Cymodocea nodosa, a Promising Seagrass of Nutraceutical Interest: Overview of Phytochemical Constituents and Potential Therapeutic Uses
Abstract
1. Introduction
2. Chemical Constituents of Cymodocea nodosa
2.1. Mineral and Water Content
2.2. Carbohydrates
2.2.1. Simple Sugars
2.2.2. Polysaccharides
2.3. Fatty Acids
2.4. Polyamines
2.5. Sterols
2.6. Phenols
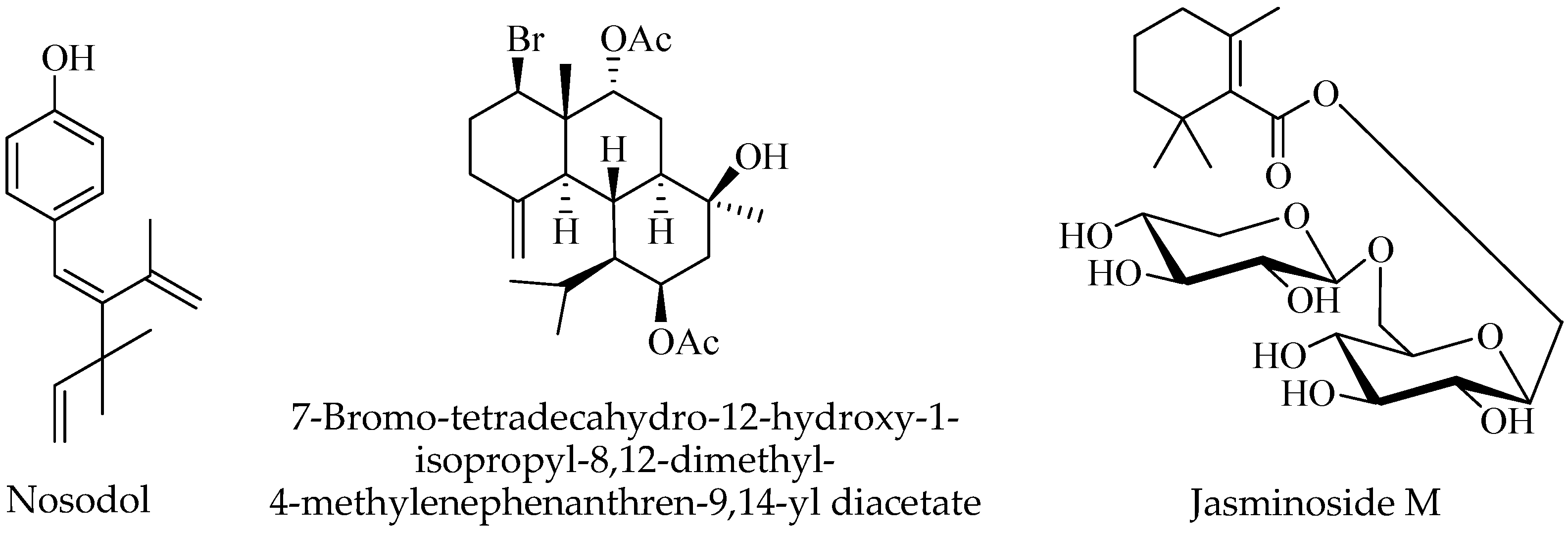
2.7. Terpenoids
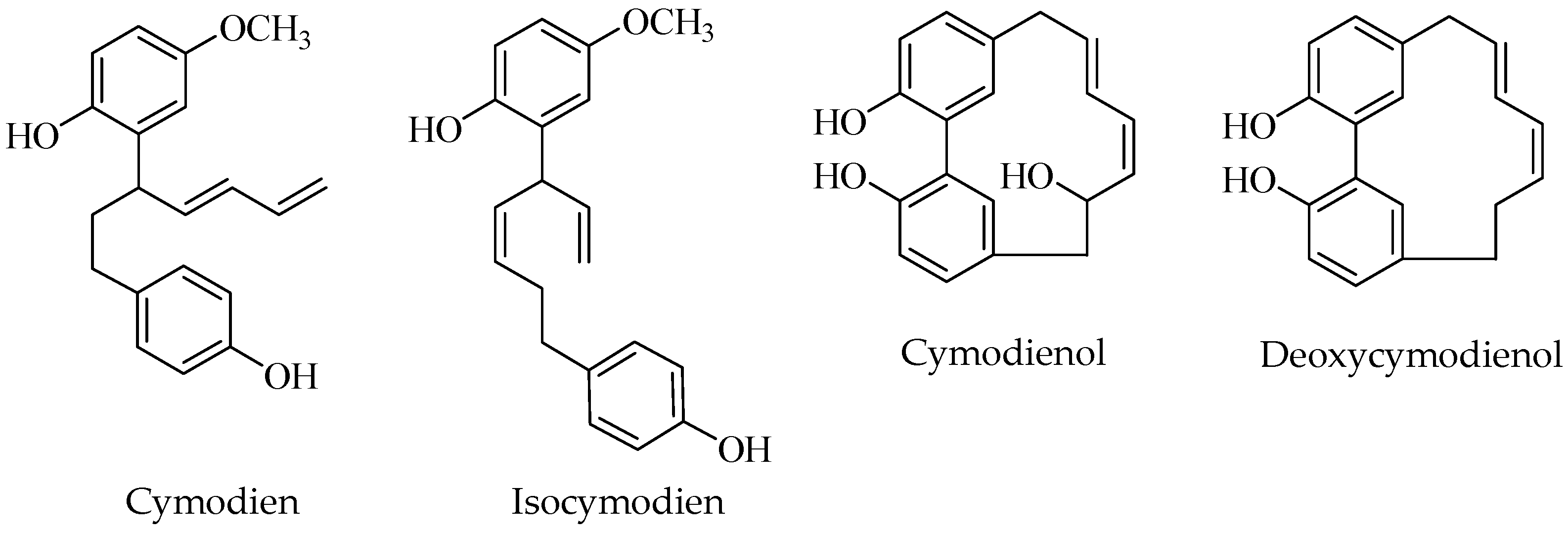
2.8. Diarylheptanoids
2.9. Volatilome
3. Therapeutic Perspectives
3.1. Antioxidant Activity
3.2. Angiotensin Converting Enzyme (ACE) Inhibitory Activity
3.3. Antidiabetic Activity
3.4. Regulation of Lipidic Profile
3.5. Antibacterial and Antifungal Activities
3.6. Antiproliferative Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- den Hartog, C.; Kuo, J. Taxonomy and Biogeography of Seagrasses. In Seagrasses: Biology, Ecologyand Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 1–23. ISBN 978-1-4020-2983-7. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and Negative Effects of Organisms as Physical Ecosystem Engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-66184-3. [Google Scholar]
- Borum, J.; Duarte, C.M.; Krause-Jensen, D.; Greve, T.M. European Seagrasses: An Introduction to Monitoring and Management; Monitoring and Managing of European Seagrasses (M&MS) Project: Copenhagen, Denmark, 2004; ISBN 87-89143-21-3. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The Value of Estuarine and Coastal Ecosystem Services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Montaño, M.N.E.; Bonifacio, R.S.; Rumbaoa, R.G.O. Proximate Analysis of the Flour and Starch from Enhalus Acoroides (L.f.) Royle Seeds. Aquat. Bot. 1999, 65, 321–325. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L.; Brun, F.G. “Sea Rice”: From Traditional Culinary Customs to Sustainable Crop for High-End Gastronomy? Int. J. Gastron. Food Sci. 2023, 34, 100814. [Google Scholar] [CrossRef]
- Shams El Din, N.G.; El-Sherif, Z.M. Nutritional Value of Cymodocea nodosa and Posidonia oceanica along the Western Egyptian Mediterranean Coast. Egypt. J. Aquat. Res. 2013, 39, 153–165. [Google Scholar] [CrossRef]
- Rengasamy, R.R.K.; Radjassegarin, A.; Perumal, A. Seagrasses as Potential Source of Medicinal Food Ingredients: Nutritional Analysis and Multivariate Approach. Biomed. Prev. Nutr. 2013, 3, 375–380. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Salah, H.B.; Saidi, S.A.; Allouche, N.; Belghith, H.; Belghith, K. Evaluation of Nutritional Value, Characteristics, Functional Properties of Cymodocea nodosa and Its Benefits on Health Diseases. Lipids Health Dis. 2017, 16, 238. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S.-W.; Choi, J. Drug Discovery Inspired by Bioactive Small Molecules from Nature. Anim. Cells Syst. 2022, 26, 254–265. [Google Scholar] [CrossRef]
- Ameen, H.M.; Jayadev, A.; Prasad, G.; Nair, D.I. Seagrass Meadows: Prospective Candidates for Bioactive Molecules. Molecules 2024, 29, 4596. [Google Scholar] [CrossRef]
- Mathakala, V.; Ullakula, T.; Palempalli, U.M.D. Seagrass as a Potential Nutraceutical to Decrease Pro-Inflammatory Markers. BMC Complement. Med. Ther. 2024, 24, 260. [Google Scholar] [CrossRef]
- Griffiths, L.L.; Melvin, S.D.; Connolly, R.M.; Pearson, R.M.; Brown, C.J. Metabolomic Indicators for Low-Light Stress in Seagrass. Ecol. Indic. 2020, 114, 106316. [Google Scholar] [CrossRef]
- Jiménez-Ramos, R.; Egea, L.G.; Pérez-Estrada, C.J.; Balart, E.F.; Vergara, J.J.; Brun, F.G. Patch Age Alters Seagrass Response Mechanisms to Herbivory Damage. Mar. Environ. Res. 2024, 197, 106443. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Taberner, M.D.M.; Mir-Rossello, P.M.; Gil, L.; Sureda, A.; Capó, X. Potential Use of Marine Plants as a Source of Bioactive Compounds. Molecules 2025, 30, 485. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.; Seberg, O.; Short, F.T.; Fortes, M.D. Complete Genomic Congruence but Non-monophyly of Cymodocea (Cymodoceaceae), a Small Group of Seagrasses. TAXON 2014, 63, 3–8. [Google Scholar] [CrossRef]
- Balestri, E.; Menicagli, V.; Lardicci, C. Managing Biotic Interactions during Early Seagrass Life Stages to Improve Seed-based Restoration. J. Appl. Ecol. 2021, 58, 2453–2462. [Google Scholar] [CrossRef]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc Uptake, Photosynthetic Efficiency and Oxidative Stress in the Seagrass Cymodocea nodosa Exposed to ZnO Nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef]
- Serrano, R.; Gras, L.; Giménez-Casalduero, F.; del-Pilar-Ruso, Y.; Grindlay, G.; Mora, J. The Role of Cymodocea nodosa on the Dynamics of Trace Elements in Different Marine Environmental Compartments at the Mar Menor Lagoon (Spain). Mar. Pollut. Bull. 2019, 141, 52–60. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Malea, P.; Sperdouli, I.; Panteris, E.; Kokkinidi, D.; Moustakas, M. Evaluation of the Spatiotemporal Effects of Bisphenol A on the Leaves of the Seagrass Cymodocea nodosa. J. Hazard. Mater. 2021, 404, 124001. [Google Scholar] [CrossRef]
- Menicagli, V.; Balestri, E.; Vallerini, F.; De Battisti, D.; Lardicci, C. Plastics and Sedimentation Foster the Spread of a Non-Native Macroalga in Seagrass Meadows. Sci. Total Environ. 2021, 757, 143812. [Google Scholar] [CrossRef]
- Menicagli, V.; Castiglione, M.R.; Balestri, E.; Giorgetti, L.; Bottega, S.; Sorce, C.; Spanò, C.; Lardicci, C. Early Evidence of the Impacts of Microplastic and Nanoplastic Pollution on the Growth and Physiology of the Seagrass Cymodocea nodosa. Sci. Total Environ. 2022, 838, 156514. [Google Scholar] [CrossRef]
- Menicagli, V.; Ruffini Castiglione, M.; Cioni, E.; Spanò, C.; Balestri, E.; De Leo, M.; Bottega, S.; Sorce, C.; Lardicci, C. Stress Responses of the Seagrass Cymodocea nodosa to Environmentally Relevant Concentrations of Pharmaceutical Ibuprofen: Ecological Implications. J. Hazard. Mater. 2024, 476, 135188. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.B.; Yacoubi, L.; Castet, S.; Grégoire, M.; Lin, Y.-J.; Attia, F.; Ayranci, K.; Baki, Z.A.; Courjault-Radé, P.; Rabaoui, L.J. Compartmentation of Trace Metals in Cymodocea nodosa from a Heavily Polluted Area (Central Gulf of Gabes; Southern Mediterranean Sea): Potential Use of the Seagrass as Environmental Monitoring and Bioremediation Tool. Reg. Stud. Mar. Sci. 2023, 65, 103056. [Google Scholar] [CrossRef]
- Llagostera, I.; Pérez, M.; Romero, J. Trace Metal Content in the Seagrass Cymodocea nodosa: Differential Accumulation in Plant Organs. Aquat. Bot. 2011, 95, 124–128. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean Seagrasses Caused by Natural Processes and Anthropogenic Disturbances and Stress: A Critical Review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Turschwell, M.P.; Connolly, R.M.; Dunic, J.C.; Sievers, M.; Buelow, C.A.; Pearson, R.M.; Tulloch, V.J.D.; Côté, I.M.; Unsworth, R.K.F.; Collier, C.J.; et al. Anthropogenic Pressures and Life History Predict Trajectories of Seagrass Meadow Extent at a Global Scale. Proc. Natl. Acad. Sci. USA 2021, 118, e2110802118. [Google Scholar] [CrossRef]
- Losciale, R.; Day, J.C.; Rasheed, M.A.; Heron, S.F. The Vulnerability of World Heritage Seagrass Habitats to Climate Change. Glob. Chang. Biol. 2024, 30, e17113. [Google Scholar] [CrossRef] [PubMed]
- ETS/STE 104, Annex I of the Bern Convention 2002. Available online: https://rm.coe.int/1680304354 (accessed on 7 March 2025).
- UNEP/MAP-SPA/RAC, SAP/RAC: SPA-BD Protocol, Annex II: List of Endangered or Threatened Species. 2018. Available online: https://www.rac-spa.org/sites/default/files/annex/annex_2_en_20182.pdf (accessed on 7 March 2025).
- Grignon-Dubois, M.; Rezzonico, B. The Economic Potential of Beach-Cast Seagrass—Cymodocea nodosa: A Promising Renewable Source of Chicoric Acid. Bot. Mar. 2013, 56, 303–311. [Google Scholar] [CrossRef]
- Zarranz, M.E.; González-Henríquez, N.; García-Jiménez, P.; Robaina, R.R. Restoration of Cymodocea nodosa Seagrass Meadows through Seed Propagation: Germination In Vitro, Seedling Culture and Field Transplants. Bot. Mar. 2010, 53, 173–181. [Google Scholar] [CrossRef]
- Balestri, E.; Lardicci, C. Nursery-propagated Plants from Seed: A Novel Tool to Improve the Effectiveness and Sustainability of Seagrass Restoration. J. Appl. Ecol. 2012, 49, 1426–1435. [Google Scholar] [CrossRef]
- Milović, S.; Stanković, I.; Nikolić, D.; Radović, J.; Kolundžić, M.; Nikolić, V.; Stanojković, T.; Petović, S.; Kundaković-Vasović, T. Chemical Analysis of Selected Seaweeds and Seagrass from the Adriatic Coast of Montenegro. Chem. Biodivers. 2019, 16, e1900327. [Google Scholar] [CrossRef]
- Milović, S.; Kundaković, T.; Macić, V.; Antić-Stanković, J.; Grozdanić, N.; Đuričić, I.; Stanković, I.; Stanojković, T. Anti α-Glucosidase, Antitumour, Antioxidative, Antimicrobial Activity, Nutritive and Health Protective Potential of Some Seaweeds from the Adriatic Coast of Montenegro. Farmacia 2017, 65, 731–740. [Google Scholar]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese Seaweed, Wakame (Undaria pinnatifida) as an Ingredient in Pasta: Chemical, Functional and Structural Evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Drew, E.A. Carbohydrate and Inositol Metabolism in the Seagrass, Cymodocea nodosa. New Phytol. 1978, 81, 249–264. [Google Scholar] [CrossRef]
- Silva, J.; Barrote, I.; Costa, M.M.; Albano, S.; Santos, R. Physiological Responses of Zostera Marina and Cymodocea nodosa to Light-Limitation Stress. PLoS ONE 2013, 8, e81058. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Fakhfakh, J.; Krichen, F.; Jribi, I.; Chiarore, A.; Patti, F.P.; Blecker, C.; Allouche, N.; Belghith, H.; Belghith, K. Structural Characterization and Functional Properties of Antihypertensive Cymodocea nodosa Sulfated Polysaccharide. Carbohydr. Polym. 2016, 151, 511–522. [Google Scholar] [CrossRef]
- Ben Abdallah Kolsi, R.; Ben Gara, A.; Jardak, N.; Chaaben, R.; El Feki, A.; El Feki, L.; Belghith, K. Inhibitory Effects of Cymodocea nodosa Sulphated Polysaccharide on α-Amylase Activity, Liver-Kidney Toxicities and Lipid Profile Disorders in Diabetic Rats. Arch. Physiol. Biochem. 2015, 121, 218–227. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Guihéneuf, F.; Marín-Guirao, L.; Bernardeau-Esteller, J.; García-Muñoz, R.; Stengel, D.B.; Ruiz, J.M. Effects of an Experimental Heat Wave on Fatty Acid Composition in Two Mediterranean Seagrass Species. Mar. Pollut. Bull. 2018, 134, 27–37. [Google Scholar] [CrossRef]
- Marián, F.D.; Garcia-Jimenez, P.; Robaina, R.R. Polyamine Levels in the Seagrass Cymodocea nodosa. Aquat. Bot. 2000, 68, 179–184. [Google Scholar] [CrossRef]
- Iatrides, M.; Artaud, J.; Vicente, N. Sterol Composition of Mediterranean Marine Plants. Oceanol. Acta 1983, 6, 73–77. [Google Scholar]
- Sica, D.; Piccialli, V.; Masullo, A. Configuration at C-24 of Sterols from the Marine Phanerogames Posidonia Oceanica and Cymodocea nodosa. Phytochemistry 1984, 23, 2609–2611. [Google Scholar] [CrossRef]
- Kontiza, I.; Abatis, D.; Malakate, K.; Vagias, C.; Roussis, V. 3-Keto Steroids from the Marine Organisms Dendrophyllia Cornigera and Cymodocea Nodosa. Steroids 2006, 71, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cariello, L.; Zanetti, L.; De Stefano, S. Posidonia Ecosystem—V. Phenolic Compounds from Marine Phanerogames, Cymodocea nodosa and Posidonia oceanica. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 62, 159–161. [Google Scholar] [CrossRef]
- Arnold, T.; Mealey, C.; Leahey, H.; Miller, A.W.; Hall-Spencer, J.M.; Milazzo, M.; Maers, K. Ocean Acidification and the Loss of Phenolic Substances in Marine Plants. PLoS ONE 2012, 7, e35107. [Google Scholar] [CrossRef]
- Ben Abdallah Kolsi, R.; Ben Salah, H.; Jardak, N.; Chaaben, R.; El Feki, A.; Rebai, T.; Jamoussi, K.; Allouche, N.; Belghith, H.; Belghith, K. Effects of Cymodocea nodosa Extract on Metabolic Disorders and Oxidative Stress in Alloxan-Diabetic Rats. Biomed. Pharmacother. 2017, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Smadi, A.; Ciavatta, M.; Bitam, F.; Carbone, M.; Villani, G.; Gavagnin, M. Prenylated Flavonoids and Phenolic Compounds from the Rhizomes of Marine Phanerogam Cymodocea nodosa. Planta Med. 2018, 84, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Chaabani, E.; Mgaidi, S.; Ben Abdennebi, A.; Dakhlaoui, S.; Hammami, M.; Selmi, S.; Zariat, M.; Shili, A.; Merah, O.; Bettaieb Rebey, I. Enhancing Antioxidant Activity from Aquatic Plant Cymodocea nodosa for Cosmetic Formulation Through Optimized Ultrasound-Assisted Extraction Using Response Surface Methodology. Cosmetics 2024, 11, 186. [Google Scholar] [CrossRef]
- Kontiza, I.; Stavri, M.; Zloh, M.; Vagias, C.; Gibbons, S.; Roussis, V. New Metabolites with Antibacterial Activity from the Marine Angiosperm Cymodocea nodosa. Tetrahedron 2008, 64, 1696–1702. [Google Scholar] [CrossRef]
- Alberti, Á.; Riethmüller, E.; Béni, S. Characterization of Diarylheptanoids: An Emerging Class of Bioactive Natural Products. J. Pharm. Biomed. Anal. 2018, 147, 13–34. [Google Scholar] [CrossRef]
- Kontiza, I.; Vagias, C.; Jakupovic, J.; Moreau, D.; Roussakis, C.; Roussis, V. Cymodienol and Cymodiene: New Cytotoxic Diarylheptanoids from the Sea Grass Cymodocea nodosa. Tetrahedron Lett. 2005, 46, 2845–2847. [Google Scholar] [CrossRef]
- Li, Y.; Mangoni, A.; Shulha, O.; Çiçek, S.S.; Zidorn, C. Cyclic Diarylheptanoids Deoxycymodienol and Isotedarene A from Zostera marina (Zosteraceae). Tetrahedron Lett. 2019, 60, 150930. [Google Scholar] [CrossRef]
- Coquin, S.; Ormeno, E.; Pasqualini, V.; Monnier, B.; Culioli, G.; Lecareux, C.; Fernandez, C.; Saunier, A. Chemical Diversity of Mediterranean Seagrasses Volatilome. Metabolites 2024, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible Seaweeds: A Potential Novel Source of Bioactive Metabolites and Nutraceuticals With Human Health Benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. IJMS 2020, 21, 1250. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. IJMS 2020, 21, 7774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Chuang, Y.-C.; Ku, Y.-H. Quantitation of Bioactive Compounds in Citrus Fruits Cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.-P.; Paquot, M.; Blecker, C.; Attia, H. Impact of Extraction Procedures on the Chemical, Rheological and Textural Properties of Ulvan from Ulva Lactuca of Tunisia Coast. Food Hydrocoll. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Mao, W.; Li, H.; Li, Y.; Zhang, H.; Qi, X.; Sun, H.; Chen, Y.; Guo, S. Chemical Characteristic and Anticoagulant Activity of the Sulfated Polysaccharide Isolated from Monostroma Latissimum (Chlorophyta). Int. J. Biol. Macromol. 2009, 44, 70–74. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Gargouri, B.; Sassi, S.; Frikha, D.; Lassoued, S.; Belghith, K. In Vitro Biological Properties and Health Benefits of a Novel Sulfated Polysaccharide Isolated from Cymodocea Nodosa. Lipids Health Dis. 2017, 16, 252. [Google Scholar] [CrossRef]
- Li, E.C.; Heran, B.S.; Wright, J.M. Angiotensin Converting Enzyme (ACE) Inhibitors versus Angiotensin Receptor Blockers for Primary Hypertension. Cochrane Database Syst. Rev. 2014, 2014, CD009096. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and Enzyme Inhibitory Properties of Finger Millet (Eleusine coracana L.) Seed Coat Phenolics: Mode of Inhibition of α-Glucosidase and Pancreatic Amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Wu, Z.; Wang, X. Chicoric Acid Attenuates Hyperglycemia-Induced Endothelial Dysfunction through AMPK-Dependent Inhibition of Oxidative/Nitrative Stresses. J. Recept. Signal Transduct. 2021, 41, 378–392. [Google Scholar] [CrossRef]
- Arab Sadeghabadi, Z.; Abbasalipourkabir, R.; Mohseni, R.; Ziamajidi, N. Chicoric Acid Does Not Restore Palmitate-Induced Decrease in Irisin Levels in PBMCs of Newly Diagnosed Patients with T2D and Healthy Subjects. Arch. Physiol. Biochem. 2022, 128, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, H.; Jung, C.H.; Lee, S.-J.; Ha, T.-Y.; Ahn, J. Chicoric Acid Mitigates Impaired Insulin Sensitivity by Improving Mitochondrial Function. Biosci. Biotechnol. Biochem. 2018, 82, 1197–1206. [Google Scholar] [CrossRef]
- Sadeghabadi, Z.A.; Ziamajidi, N.; Abbasalipourkabir, R.; Mohseni, R.; Borzouei, S. Palmitate-Induced IL6 Expression Ameliorated by Chicoric Acid through AMPK and SIRT1-Mediated Pathway in the PBMCs of Newly Diagnosed Type 2 Diabetes Patients and Healthy Subjects. Cytokine 2019, 116, 106–114. [Google Scholar] [CrossRef]
- Idres, A.Y.; Tousch, D.; Dhuyque-Mayer, C.; Hammad, I.; Lambert, K.; Cazals, G.; Portet, K.; Ferrare, K.; Bidel, L.P.R.; Poucheret, P. An Original Asteraceae Based Infused Drink Prevents Metabolic Syndrome in Fructose-Rat Model. Antioxidants 2023, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK Signaling in Diabetes Mellitus, Insulin Resistance and Diabetic Complications: A Pre-Clinical and Clinical Investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Ben Abdallah Kolsi, R.; Ben Gara, A.; Chaaben, R.; El Feki, A.; Paolo Patti, F.; El Feki, L.; Belghith, K. Anti-Obesity and Lipid Lowering Effects of Cymodocea nodosa Sulphated Polysaccharide on High Cholesterol-Fed-Rats. Arch. Physiol. Biochem. 2015, 121, 210–217. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A Review of the Antibacterial Activity and Mechanisms of Plant Polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Mohanta, B.; Nayak, A.K.; Dhara, A.K. Plant Polysaccharides as Antiviral Agents. In Viral Infections and Antiviral Therapies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 567–579. ISBN 978-0-323-91814-5. [Google Scholar]
- Vijayabaskar, P.; Vaseela, N.; Thirumaran, G. Potential Antibacterial and Antioxidant Properties of a Sulfated Polysaccharide from the Brown Marine Algae Sargassum Swartzii. Chin. J. Nat. Med. 2012, 10, 421–428. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Krichen, F.; Karoud, W.; Martinez-Alvarez, O.; Ellouz-Chaabouni, S.; Ayadi, M.A.; Bougatef, A. Sulfated Polysaccharides from Loligo Vulgaris Skin: Potential Biological Activities and Partial Purification. Int. J. Biol. Macromol. 2015, 72, 1143–1151. [Google Scholar] [CrossRef] [PubMed]


| Putrescine | Spermidine | Spermine | |
|---|---|---|---|
| Free | 1583 ± 147 | 19 ± 1.4 | 0.8 ± 0.1 |
| Bound-soluble | 25,886 ± 488 | 35 ± 2.05 | 63 ± 2.99 |
| Bound-unsoluble | 7530 ± 136 | 3.29 ± 0.14 | 8.43 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leo, M.; Ciccone, L.; Menicagli, V.; Balestri, E.; Braca, A.; Nieri, P.; Testai, L. Cymodocea nodosa, a Promising Seagrass of Nutraceutical Interest: Overview of Phytochemical Constituents and Potential Therapeutic Uses. Nutrients 2025, 17, 1236. https://doi.org/10.3390/nu17071236
De Leo M, Ciccone L, Menicagli V, Balestri E, Braca A, Nieri P, Testai L. Cymodocea nodosa, a Promising Seagrass of Nutraceutical Interest: Overview of Phytochemical Constituents and Potential Therapeutic Uses. Nutrients. 2025; 17(7):1236. https://doi.org/10.3390/nu17071236
Chicago/Turabian StyleDe Leo, Marinella, Lidia Ciccone, Virginia Menicagli, Elena Balestri, Alessandra Braca, Paola Nieri, and Lara Testai. 2025. "Cymodocea nodosa, a Promising Seagrass of Nutraceutical Interest: Overview of Phytochemical Constituents and Potential Therapeutic Uses" Nutrients 17, no. 7: 1236. https://doi.org/10.3390/nu17071236
APA StyleDe Leo, M., Ciccone, L., Menicagli, V., Balestri, E., Braca, A., Nieri, P., & Testai, L. (2025). Cymodocea nodosa, a Promising Seagrass of Nutraceutical Interest: Overview of Phytochemical Constituents and Potential Therapeutic Uses. Nutrients, 17(7), 1236. https://doi.org/10.3390/nu17071236











