Yang, Q.; et al. Diabetes in China part 2: Prevention, challenges, and progress. Lancet Public Health 2024, 9, e1098–e1104. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, Y. Research on the changes and predictions of the burden of type 2 diabetes mellitus in Pacific Island countries from 1990 to 2019. PLoS ONE 2023, 18, e0293681. [Google Scholar] [CrossRef]
- Hickey, G.M.; Unwin, N. Addressing the triple burden of malnutrition in the time of COVID-19 and climate change in Small Island Developing States: What role for improved local food production? Food Secur. 2020, 12, 831–835. [Google Scholar] [CrossRef]
- Gouda, H.N.; Charlson, F.; Sorsdahl, K.; Ahmadzada, S.; Ferrari, A.J.; Erskine, H.; Leung, J.; Santamauro, D.; Lund, C.; Aminde, L.N.; et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Glob. Health 2019, 7, e1375–e1387. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.D.S.; Lopes, S.O.; Araújo, S.P.; Priore, S.E.; Alfenas, R.C.G.; Hermsdorff, H.H.M. Association between food insecurity and cardiometabolic risk in adults and the elderly: A systematic review. J. Glob. Health 2020, 10, 020402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cai, J.; Feng, Y.H.; Su, X.; Chen, S.Y.; Liu, J.Z.; Li, W.L.; Ouyang, R.Q.; Ma, J.R.; Cheng, C.; et al. The association between dietary branched-chain amino acids and the risk of cardiovascular diseases in Chinese patients with type 2 diabetes: A hospital-based case-control study. Front. Nutr. 2022, 9, 999189. [Google Scholar] [CrossRef]
- Mouanness, M.; Nava, H.; Dagher, C.; Merhi, Z. Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review. Nutrients 2022, 14, 3578. [Google Scholar] [CrossRef]
- Lee, S.H.; Moore, L.V.; Park, S.; Harris, D.M.; Blanck, H.M. Adults Meeting Fruit and Vegetable Intake Recommendations—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J. Principles and suggestions on diet and nutrition—Interpretation of dietary guide for adults with diabetes mellitus (2023 edition). Chin. J. Diabetes 2023, 31, 873–876. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, S.; Su, Y.; Ying, C.; Luo, H. Effect of fruit on glucose control in diabetes mellitus: A meta-analysis of nineteen randomized controlled trials. Front. Endocrinol. 2023, 14, 1174545. [Google Scholar] [CrossRef]
- Chen, Y.; Qie, X.; Quan, W.; Zeng, M.; Qin, F.; Chen, J.; Adhikari, B.; He, Z. Omnifarious fruit polyphenols: An omnipotent strategy to prevent and intervene diabetes and related complication? Crit. Rev. Food Sci. Nutr. 2023, 63, 4288–4324. [Google Scholar] [CrossRef]
- Wu, Y.; Wong, C.W.; Chiles, E.N.; Mellinger, A.L.; Bae, H.; Jung, S.; Peterson, T.; Wang, J.; Negrete, M.; Huang, Q.; et al. Glycerate from intestinal fructose metabolism induces islet cell damage and glucose intolerance. Cell Metab. 2022, 34, 1042–1053.e1046. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Tresserra-Rimbau, A.; Martínez-González, M.; Barrubés, L.; Corella, D.; Muñoz-Martínez, J.; Romaguera, D.; Vioque, J.; Alonso-Gómez, M.Á.; et al. Fruit consumption and cardiometabolic risk in the PREDIMED-plus study: A cross-sectional analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.M.; Edirisinghe, I.; Wilcox, M.L.; Burton-Freeman, B.; Xiao, D.; Maki, K.C. A Lean Pork-Containing Breakfast Reduces Hunger and Glycemic Response Compared to a Refined Carbohydrate-Containing Breakfast in Adults with Prediabetes. J. Am. Coll. Nutr. 2018, 37, 293–301. [Google Scholar] [CrossRef]
- He, J.; Fang, A.; Yu, S.; Shen, X.; Li, K. Dietary Nonheme, Heme, and Total Iron Intake and the Risk of Diabetes in Adults: Results From the China Health and Nutrition Survey. Diabetes Care 2020, 43, 776–784. [Google Scholar] [CrossRef]
- Gu, X.; Drouin-Chartier, J.P.; Sacks, F.M.; Hu, F.B.; Rosner, B.; Willett, W.C. Red meat intake and risk of type 2 diabetes in a prospective cohort study of United States females and males. Am. J. Clin. Nutr. 2023, 118, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans 2020–2025. Available online: http://www.dietaryguidelines.gov (accessed on 1 November 2024).
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Palafox, B.; McKee, M.; Balabanova, D.; AlHabib, K.F.; Avezum, A.J.; Bahonar, A.; Ismail, N.; Chifamba, J.; Chow, C.K.; Corsi, D.J.; et al. Wealth and cardiovascular health: A cross-sectional study of wealth-related inequalities in the awareness, treatment and control of hypertension in high-, middle- and low-income countries. Int. J. Equity Health 2016, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Health Financing Collaborator Network. Health sector spending and spending on HIV/AIDS, tuberculosis, and malaria, and development assistance for health: Progress towards Sustainable Development Goal 3. Lancet 2020, 396, 693–724. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Gallardo-Gómez, D.; Salazar-Martínez, E.; Alfonso-Rosa, R.M.; Ramos-Munell, J.; Del Pozo-Cruz, J.; Del Pozo Cruz, B.; Álvarez-Barbosa, F. Optimal Dose and Type of Physical Activity to Improve Glycemic Control in People Diagnosed With Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2024, 47, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.F.J. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.Q.; Srivastava, R.; Morvant, J.; Shankar, S.; Srivastava, R.K. Association of Diabetes Mellitus and Alcohol Abuse with Cancer: Molecular Mechanisms and Clinical Significance. Cells 2021, 10, 3077. [Google Scholar] [CrossRef] [PubMed]

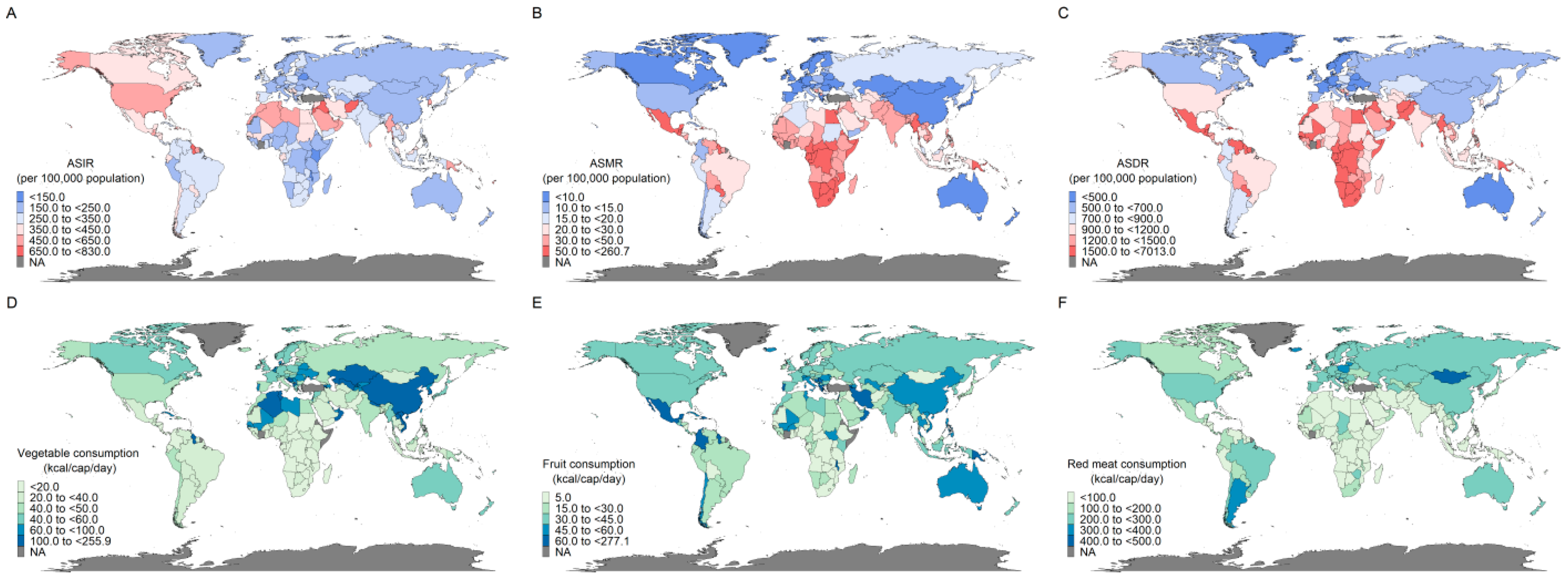
| Region | 2010 | 2021 | Relative Value of Change (%) b | Pattern | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASIR | ASMR | ASDR | ASIR | ASMR | ASDR | ASIR | ASMR | ASDR | ||
| Global | 237.94 | 19.12 | 803.41 | 287.31 | 19.61 | 916.25 | 20.80 | 2.60 | 14.00 | \ |
| Andean Latin America | 771.21 | 96.42 | 3306.76 | 930.48 | 89.31 | 3433.61 | 20.80 | −70.90 | 97.20 | 4 |
| Australasia | 341.80 | 20.04 | 885.57 | 429.95 | 16.16 | 969.10 | 15.00 | −24.70 | −46.90 | 2 |
| Caribbean | 8613.66 | 1171.72 | 41,557.15 | 9840.88 | 1055.52 | 42,079.17 | 22.70 | 286.60 | −11.40 | 3 |
| Central Asia | 1879.00 | 179.84 | 7939.18 | 2350.52 | 156.66 | 8163.55 | 13.20 | −64.50 | 32.70 | 4 |
| Central Europe | 3347.95 | 207.59 | 9731.14 | 3719.62 | 213.79 | 10,401.60 | 13.70 | −25.90 | 60.40 | 4 |
| Central Latin America | 2900.18 | 302.04 | 12,207.56 | 3331.36 | 337.78 | 14,115.18 | 21.10 | 86.80 | 7.50 | 1 |
| Central Sub-Saharan Africa | 1619.17 | 352.13 | 10,678.62 | 1894.98 | 366.75 | 11,557.65 | 24.70 | −81.20 | 10.20 | 4 |
| East Asia | 657.71 | 56.17 | 2271.89 | 782.99 | 48.07 | 2354.17 | 28.00 | 50.00 | −6.60 | 3 |
| Eastern Europe | 1105.74 | 31.25 | 2704.18 | 1360.24 | 68.57 | 4008.63 | 18.70 | 79.50 | −5.00 | 3 |
| Eastern Sub-Saharan Africa | 2452.37 | 688.16 | 19,027.46 | 2807.69 | 686.88 | 19,575.82 | 25.70 | 293.90 | 113.90 | 1 |
| High-income Asia Pacific | 1427.55 | 95.14 | 4150.01 | 1815.18 | 67.30 | 4298.09 | 19.50 | −69.50 | 116.60 | 4 |
| High-income North America | 720.94 | 37.90 | 1796.89 | 1031.02 | 30.16 | 2120.67 | 33.50 | 76.00 | −41.60 | 3 |
| North Africa and Middle East | 8949.61 | 892.83 | 31,445.52 | 11,193.22 | 801.01 | 33,656.39 | 21.10 | −77.30 | −86.50 | 2 |
| Oceania | 10,355.97 | 2109.40 | 67,391.16 | 11,905.06 | 2101.08 | 70,709.09 | 26.00 | 120.00 | 384.20 | 1 |
| South Asia | 1226.03 | 167.78 | 5464.40 | 1477.91 | 178.23 | 6160.72 | 8.30 | 1266.80 | 3.00 | 1 |
| Southeast Asia | 4134.20 | 504.79 | 17,626.73 | 5148.67 | 493.28 | 19,133.57 | 20.90 | −15.10 | 13.20 | 4 |
| Southern Latin America | 749.43 | 52.72 | 2124.25 | 955.58 | 44.02 | 2260.86 | 36.70 | −24.50 | 48.50 | 4 |
| Southern Sub-Saharan Africa | 1613.00 | 556.98 | 14,463.18 | 1896.95 | 493.35 | 13,606.41 | 19.10 | −4.40 | −16.70 | 2 |
| Tropical Latin America | 564.35 | 87.10 | 2833.34 | 647.93 | 83.53 | 2923.87 | 17.80 | 122.40 | −27.90 | 3 |
| Western Europe | 4876.75 | 265.85 | 11,773.05 | 6014.55 | 206.43 | 12,554.87 | 13.60 | 239.80 | 131.70 | 1 |
| Western Sub-Saharan Africa | 4367.65 | 731.09 | 23,206.38 | 5154.96 | 745.13 | 24,987.03 | 13.40 | −86.20 | −17.20 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Wang, J.; Jin, L.; Zhang, L.; Fang, Y. Longitudinal Association Between the Consumption of Vegetables, Fruits, and Red Meat and Diabetes Disease Burden: An Analysis of Multiple Global Datasets. Nutrients 2025, 17, 1256. https://doi.org/10.3390/nu17071256
Yuan M, Wang J, Jin L, Zhang L, Fang Y. Longitudinal Association Between the Consumption of Vegetables, Fruits, and Red Meat and Diabetes Disease Burden: An Analysis of Multiple Global Datasets. Nutrients. 2025; 17(7):1256. https://doi.org/10.3390/nu17071256
Chicago/Turabian StyleYuan, Manqiong, Juan Wang, Lifen Jin, Liangwen Zhang, and Ya Fang. 2025. "Longitudinal Association Between the Consumption of Vegetables, Fruits, and Red Meat and Diabetes Disease Burden: An Analysis of Multiple Global Datasets" Nutrients 17, no. 7: 1256. https://doi.org/10.3390/nu17071256
APA StyleYuan, M., Wang, J., Jin, L., Zhang, L., & Fang, Y. (2025). Longitudinal Association Between the Consumption of Vegetables, Fruits, and Red Meat and Diabetes Disease Burden: An Analysis of Multiple Global Datasets. Nutrients, 17(7), 1256. https://doi.org/10.3390/nu17071256






