Blueberry Extract and Resistance Training Prevent Left Ventricular Redox Dysregulation and Pathological Remodeling in Experimental Severe Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Materials and Methods
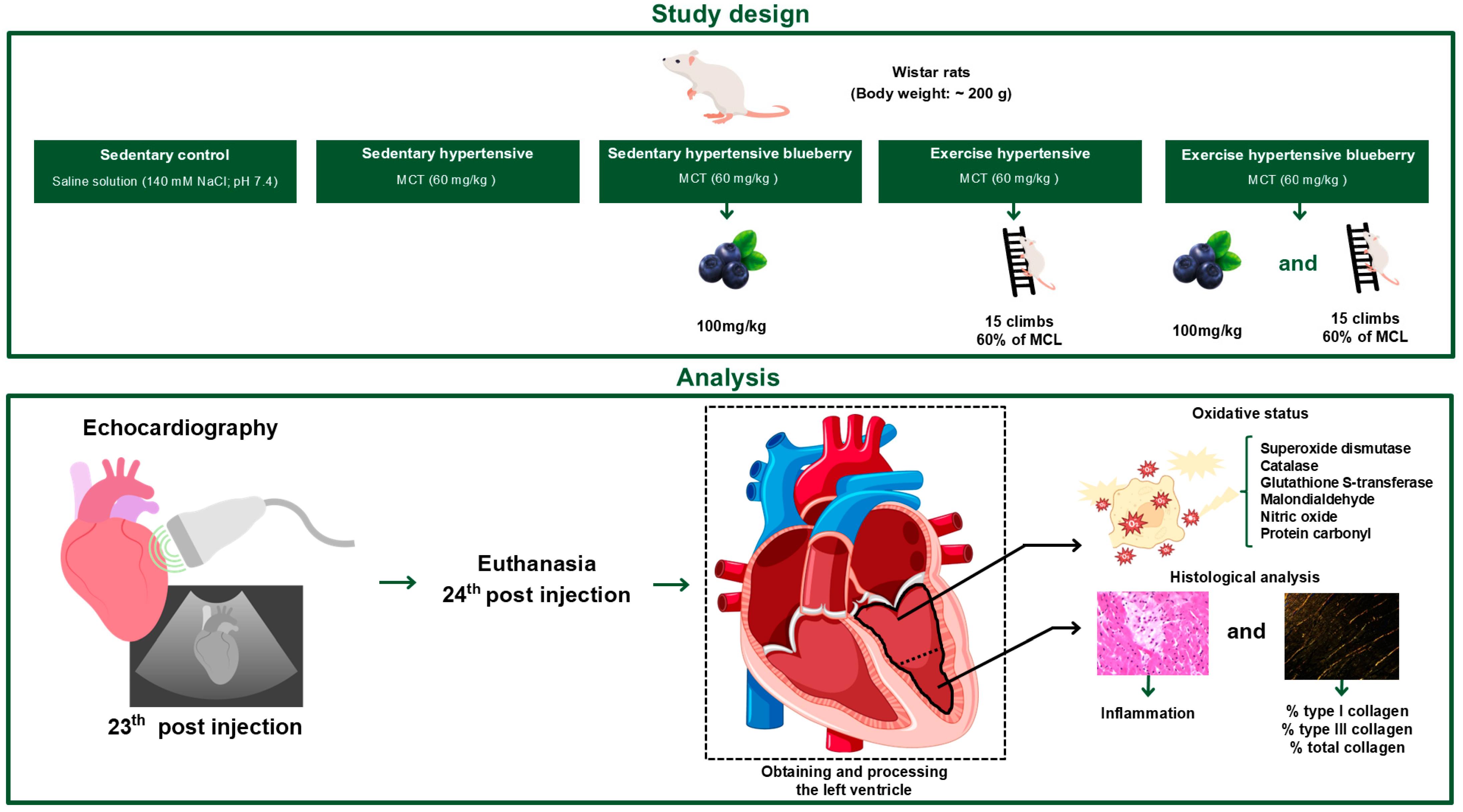
2.1. Animals
2.2. PAH Induction
2.3. Preparation and Characterization of Blueberry Extract
2.4. Composition and Administration of Blueberry Extract
2.5. Blueberry Extract Analysis
2.6. Resistance Exercise Training
2.7. Echocardiography
2.8. Sample Collection
2.9. Histological Processing and Histopathological Analysis
2.10. Quantification of Collagen Fibers
2.11. Evaluation of Antioxidant Enzyme Activity and Oxidative Stress Indicators
2.11.1. Superoxide Dismutase
2.11.2. Catalase
2.11.3. Glutathione S-Transferase
2.11.4. Nitric Oxide
2.11.5. Malondialdehyde
2.11.6. Carbonylated Proteins
2.11.7. Total Protein
2.12. Statistical Analysis
3. Results
3.1. Validation of Pulmonary Hypertension
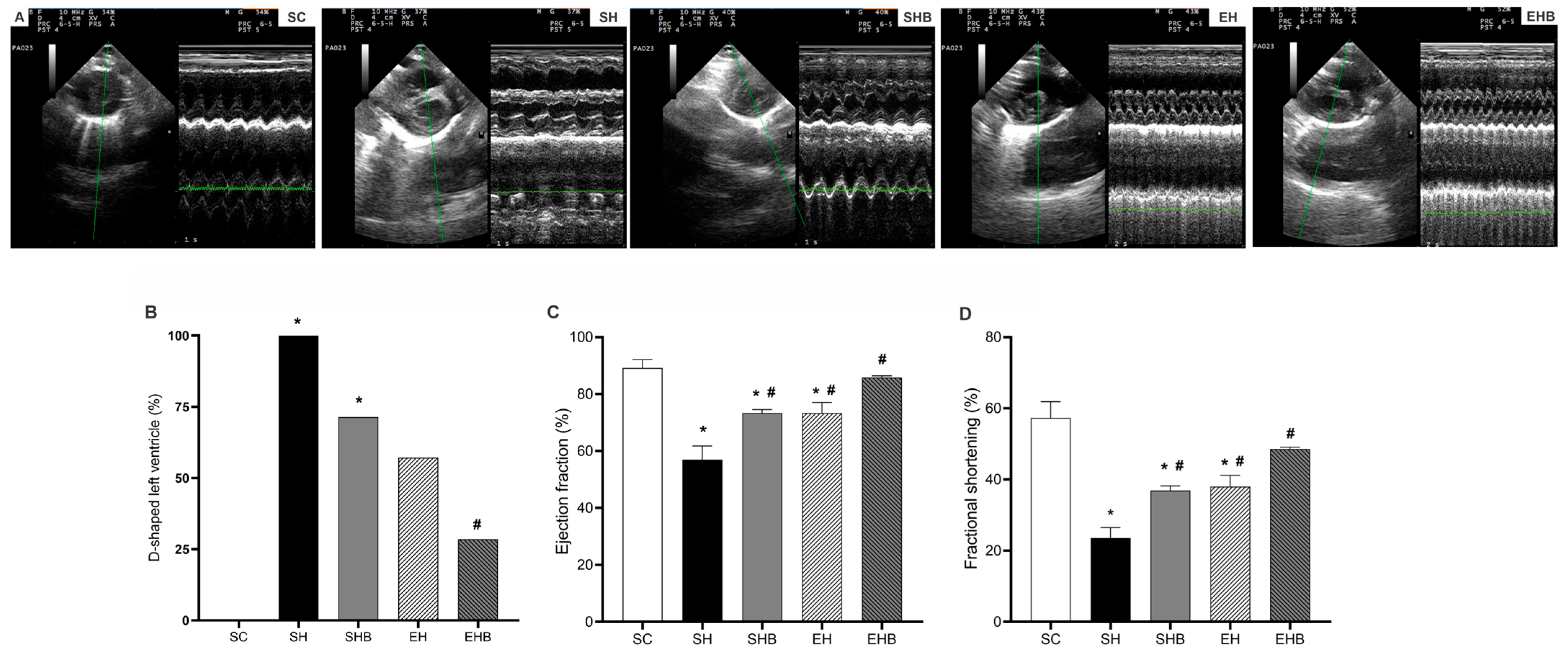
3.2. LV Echocardiographic Data
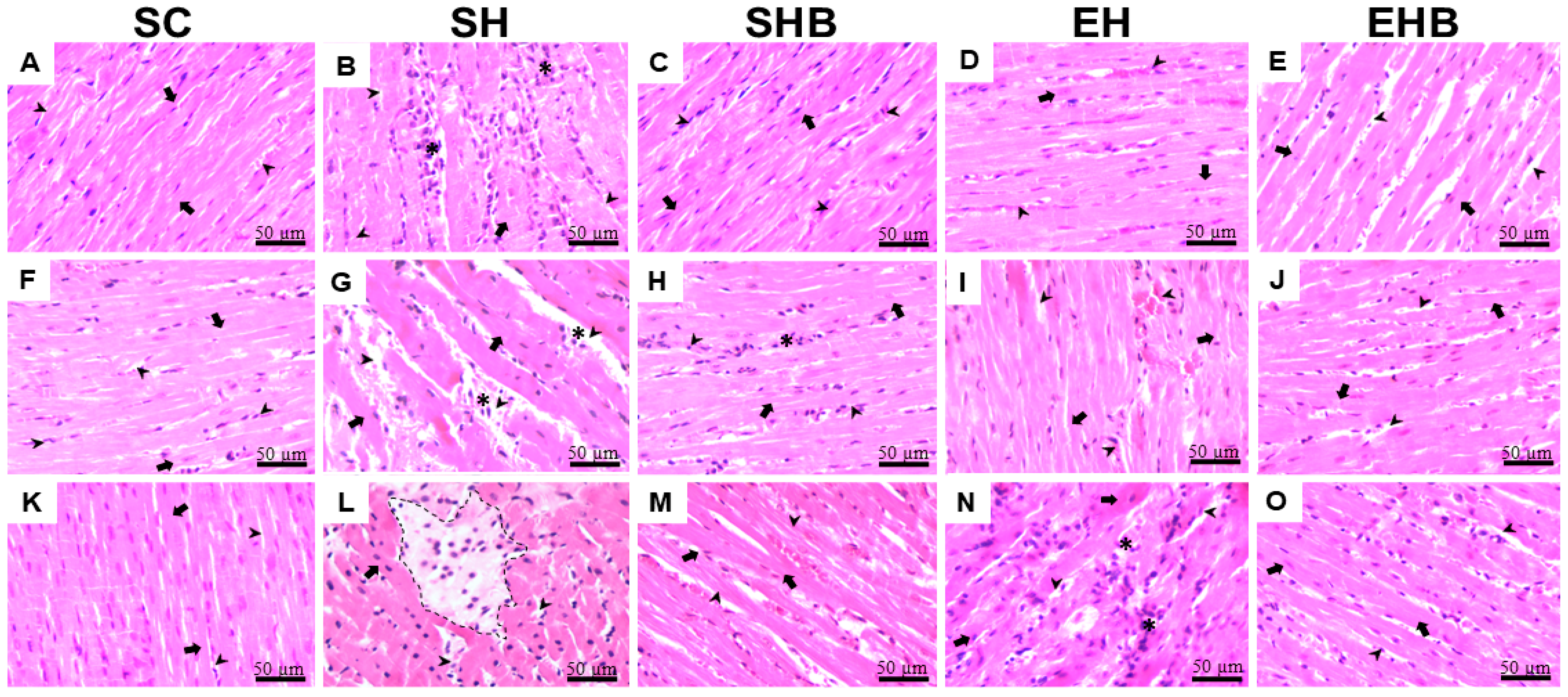
3.3. Morphology and Adverse Remodeling of the LV Tissue
3.4. Oxidative Stress Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maron, B.A. Revised definition of pulmonary hypertension and approach to management: A clinical primer. J. Am. Heart Assoc. 2023, 12, e029024. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, Y.H.; Gou, X.; Li, F.Y.; Yang, X.Y.C.; Li, Y.M.; Chen, F. Oxidative stress and antioxidative therapy in pulmonary arterial hypertension. Molecules 2022, 27, 3724. [Google Scholar] [CrossRef]
- Kishiki, K.; Singh, A.; Narang, A.; Gomberg-Maitland, M.; Goyal, N.; Maffessanti, F.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Addetia, K. Impact of severe pulmonary arterial hypertension on the left heart and prognostic implications. J. Am. Soc. Echocardiogr. 2019, 32, 1128–1137. [Google Scholar] [CrossRef]
- Redout, E.M.; Wagner, M.J.; Zuidwijk, M.J.; Boer, C.; Musters, R.J.; van Hardeveld, C.; Paulus, W.; Simonides, W.S. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc. Res. 2007, 75, 770–781. [Google Scholar] [CrossRef]
- Souza-Rabbo, M.P.; Silva, L.F.; Auzani, J.A.; Picoral, M.; Khaper, N.; Belló-Klein, A. Effects of a chronic exercise training protocol on oxidative stress and right ventricular hypertrophy in monocrotaline-treated rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Redout, E.M.; van der Toorn, A.; Zuidwijk, M.J.; van de Kolk, C.W.; van Echteld, C.J.; Musters, R.J.; van Hardeveld, C.; Paulus, W.J.; Simonides, W.S. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1038–H1047. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.B.; Soares, L.L.; Portes, A.M.O.; Soares, T.I.; da Silva, B.A.F.; Dias, T.R.; Natali, A.J. Combined physical training protects the left ventricle from structural and functional damages in experimental pulmonary arterial hypertension. Clin. Hypertens. 2024, 30, 12. [Google Scholar] [CrossRef]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef]
- Hardegree, E.L.; Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McGoon, M.D.; Kushwaha, S.S.; Hsiao, J.-F.; McCully, R.B.; Oh, J.K.; Pellikka, P.A.; et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am. J. Cardiol. 2013, 111, 143–148. [Google Scholar] [CrossRef]
- Puwanant, S.; Park, M.; Popovic, Z.B.; Tang, W.W.; Farha, S.; George, D.; Sharp, J.; Puntawangkoon, J.; Loyd, J.E.; Erzurum, S.C.; et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010, 121, 259–266. [Google Scholar] [CrossRef]
- Haeck, M.L.; Höke, U.; Marsan, N.A.; Holman, E.R.; Wolterbeek, R.; Bax, J.J.; Schalij, M.J.; Vliegen, H.W.; Delgado, V. Impact of right ventricular dyssynchrony on left ventricular performance in patients with pulmonary hypertension. Int. J. Cardiovasc. Imaging 2014, 30, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Louie, E.K.; Rich, S.; Brundage, B.H. Doppler echocardiography assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J. Am. Coll. Cardiol. 1986, 8, 1298–1306. [Google Scholar] [CrossRef]
- Tji-Joong Gan, C.; Lankhaar, J.W.; Marcus, J.T.; Westerhof, N.; Marques, K.M.; Bronzwaer, J.G.; Boonstra, A.; Postmus, P.E.; Vonk-Noordegraaf, A. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1528–H1533. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.T.; Noordegraaf, A.V.; Roeleveld, R.J.; Postmus, P.E.; Heethaar, R.M.; Van Rossum, A.C.; Boonstra, A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: Noninvasive monitoring using MRI. Chest 2001, 119, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc. Res. 2009, 81, 449–456. [Google Scholar] [CrossRef]
- Hessel, M.H.; Steendijk, P.; den Adel, B.; Schutte, C.I.; van der Laarse, A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2424–H2430. [Google Scholar] [CrossRef]
- Farahmand, F.; Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes in experimental cor pulmonale. Mol. Cell. Biochem. 2004, 260, 21–29. [Google Scholar] [CrossRef]
- Sitbon, O.; Gomberg-Maitland, M.; Granton, J.; Lewis, M.I.; Mathai, S.C.; Rainisio, M.; Stockbridge, N.L.; Wilkins, M.R.; Zamanian, R.T.; Rubin, L.J. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801908. [Google Scholar] [CrossRef]
- Türck, P.; Fraga, S.; Salvador, I.; Campos-Carraro, C.; Lacerda, D.; Bahr, A.; Ortiz, V.; Hickmann, A.; Koetz, M.; Belló-Klein, A.; et al. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition 2020, 70, 110579. [Google Scholar] [CrossRef]
- Türck, P.; Salvador, I.S.; Campos-Carraro, C.; Ortiz, V.; Bahr, A.; Andrades, M.; da Rosa Araujo, A.S. Blueberry extract improves redox balance and functional parameters in the right ventricle from rats with pulmonary arterial hypertension. Eur. J. Nutr. 2022, 61, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Tan, D.H.; Shi, L.; Liu, X.W.; Zhang, Y.B.; Tong, C.T.; Hou, M.X. Blueberry anthocyanins-enriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS ONE 2015, 10, e0127813. [Google Scholar] [CrossRef]
- Jesus Silva, F.; Drummond, F.R.; Fidelis, M.R.; Freitas, M.O.; Leal, T.F.; De Rezende, L.M.T.; de Moura, A.G.M.; Reis, E.C.D.C.; Natali, A.J. Continuous aerobic exercise prevents detrimental remodeling and right heart myocyte contraction and calcium cycling dysfunction in pulmonary artery hypertension. J. Cardiovasc. Pharmacol. 2021, 77, 69–78. [Google Scholar] [CrossRef]
- Natali, A.J.; Fowler, E.D.; Calaghan, S.C.; White, E. Voluntary exercise delays heart failure onset in rats with pulmonary artery hypertension. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H421–H424. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Hornberger Jr, T.A.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef]
- Leite, L.B.; Soares, L.L.; Portes, A.M.O.; da Silva, B.A.F.; Dias, T.R.; Soares, T.I.; Assis, M.Q.; Guimarães-Ervilha, L.O.; Carneiro-Júnior, M.A.; Forte, P.; et al. Combined exercise hinders the progression of pulmonary and right heart harmful remodeling in monocrotaline-induced pulmonary arterial hypertension. J. Appl. Physiol. 2025, 138, 182–194. [Google Scholar] [CrossRef]
- Soares, L.L.; Leite, L.B.; Ervilha, L.O.G.; da Silva, B.A.F.; de Freitas, M.O.; Portes, A.M.O.; Natali, A.J. Resistance exercise training mitigates left ventricular dysfunctions in pulmonary artery hypertension model. Arq. Bras. Cardiol. 2022, 119, 574–584. [Google Scholar]
- Soares, L.L.; Leite, L.B.; Ervilha, L.O.G.; Pelozin, B.R.A.; Pereira, N.P.; da Silva, B.A.F.; Portes, A.M.O.; Drummond, F.R.; de Rezende, L.M.T.; Fernandes, T.; et al. Resistance exercise training benefits pulmonary, cardiac, and muscular structure and function in rats with stable pulmonary artery hypertension. Life Sci. 2023, 332, 122128. [Google Scholar]
- Sahn, D.J.; DeMaria, A.N.T.H.O.N.Y.; Kisslo, J.O.S.E.P.H.; Weyman, A.F. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978, 58, 1072–1083. [Google Scholar] [CrossRef]
- Wang, Z.; Patel, J.R.; Schreier, D.A.; Hacker, T.A.; Moss, R.L.; Chesler, N.C. Organ-level right ventricular dysfunction with preserved Frank-Starling mechanism in a mouse model of pulmonary arterial hypertension. J. Appl. Physiol. 2018, 124, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.F.; de Paiva Coimbra, J.L.; Ervilha, L.O.G.; Bastos, D.S.S.; Cossolin, J.F.S.; Santos, E.C.; de Oliveira, L.L.; Machado-Neves, M. Arsenic induces dose-dependent structural and ultrastructural pathological remodeling in the heart of Wistar rats. Life Sci. 2020, 257, 118132. [Google Scholar] [CrossRef]
- Novaes, R.D.; Mouro, V.G.; Gonçalves, R.V.; Mendonça, A.A.; Santos, E.C.; Fialho, M.C.; Machado-Neves, M. Aluminum: A potentially toxic metal with dose-dependent effects on cardiac bioaccumulation, mineral distribution, DNA oxidation and microstructural remodeling. Environ. Pollut. 2018, 242, 814–826. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Ramírez-Tortosa, C.L.; Varela-López, A.; Romero-Márquez, J.M.; Ochoa, J.J.; Ramírez-Tortosa, M.; Forbes-Hernández, T.Y.; Granados-Principal, S.; Battino, M.; Quiles, J.L. Heart histopathology and mitochondrial ultrastructure in aged rats fed for 24 months on different unsaturated fats (virgin olive oil, sunflower oil or fish oil) and affected by different longevity. Nutrients 2019, 11, 2390. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Levine, R.L. Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 246–257. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.; McLay, J.; Chopra, M.; Bridges, A.; Belch, J.J.F. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am. J. Cardiol. 1990, 65, 1261–1262. [Google Scholar]
- Diaz-Velez, C.R.; García-Castiñeiras, S.; Mendoza-Ramos, E.; Hernández-López, E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am. Heart J. 1996, 131, 146–152. [Google Scholar] [CrossRef]
- Correia-Pinto, J.; Henriques-Coelho, T.; Roncon-Albuquerque, R.; Lourenço, A.P.; Melo-Rocha, G.; Vasques-Nóvoa, F.; Gillebert, T.C.; Leite-Moreira, A.F. Time course and mechanisms of left ventricular systolic and diastolic dysfunction in monocrotaline-induced pulmonary hypertension. Basic Res. Cardiol. 2009, 104, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bovolini, J.A.; Gonçalves, N.; Vasques-Nóvoa, F.; do Amparo Andrade, M.; Santos, M.; Leite-Moreira, A.M.; Henriques-Coelho, T.M.; Duarte, J.A.; Moreira-Gonçalves, D. Exercise preconditioning prevents left ventricular dysfunction and remodeling in monocrotaline-induced pulmonary hypertension. Porto Biomed. J. 2020, 5, e081. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The dynamic interplay between cardiac inflammation and fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Zima, A.V.; Blatter, L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006, 71, 310–321. [Google Scholar] [CrossRef]
- Rajabi, S.; Najafipour, H.; Jafarinejad-Farsangi, S.; Joukar, S.; Beik, A.; Askaripour, M.; Jafari, E.; Safi, Z. Quercetin, perillyl alcohol, and berberine ameliorate right ventricular disorders in experimental pulmonary arterial hypertension: Effects on miR-204, miR-27a, fibrotic, apoptotic, and inflammatory factors. J. Cardiovasc. Pharmacol. 2021, 77, 777–786. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Al Arqam, Z.O.; Zaki, H.F.; Agha, A.M. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation and nitric oxide. Eur. J. Pharm. Sci. 2014, 62, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chen, G.X.; Liang, M.Y.; Yao, J.P.; Wu, Z.K. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. Int. J. Cardiol. 2015, 180, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Tawa, M.; Yano, Y.; Yamanaka, M.; Sawano, T.; Iesaki, K.; Murata, Y.; Tanaka, R.; Nakagawa, K.; Ohkita, M.; Matsumura, Y. Effects of beet juice supplementation on monocrotaline-induced pulmonary hypertension in rats. Am. J. Hypertens. 2019, 32, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gloria, J.L.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Carbó, R.; Hernández-Díazcouder, A.; Guzmán-Martín, C.A.; Rubio-Gayosso, I.; Sánchez-Muñoz, F. Nutraceuticals in the treatment of pulmonary arterial hypertension. Int. J. Mol. Sci. 2020, 21, 4827. [Google Scholar] [CrossRef]
- Colombo, R.; Siqueira, R.; Conzatti, A.; Fernandes, T.R.G.; Tavares, A.M.V.; da Rosa Araújo, A.S.; Belló-Klein, A. Aerobic exercise promotes a decrease in right ventricle apoptotic proteins in experimental cor pulmonale. J. Cardiovasc. Pharmacol. 2015, 66, 246–253. [Google Scholar] [CrossRef]



| Compound | Concentration |
|---|---|
| Total phenolic compounds (mg GAE/g) | 38.89 |
| Total flavonoid compounds | |
| Cyanidin (µg/mL) | 219.21 ± 5.38 |
| Malvidin (µg/mL) | 128.68 ± 1.27 |
| Rutin | ND |
| Gallic acid | ND |
| Catechin | ND |
| Epicatechin | ND |
| Grade | SC | SH | SHB | EH | EHB |
|---|---|---|---|---|---|
| 0 | 2 (28.57%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| I | 4 (57.14%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| II | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (28.57%) |
| III | 1 (14.20%) | 0 (0.00%) | 2 (28.57%) | 1 (14.20%) | 1 (14.20%) |
| IV | 0 (0.00%) | 0 (0.00%) | 5 (71.42%) | 6 (85.71%) | 4 (57.14%) |
| V | 0 (0.00%) | 7 (100.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, L.B.; Soares, L.L.; Guimarães-Ervilha, L.O.; Costa, S.F.F.; Generoso, S.C.d.L.; Xavier, M.A.M.; Iasbik-Lima, T.; Oliveira, L.L.d.; Della Lucia, C.M.; Bianchi, S.E.; et al. Blueberry Extract and Resistance Training Prevent Left Ventricular Redox Dysregulation and Pathological Remodeling in Experimental Severe Pulmonary Arterial Hypertension. Nutrients 2025, 17, 1145. https://doi.org/10.3390/nu17071145
Leite LB, Soares LL, Guimarães-Ervilha LO, Costa SFF, Generoso SCdL, Xavier MAM, Iasbik-Lima T, Oliveira LLd, Della Lucia CM, Bianchi SE, et al. Blueberry Extract and Resistance Training Prevent Left Ventricular Redox Dysregulation and Pathological Remodeling in Experimental Severe Pulmonary Arterial Hypertension. Nutrients. 2025; 17(7):1145. https://doi.org/10.3390/nu17071145
Chicago/Turabian StyleLeite, Luciano Bernardes, Leôncio Lopes Soares, Luiz Otávio Guimarães-Ervilha, Sebastião Felipe Ferreira Costa, Sara Caco dos Lúcio Generoso, Mirielly Alexia Miranda Xavier, Thainá Iasbik-Lima, Leandro Licursi de Oliveira, Ceres Mattos Della Lucia, Sara Elis Bianchi, and et al. 2025. "Blueberry Extract and Resistance Training Prevent Left Ventricular Redox Dysregulation and Pathological Remodeling in Experimental Severe Pulmonary Arterial Hypertension" Nutrients 17, no. 7: 1145. https://doi.org/10.3390/nu17071145
APA StyleLeite, L. B., Soares, L. L., Guimarães-Ervilha, L. O., Costa, S. F. F., Generoso, S. C. d. L., Xavier, M. A. M., Iasbik-Lima, T., Oliveira, L. L. d., Della Lucia, C. M., Bianchi, S. E., Bassani, V. L., Herter, F. G., Turck, P., da Rosa Araujo, A. S., Forte, P., Reis, E. C. C., Machado-Neves, M., & José Natali, A. (2025). Blueberry Extract and Resistance Training Prevent Left Ventricular Redox Dysregulation and Pathological Remodeling in Experimental Severe Pulmonary Arterial Hypertension. Nutrients, 17(7), 1145. https://doi.org/10.3390/nu17071145









