Considerable Variation in Intake of Live Food Microbes in Dutch Adults
Abstract
1. Introduction
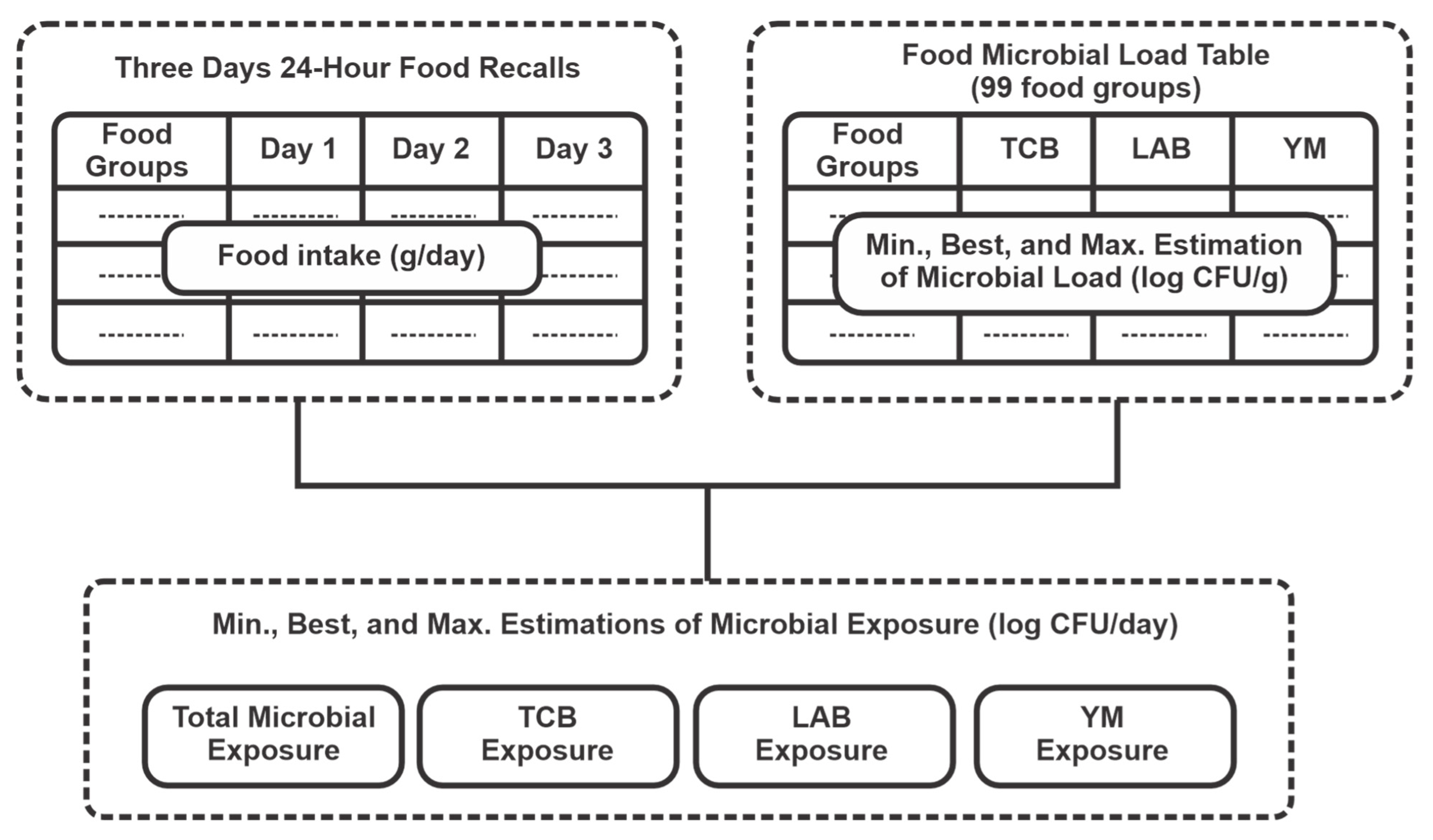
2. Materials and Methods
2.1. Study Population and Consumption Data
2.2. Compilation of a Food Microbial Content Table
2.3. Estimation of Food Microbial Intake in the Study Population
2.4. Identification of Major Contributing Foods to Total Microbial Intake and Its Variation
2.5. Collection of 24 h Duplicate Foods
2.6. Evaluation of Estimated Food Microbial Intake Using Conventional Culturing Techniques (Plate Counting) of Duplicate Foods
2.7. Statistics
3. Results
3.1. Estimated Individual Food Microbial Intake at Minimum, Best, and Maximum Levels
3.2. Estimated Intraindividual Variation in Food Microbial Intake at Minimum, Best, and Maximum Level
3.3. Determination of Food Microbial Content of 24 h Duplicate Foods Using Plate Counting as a Reference
3.4. Agreement Between Estimated and Measured Microbial Content
3.5. Identification of Foods Contributing Most to the Level and Variation of Microbial Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBC | total contaminating bacteria |
| LAB | lactic acid bacteria |
| YM | yeasts/moulds |
| CFU | colony-forming units |
| 24-HDRs | 24 h dietary recalls |
| NEVO | Dutch food composition table |
| PPS | Peptone Physiological Salt Solution |
| MRSA | Man Rogosa Sharpe Agar |
| PCA | Plate Count Agar |
| OGYE | Oxytetracycline Glucose Yeast Extract Agar |
References
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- Nauta, A.J.; Ben Amor, K.; Knol, J.; Garssen, J.; van der Beek, E.M. Relevance of pre- and postnatal nutrition to development and interplay between the gut microbiome and metabolic and immune systems. Am. J. Clin. Nutr. 2013, 98, 586S–593S. [Google Scholar] [PubMed]
- Von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. WAO Special Committee on Climate Change and Biodiversity. The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 467–485. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652. [Google Scholar]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar]
- Lambrecht, B.N.; Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar]
- Carlino, N.; Blanco-Míguez, A.; Punčochář, M.; Mengoni, C.; Pinto, F.; Tatti, A.; Manghi, P.; Armanini, F.; Avagliano, M.; Barcenilla, C.; et al. Unexplored microbial diversity from 2,500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795.e15. [Google Scholar] [CrossRef]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Mitchell, P.C., Translator; William Heinemann: London, UK, 1907. [Google Scholar]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Breton, J.; Daniel, C.; Foligné, B. Maintaining gut ecosystems for health: Are transitory food bugs stowaways or part of the crew? Int. J. Food Microbiol. 2015, 213, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Hill, C.; Tancredi, D.J.; Cifelli, C.J.; Slavin, J.L.; Gahche, J.; Marco, M.L.; Hutkins, R.; Fulgoni, V.L.; Merenstein, D.; Sanders, M.E. Positive health outcomes associated with live microbe intake from foods, including fermented foods, Assessed using the NHANES Database. J. Nutr. 2023, 153, 1143–1149. [Google Scholar] [CrossRef]
- Iyer, A.; Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. The impact of live dietary microbes on health: A scoping review. J. Food Sci. 2024, 89, 773–792. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Lang, J.M.; Eisen, J.A.; Zivkovic, A.M. The microbes we eat: Abundance and taxonomy of microbes consumed in a day’s worth of meals for three diet types. PeerJ 2014, 2, e659. [Google Scholar] [CrossRef]
- Zuccotti, G.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Italian Society of Neonatology. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371. [Google Scholar] [PubMed]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.K.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11, CD006135. [Google Scholar] [PubMed]
- Roduit, C.; Frei, R.; Loss, G.; Büchele, G.; Weber, J.; Depner, M.; Loeliger, S.; Dalphin, M.L.; Roponen, M.; Hyvärinen, A.; et al. Development of atopic dermatitis according to age of onset and association with early-life exposures. J. Allergy Clin. Immunol. 2012, 130, 130–136. [Google Scholar] [PubMed]
- Nicklaus, S.; Divaret-Chauveau, A.; Chardon, M.L.; Roduit, C.; Kaulek, V.; Ksiazek, E.; Dalphin, M.; Karvonen, A.M.; Kirjavainen, P.; Pekkanen, J.; et al. The protective effect of cheese consumption at 18 months on allergic diseases in the first 6 years. Allergy 2018, 74, 788–798. [Google Scholar]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Mills, E.N.C.; Margetts, B.M.; Roberts, G. Diet and food allergy development during infancy: Birth cohort study findings using prospective food diary data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar]
- Marco, M.L.; Hill, C.; Hutkins, R.; Slavin, J.; Tancredi, D.J.; Merenstein, D.; Sanders, M.E. Should there be a recommended daily intake of microbes? J. Nutr. 2020, 150, 3061–3067. [Google Scholar]
- Marco, M.L.; Hutkins, R.; Hill, C.; Fulgoni, V.L.; Cifelli, C.J.; Gahche, J.; Slavin, J.L.; Merenstein, D.; Tancredi, D.J.; E Sanders, M. A classification system for defining and estimating dietary intake of live microbes in US adults and children. J. Nutr. 2022, 152, 1729–1736. [Google Scholar]
- Trijsburg, L.; de Vries, J.H.M.; Boshuizen, H.C.; Hulshof, P.J.M.; Hollman, P.C.H.; van’t Veer, P.; Geelen, A. Comparison of duplicate portion and 24 h recall as reference methods for validating a FFQ using urinary markers as the estimate of true intake. Br. J. Nutr. 2015, 114, 1304–1312. [Google Scholar]
- National Institute for Public Health and the Environment. Dutch Food Composition Database; Nevo: Bilthoven, The Netherlands, 2012.
- Ocké, M.C.; Hulshof, K.F.A.M.; van Rossum, C.T.M. The Dutch National Food Consumption Survey 2003. Arch Public Health 2005, 63, 227–241. [Google Scholar]
- Adams, M.R.; Moss, M.O. Food Microbiology, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- Catellani, P.; Giaccone, V.; Novelli, E. Microbial flora of freshly-produced vegetables: A preliminary report. Vet. Res. Commun. 2006, 30, 345–347. [Google Scholar]
- Molag, M.L.; de Vries, J.H.M.; Duif, N.; Ocké, M.C.; Dagnelie, P.C.; Goldbohm, R.A.; van’t Veer, P. Selecting informative food items for compiling food-frequency questionnaires: Comparison of procedures. Br. J. Nutr. 2010, 104, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Poslusna, K.; Ruprich, J.; de Vries, J.H.M.; Jakubikova, M.; Van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101 (Suppl. S2), S73–S85. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Southgate, D.A.T.; Biessy, C.; Chajès, V.; van Bakel, M.M.E.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S4), S206–S225. [Google Scholar] [CrossRef] [PubMed]

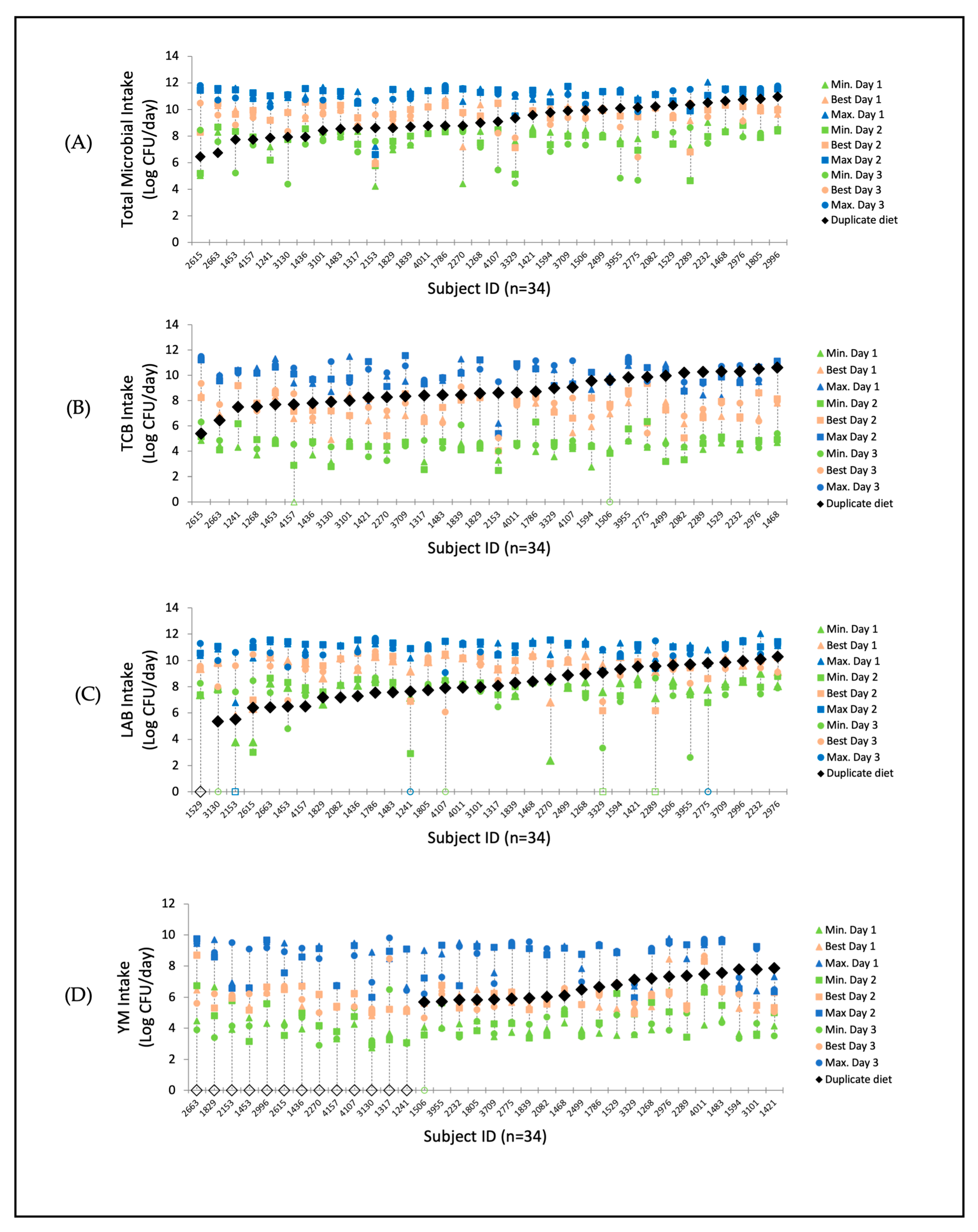

| A. Mean of Estimated Individual Food Microbial Intake Based on Three 24 hRs (n = 34) | ||||
|---|---|---|---|---|
| Group of Microbes | Day | Mean and Range of Microbial Exposure (Log CFU/Day) | ||
| Minimum Estimation | Best Estimation | Maximum Estimation | ||
| Total Contaminating Bacteria (TCB) | 1 | 4.2 (0–4.9) Aa | 7.0 (4.9–8.5) Ba | 9.8 (6.2–11.5) Ca |
| 2 | 4.5 (2.5–6.4) Aab | 7.3 (0.3–9.4) Bab | 10.1 (5.4–11.6) Cab | |
| 3 | 4.6 (0–6.3) Ab | 7.6 (5.1–9.4) Bb | 10.4 (9.3–11.5) Cb | |
| Average | 4.4 (2.5–5.6) * | 7.3 (4.8–8.7) * | 10.1 (7.0–11.4) * | |
| Lactic acid bacteria (LAB) | 1 | 7.4 (0–9.0) Aa | 9.4 (5.8–10.5) Ba | 10.9 (6.8–12.0) Ca |
| 2 | 7.3 (0–8.8) Aa | 9.3 (0–10.5) Ba | 10.7 (0–11.6) Ca | |
| 3 | 6.5 (0–9.1) Aa | 8.7 (0–10.7) Ba | 10.1 (0–11.7) Ca | |
| Average | 7.0 (3.4–8.5) | 9.2 (5.1–10.4) * | 10.6 (5.8–11.5) | |
| Yeasts and Mould (YM) | 1 | 4.1 (2.7–6.7) Aa | 6.0 (4.8–8.7) Ba | 8.1 (4.4–9.8) Ca |
| 2 | 4.5 (2.9–6.7) Aa | 6.0 (5.0–8.7) Ba | 8.3 (3.9–9.7) Ca | |
| 3 | 4.1 (0–9.4) Aa | 6.0 (4.7–8.7) Ba | 8.3 (4.5–9.8) Ca | |
| Average | 4.2 (2.6–5.7) | 6.0 (5.0–7.8) * | 8.4 (6.5–9.6) * | |
| Total microbial exposure | 1 | 7.7 (4.2–9.0) Aa | 9.6 (6.2–10.5) Ba | 11.1 (7.2–12.0) Ca |
| 2 | 7.6 (4.6–8.8) Aa | 9.6 (5.9–10.5) Ba | 11.0 (6.6–11.7) Ca | |
| 3 | 7.2 (4.4–8.7) Aa | 9.4 (6.4–10.7) Ba | 11.1 (9.8–11.8) Ca | |
| Average | 7.5 (5.7–8.5) | 9.5 (7.2–10.4) * | 11.1 (8.2–11.6) * | |
| B. Mean of Measured Food Microbial Intake Based on One Day Duplicate Portion per Person (n = 34) | ||||
| Group of Microbes | Mean, Median, and Range of Microbial Exposure (Log CFU/day) | |||
| Total aerobic bacteria (TAB) ** | 10.0; 8.6; 5.4–11.0 | |||
| Lactic acid bacteria (LAB) | 9.4; 8.1; Appr. 4–10.3 *** | |||
| Yeasts and moulds (YM) | 7.0; 5.6; Appr. 4–7.9 *** | |||
| Total food microbial intake | 10.1; 9.0; 6.4–11.0 | |||
| Food Items | Contribution to Exposure Level (%) | Contribution to Variance (%) | ||
|---|---|---|---|---|
| Best | Max. | Best | Max. | |
| Intake of LAB | ||||
| Yoghurt | 43.4 | 25.6 | 65.8 | 24.8 |
| Cheese (excl. mould cheese) | 35.5 | 21.0 | 15.0 | 5.6 |
| Quark, fresh cheese | 14.0 | 8.3 | 17.1 | 6.4 |
| Buttermilk | 3.6 | 21.1 | 1.5 | 55.4 |
| Drink yoghurt | 2.5 | 1.5 | 0.6 | 0.2 |
| Salami and other fermented meats | 0.6 | 3.7 | 0.0 | 1.2 |
| Mould cheese | 0.3 | 1.4 | 0.0 | 0.3 |
| Meat products (Sliced meats) | 0.0 | 16.2 | 0.0 | 6.0 |
| Milk | 0.0 | 0.8 | 0.0 | 0.0 |
| Intake of TCB | ||||
| Raw fish | 51.5 | 1.6 | 89.8 | 0.2 |
| Raw vegetables | 27.3 | 83.9 | 4.0 | 97.8 |
| Cold meals (uncooked) | 14.5 | 4.4 | 5.8 | 1.4 |
| Meat products (Sliced meats) | 1.4 | 4.4 | 0.0 | 0.2 |
| Raw milk | 1.2 | 0.4 | 0.2 | 0.1 |
| Milk | 0.7 | 2.2 | 0.0 | 0.1 |
| Vegetarian meat on bread | 0.3 | 0.1 | 0.0 | 0.0 |
| Cut vegetables | 0.2 | 0.5 | 0.0 | 0.1 |
| Unpeeled fruits (fresh) | 0.0 | 1.1 | 0.0 | 0.0 |
| Peeled fruits (fresh) | 0.0 | 1.2 | 0.0 | 0.0 |
| Intake of YM | ||||
| Mould cheese | 93.3 | 13.5 | 100.0 | 28.7 |
| Unpeeled fruits | 2.6 | 37.6 | 0.0 | 31.1 |
| Fruits juice | 2.5 | 0.4 | 0.0 | 0.0 |
| Bread and bread substitutes (excl. sourdough) | 0.6 | 0.1 | 0.0 | 0.0 |
| Peeled fruits | 0.3 | 40.6 | 0.0 | 35.9 |
| Savory bread salad | 0.2 | 0 | 0.0 | 0.0 |
| Fruit salad (excl. canned fruits) | 0.1 | 1.2 | 0.0 | 1.2 |
| Beer, cider | 0.0 | 6.4 | 0.0 | 2.9 |
| Margarines | 0.0 | 0.1 | 0.0 | 0.0 |
| Sample Menu with Low Microbial Content | Sample Menu with High Microbial Content |
|---|---|
| Breakfast: | Breakfast: |
| UHT milk, breakfast cereals | Fresh milk, muesli, |
| Crackers | fresh fruit |
| Cheese spread, jam | |
| Lunch: | Lunch: |
| Toasted or bread microwaved for defrosting | Two-day old fresh bread |
| Canned fish | Raw fish, salami |
| Canned meat | Fresh salad, sprouts |
| Ultra-processed foods, e.g., pizza/hot dog | Cheese, mould cheese |
| Snacks: | Snacks: |
| Chips, confectionery | Fresh fruits, fresh olives, |
| Soda | Fresh orange juice |
| Chocolate bar | Nuts |
| Dinner: | Dinner: |
| Instant soup | Home cooked soup |
| Ready meals/meat/sauces | Home cooked dinner |
| Canned or jarred vegetables/apple sauce | Fermented vegetables, mushrooms, sauerkraut, home-cooked apple sauce |
| No use of leftovers | Leftovers |
| Ready-to-use pudding | Yoghurt |
| General: | General: |
| Ultra-processed foods | Home-made foods |
| Packages not opened until consumption and consumed long before the end of shelf life | Opened packages until the end of shelf life in the fridge |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlieg-Boerstra, B.J.; de Vries, J.H.M.; Yulviatun, A.; Zwietering, M.H. Considerable Variation in Intake of Live Food Microbes in Dutch Adults. Nutrients 2025, 17, 1248. https://doi.org/10.3390/nu17071248
Vlieg-Boerstra BJ, de Vries JHM, Yulviatun A, Zwietering MH. Considerable Variation in Intake of Live Food Microbes in Dutch Adults. Nutrients. 2025; 17(7):1248. https://doi.org/10.3390/nu17071248
Chicago/Turabian StyleVlieg-Boerstra, Berber J., Jeanne H. M. de Vries, Anastriyani Yulviatun, and Marcel H. Zwietering. 2025. "Considerable Variation in Intake of Live Food Microbes in Dutch Adults" Nutrients 17, no. 7: 1248. https://doi.org/10.3390/nu17071248
APA StyleVlieg-Boerstra, B. J., de Vries, J. H. M., Yulviatun, A., & Zwietering, M. H. (2025). Considerable Variation in Intake of Live Food Microbes in Dutch Adults. Nutrients, 17(7), 1248. https://doi.org/10.3390/nu17071248







