Abstract
There has been a growing interest globally in vegan and vegetarian diets over the last decade for a combination of health, ethical, environmental, spiritual, and social reasons. In line with this popularity, research examining the role of plant-based food sources, including vegan and vegetarian diets, in supporting skeletal muscle remodeling and anabolism in humans has also received considerable attention. The emergence of the microbiota-gut–muscle axis, a bidirectional pathway where the gut microbiota impacts skeletal muscle and vice versa, has been suggested as a potential mediator of food and nutrition’s influence on the mechanistic processes that regulate muscle mass and function. Considering inherent nutritional differences between vegan, vegetarian, and omnivorous diets related to the fiber and macronutrient content, presence of anti-nutritional factors, and diverse food and supplemental sources for obtaining protein, it stands to reason that the regulation of the microbiota–gut–muscle axis via diet-induced changes in gut microbiota composition and function may be dissimilar. However, whether this translates into differential effects on the skeletal muscle is unclear. This review article aims to provide a contemporary perspective for how variations in gut microbiota linked to vegan, vegetarian, and omnivorous diets may be a potential mechanism for influencing protein metabolism in skeletal muscle mass via a purported microbiota-gut–muscle axis.
1. Introduction
Over the past few years, there has been a growing interest in vegan and vegetarian diets in many Western countries, including the United Kingdom, the United States, and Australia [1]. This increase can be attributed to several factors, namely, health, ethical, environmental, spiritual, and social. Vegan diets are not to be mistaken for a vegetarian diet, which allows a small-to-moderate amount of animal products to be consumed (i.e., a flexitarian diet, lacto-ovo-vegetarian, etc.). More recently, the term “plant-based” diet has received a lot of interest coinciding with the publication of the EAT–Lancet report in 2019 [2]. Similar to other initiatives where recommended dietary guidelines are presented visually on a plate schematic, the report recommended a “planetary health” plate that consists of approximately half a plate of vegetables and fruits, with the remainder made up of whole grains, plant-based protein sources, unsaturated plant oils, and smaller amounts of dairy, animal proteins, starchy vegetables, and added sugars [2,3]. The term plant-based diet is often used interchangeably with vegan diets, implying that both are characterized by the avoidance of all flesh foods and animal-derived ingredients [4]. On the contrary, others explicitly note that the term plant-based diet does not mean vegan but rather prioritizes foods that come from plants [5]. Regardless, accumulating evidence suggests that incorporating more plant-based options into a habitual diet can have numerous health benefits and lower risk of cardiovascular disease and cancer [2,6,7].
The popularity of vegan and vegetarian diets amongst athletic populations, especially endurance athletes, continues to grow, with previous studies observing that 10% of marathon runners [8] and 13% of ultramarathon runners [9] follow vegan or vegetarian diets. While more athletes appear to be adopting vegan and vegetarian dietary lifestyles, such diets can lead to inadequacies in specific macro- and/ or micronutrients (i.e., protein, n-3 fatty acids, Vitamin B12, iron, etc.) compared to an omnivorous diet and, as such, may negatively impact performance and training adaptations [10]. Conversely, others claim that any dietary deficiencies are likely due to poorly constructed diet plans rather than vegan and vegetarian diets [1,11]. In fact, well-balanced vegan and vegetarian diets can provide adequate amounts of these macro- and micronutrients and, in some situations, even greater amounts of carbohydrate and antioxidant-rich foods compared to omnivorous diets, thus assisting in training and recovery [12].
The role of vegan and vegetarian diets in supporting resistance training-induced skeletal muscle remodeling and anabolism in humans has also received recent attention. Studies examining isolated plant proteins (soy, pea, wheat, and corn) have demonstrated increased muscle protein synthesis responses following intake, both with and without exercise [13,14,15]. While most research in this area has focused on isolated protein supplements, emerging evidence suggests that whole-food plant meals can support anabolic muscle adaptations, especially when adequate vegan/plant protein intake is provided. The interplay between diet and exercise is complex, with many exogenous factors such as exercise (sets, repetitions, volume, and frequency) and diet (protein type and amount and energy intake), as well as endogenous factors, including genomic, epigenetic, transcriptomic, and proteomic regulation, all influencing the magnitude of muscle hypertrophy and stimulation of muscle protein synthesis (MPS) with exercise. The gut microbiome has recently been identified as another important factor, with the emergence of the microbiota–gut–muscle axis. Evidence from both animal and, to a lesser degree, human models of sarcopenia suggest a bidirectional relationship between microbiome and skeletal muscle physiology exists [16,17]. Modulation of the immune system, reductions in oxidative stress and inflammation, and/or the production of short-chain fatty acids (SCFA) (especially butyrate) and pro-anabolic mediators (i.e., increased availability of systemic amino acids and uptake) have all been suggested as possible mechanisms of action by which the microbiota directly and/or indirectly influences muscle physiology [18]. Given vegan and vegetarian diets can be higher in fiber, polyphenols, and other “healthy” microbiome-promoting ingredients compared to omnivorous diets, it could be hypothesized that vegan and vegetarian diets are superior for enhancing the microbiota–gut–muscle axis [19,20]. However, whether this translates into greater anabolic adaptations in muscles with exercise is unclear. Conversely, omnivorous diets, which are higher in animal proteins and thus essential amino acid (EAA) profiles that are more optimally suitable for muscle uptake and assimilation into muscle tissue, could be superior in promoting muscle adaptations to resistance exercise, independent of significantly modulating the microbiota–gut–muscle axis.
The primary objective of this narrative review is to, therefore, assess variations in gut microbiota linked to vegan, vegetarian, and omnivorous diets as potential mechanisms for influencing acute MPS responses in skeletal muscle mass via the purported microbiota–gut–muscle axis. We first compare the effects of vegan, vegetarian, and omnivorous diets on various aspects of the gut microbiome, including microbial diversity, composition, and metabolites, along with identification of specific bacteria or bacterial “signatures” (multiple bacteria genera) and link these to mechanisms of action of the microbiota–gut–muscle axis. Subsequently, we will discuss the direct effects of plant-based protein sources, including vegan, vegetarian, and omnivorous diets, on MPS responses to put forward putative evidence that may link the direct physiological action of distinct gut microbiota phenotypes associated with plant- and meat-based dietary interventions with the stimulation of MPS in adults.
2. Effects of Vegan, Vegetarian, and Omnivorous Diets on Gut Microbiota Diversity, Composition, and Associated Bacteria-Derived Metabolites
2.1. Modulation of the Microbiome with Diet
It is well-known that the organisms within a microbiome consume the same food as the host, so it is unsurprising that diet affects the composition of microbes present in the human intestinal tract. The long-term dietary habits of an individual, such as the consumption of protein and animal fat versus carbohydrates, are key factors in determining their core microbial composition. However, both rapid and drastic changes in dietary habits can cause detectable shifts in the subsets of microorganisms within 24 h, although these changes are not considered permanent and require continued consumption of the altered diet to be maintained [21]. Over longer periods, evidence suggests that the microbiome co-evolves with the host’s diet to maximize nutrient utilization [22,23].
The impact of vegan and vegetarian diets on gut microbiota, microbial fermentation products, and subsequent host physiology is a topic of debate with no clear consensus. To date, the majority of information comes from cross-sectional studies, with few well-designed intervention studies published. It is currently unclear whether the health benefits of vegan and vegetarian diets are solely due to their nutritional composition or also partly due to impacts on the gut microbiome. Notwithstanding, the following section summarizes recent findings from both observational and intervention studies in humans, focusing on differences in microbiota composition and/or associated bacteria-derived metabolites between vegan, vegetarian, and omnivorous diets. The characteristics of cross-sectional and interventional studies can be found in Table 1 and Table 2, respectively.

Table 1.
Exploratory human studies describing correlations/interactions between short- and long-term dietary habits, gut microbiota, and associated metabolites.

Table 2.
Human studies describing dietary interventions on the gut microbiota composition and associated metabolites.
2.2. Cross-Sectional Studies Comparing Vegan and Omnivorous Diets
The earliest study to investigate the relationship between diet and gut microbiota was in the early 1970s, with diets high in meat (“mixed Western diet”) compared to a not-so-well-defined “non-meat diet” [24]. Using a very small number of participants, eight volunteers switched their usual mixed Western diet to a non-meat diet for four weeks. Though not a cross-sectional study by definition, this study found that those who consumed meat displayed an anaerobic microbiota profile enriched in Bacteroides, Bifidobacterium, Peptococcus, and Lactobacillus compared to non-meat eaters. Approximately 37 years after this study by Reddy et al. [24], Zimmer and colleagues [25] set out to distinguish differences in the fecal microbiota profiles of adult vegans, vegetarians, and omnivores (control) within a German cohort that were age and sex-matched. Self-proclaimed adherence to both vegan and vegetarian diets was short and only 4 weeks prior to the study. Fecal pH was significantly lower in vegans compared to omnivores, which was strongly correlated with reduced counts of E. coli and Enterobacteriacea species that are not tolerant of an acidic environment [25]. Low fecal pH is also likely a reflection of higher fiber intake within vegans, as shown in more recent studies [32]; however, fiber and dietary intake were not measured in the study by Zimmer and colleagues. Longer term adherence (minimum one year) within a Slovenian adult population showed higher ratios (percentage of group-specific DNA in all bacterial DNA) of Bacteroides–Prevotella, Bacteroides thetaiotaomicron, Clostridium clostridioforme, and Faecalibacterium prausnitzii in vegetarians and/or vegans compared to omnivores [27]. Additionally, both vegan and vegetarian participants displayed a lower ratio of Clostridium cluster XIVa when compared to omnivores, a result similarly noted in another study in vegetarian women from southern India [26]. Faecalibacterium prausnitzii has been identified as a key bacterial strain linked to skeletal muscle mass, given it is a butyrate producer and displays anti-inflammatory properties [42].
A series of independent studies compared gut microbiota profiles between vegan, vegetarian, and omnivorous populations in Italy. Lossaso and colleagues observed higher counts of the phylum Bacteroidota (synonym Bacteroidetes) in vegans and vegetarians compared to omnivores (all of which had been strictly following their respective diets for more than 12 months) [31]. Bacteroidota phylum are typically commensal bacteria and proficient in polysaccharide fermentation that leads to the production of SCFAs and other important molecules. However, at the genus level of this bacteria, contrary findings were observed in the aforementioned studies by Reddy [24] and Zimmer [25], as well as more recent studies [28,32], with a lower abundance of Bacteroides in vegans compared to omnivores. Furthermore, Tarallo and colleagues observed lower counts of Bacteroides but at the species level, with a lower abundance of Bacteroides dorei (currently Phocaeicola dorei) in vegans compared to omnivores [35]. Despite higher abundance levels at the phylum level, at the lower levels (i.e., species), reduced abundance could indicate a lack of diversity. This could reflect a diet low in protein and animal fat and high in fiber, as comparable patterns in Bacteroides abundance have been observed across both the short-term (4 weeks) [24,25] and long-term (1+ year) [28,31,32,35].
Geographic location is an important consideration when studying the impact of diet on the microbiome and resistome composition, as even different locations within the same country (i.e., north versus south regions) can have a significant impact. Within a Dutch cohort, differences in the relative abundance of lactic acid bacteria were observed when comparing microbiomes between diets, with omnivores and pescatarians displaying higher levels of Streptococcus thermophilus and Lactococcus lactis (pescatarian only) when compared to vegans [34]. Given the relatively high consumption of dairy products in the Netherlands [43], it is not surprising to see omnivores and pescatarians (who include dairy products in their diet) demonstrate higher levels compared to vegans (excluded from such diet). Prochazkova and colleagues used a multi-omics approach to investigate differences between self-reported (past 3 years) vegans and omnivores within central Europe [33]. Ten genera that belong to the core microbiome were different between groups, with enrichment of Lachnospira, Lachnospiraceae NK4A136 group, and Ruminiclostridium and depletion of Alistipes, Bifidobacterium, Blautia, Fusicatenibacter, Dorea, Anaerostipes, and Ruminococcaceae uncultured groups in vegans compared with omnivores. There were also several differences in the fecal metabolome between vegans and omnivores. Specifically, vegans displayed lower levels of the amino acid fermentation products p-cresol, scatole, indole, and methional, but higher levels of polysaccharide fermentation produced SCFAs and medium-chain fatty acids (MCFAs) and their derivatives. Reported levels of SCFA concentrations in populations following a vegan diet over the long term have been indifferent. While a study by De Filippis and colleagues demonstrated significant associations between the consumption of vegetable-based diets and increased levels of fecal SCFAs [29], a study by Trefflich and colleagues failed to show any difference in fecal SCFA and branch-chain fatty acid (BCFA) levels between vegans and omnivores [32]. This is also in line with another cross-sectional study in a healthy Western vegan population who followed their diet for at least six months [30]. The authors suggested that the microbiota structure of the Western population is “restrictive” and SCFA production does not increase linearly with fiber availability, which contrasts with an agrarian society characterized by a more “permissive” microbiota structure [30].
Most recently, findings from a 24-month observational study in a German population showed no significant difference in the richness (alpha diversity) of the gut microbiome across vegan, vegetarian, and omnivorous diets when cross-sectionally analyzed using an ANOVA statistical approach [36]. While this is in line with previous studies [29,30], it is contradictory to others that have reported greater richness in vegetarians compared to omnivores [31], as well as greater alpha-diversity in non-vegans compared to vegans based on different measures of diversity [33]. Another notable finding from the German population study was that vegan and vegetarian dietary patterns differed significantly in their composition (beta diversity) when compared to omnivores. Omnivores displayed the highest abundance of the Ruminococcus torques group, Eubacterium ruminantium group, Ruminococcaceae, Lachnospiraceae_2, Lactobacillus, and Senegalimassilia, while these were lowest in vegans [36]. Others have also reported that Ruminococcus and the family of Ruminococcaceae were positively associated with an omnivorous diet [29]. Ruminococcus plays a role in converting animal-derived choline and carnitine, primarily sourced from eggs, beef, pork, and fish, into trimethylamine (TMA). The liver oxidizes TMA, releasing it as trimethylamine oxide (TMAO), a compound that has been associated with cardiovascular disease [44]. This contributes to the elevated urinary TMAO levels observed among omnivores [29,45]. Finally, in one of the largest cross-sectional studies, Fackelmann and colleagues [37] analyzed 21,561 individuals spanning five independent, multinational, human cohorts (including previously mentioned Italian cohorts) to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes. Several species were higher in omnivore microbiomes and were linked to meat consumption (i.e., Alistipes putredinis) and inflammation (i.e., Ruminococcus torques), as previously observed [29,36]. In contrast, several species were over-represented in vegan microbiomes that are both known butyrate producers (Lachnospiraceae, Butyricicoccus sp., and Roseburia hominis) and highly specialized for fiber degradation (Lachnospiraceae) [37].
2.3. Intervention Studies Comparing Gut Microbiome Responses to Vegan and Omnivorous Diets
In contrast to observational studies, significantly fewer dietary intervention studies have explored and compared the short- or long-term impacts of vegan, vegetarian, and omnivorous diets on microbiota composition and/or function. The earliest studies to include a vegan diet as one of their experimental diet conditions was by Van Faassen and colleagues in 1987 [38]. In this study, the authors used a cross-over design to compare a vegan diet, lacto-ovo-vegetarian diet, and mixed Western diet for 20 days, each under very controlled conditions. Following the vegan diet, participants demonstrated significantly lower levels of fecal Lactobacilli and Enterococci, along with lower concentrations of bile acids, coprostanol, and coprostanol plus cholesterol compared to the mixed Western diet. While no details of the washout period were provided (limiting the ability to determine whether there were any carryover effects between interventions), it has been suggested that 2–3 weeks is enough time to return gut microbiota to pre-intervention states after short-term dietary alterations [21]. David and colleagues examined the short-term consumption (5 days) of diets composed entirely of animal or plant products on microbial community structure, short-chain fatty acid production, and microbial gene expression [39]. Although no significant differences in alpha diversity were detected in either diet, beta diversity was increased (within 24 h) following the animal-based diet. Further, the animal-based diet appeared to induce a more pronounced effect on the relative abundance of clustered species-level bacterial phylotypes (22 clusters vs. 3 clusters), specifically in bile-resistant taxa (Bilophila wadsworthia, Cluster 28; Alistipes putredinis, Cluster 26; and a Bacteroides sp., Cluster 29). The animal-based diet also led to a decrease in Firmicutes that break down plant polysaccharides (such as Roseburia, Eubacterium rectale, and Ruminococcus bromii). These microbial changes mirrored the differences seen in other studies [35,46] and suggest microbes find a compromise between carbohydrate and protein fermentation [39]. In contrast, Zhang and co-workers observed no effect on gut enterotype or gut microbiota alpha diversity by changing to a lacto-ovo-vegetarian diet (previously omnivorous) for 3 months [40]. However, beta diversity at the genus and species level did decrease. Such differences are likely due to the strictness of the diets, with the study by David and colleagues [39] only comparing plant (grains, fruits, and vegetables) or animal products (meats, eggs, and cheese), whereas the vegetarian diet in the work by Zhang and colleagues [40] was only devoid of meat, and the omnivores consumed both animal and plant products.
Finally, Kohnert and colleagues [41] examined 4 weeks of strict vegan and meat-rich diets in healthy adults. Both alpha and beta diversity were not significantly different between diets. Similarly, changes in gut microbiota composition were minimal after the intervention. The observed changes in amplicon sequence variants (ASVs) were attributed to a few participants, predominantly in ASVs occurring in less than 40% of samples. These were Faecalibacterium (unspecified species) and Roseburia faecis, which were depleted in the vegan diet, and Blautia (unspecified species) as well as Faecalibacterium (another unspecified species), which were enriched in the meat-rich diet. In contrast, a study by David and colleagues [39] found higher levels of Roseburia in vegetarians due to their higher fiber intake. However, factors beyond overall dietary intake patterns, such as specific food choices, can influence the abundance of butyrate-producing Roseburia, with wholegrain foods reported to enrich species.
2.4. Limitations of Current Evidence Comparing Gut Microbiome Responses Between Vegan and Omnivorous Diets
Contextualizing the findings from the above studies requires consideration of factors that may impact the gut microbiome and changes in the gut microbiome in response to dietary challenges. These include, but are likely not limited to, geographical location and environment (which impact baseline and long-term dietary patterns and subsequent microbiome profiles and responses to changes in diet) and individual differences, such as specific food choices. Furthermore, analytical methods and variability in study design also make it difficult to draw firm conclusions based on the current evidence base. A large proportion of the studies were based in Europe; however, even across regions of Europe, baseline dietary patterns varied considerably. The European country most represented was Italy [28,31,35], while others were conducted in Germany, the Netherlands, and Czechia [25,33,41]. Although on the same continent, typical dietary patterns in Italy are characterized by an increased intake of plant foods (except potatoes) and lower consumption of animal and processed foods, whereas this is the opposite in Germany and the Netherlands [47]. Similarly, dietary patterns in the US generally include even higher intakes of animal foods (in particular red and processed meat) [48], alongside low intake of plant foods, aside from (non-wholegrain) grains [49]. This is important given the possibility that certain “keystone” species may be required for the divergence in gut microbiota in response to increased dietary consumption of plant-based foods and that the presence of these species may vary depending on geographical location [30]. Although we could discern no clear pattern from the studies included, this remains an important consideration when interpreting the results of previous research.
While geographic location may exert a large influence over the dietary patterns of populations, even within those dietary patterns, specific dietary choices may also heavily influence the gut microbiome. As noted, significant consumption of dairy products in a Dutch population likely led to a greater relative abundance of lactic acid bacteria, while red meat is a strong driver of omnivorous microbiome signatures [37]. Although each of these food types might be consumed in an omnivorous diet and/or vegetarian diet (excluding meat), the influence of the relative proportions of these food types may not be obvious if this intake data is not captured. Similarly, given vegan microbiome signatures may be related to soil microbes [37], it is also likely that this may be influenced by food choices (e.g., organic versus non-organic, local versus non-local). This adds further complexity when interpreting the effects of vegan versus omnivorous diets on the gut microbiome.
The lack of consistency in analytic methods across studies also makes the interpretation of results difficult. For example, traditional 16S rRNA gene sequencing (a common PCR-based method) allows researchers to identify and quantify bacterial taxa at the genus level but offers limited insight into functional capabilities [50]. In contrast, shotgun metagenomic sequencing may provide greater resolution, including details on which genes the microbes carry and additional information on metabolic pathways associated with different community members [51]. Other methods focus on metabolite profiling, for example, measuring short-chain fatty acid production or amino acid byproducts, which provides important information on microbial activity and functionality, without necessarily identifying the organisms responsible. Multi-omics approaches often combine metagenomics, transcriptomics, proteomics, and metabolomics, giving a more comprehensive picture of the microbiome’s functional output [52]. While findings from different methods can be integrated to build a more comprehensive picture, it is crucial to interpret these results within the context of the limitations inherent to each analytical technique.
3. Effects of Vegan, Vegetarian, and Omnivorous Diets on Muscle Protein Synthesis Responses
The following section discusses studies that have investigated the effects of isolated plant-based (i.e., non-animal-derived) protein supplements, along with whole-food vegan meals and diets, on MPS responses, with comparisons to animal-based proteins/diets made where applicable. For the sake of simplicity, in the next section, non-animal sources are limited to protein sources of either plant or fungi origins, while omnivorous sources are based on food sources of animal origin, excluding alternative sources, such as insects, worms, etc. The characteristics of these studies can be found in Table 3.

Table 3.
Muscle protein synthesis (MPS) responses to plant-based and omnivorous supplement and whole-food dietary protein sources.
3.1. Effects of Supplement Vegan and Omnivorous Protein Sources on Muscle Protein Synthesis Responses
The majority of research in this growing area has focused on the use of isolated protein supplement sources. Early work incorporated soy protein with one study in males aged in their early 20s, showing a greater MPS response with skimmed milk compared to soy protein (both containing ~18 g protein) after a single session of resistance exercise [53]. Another study by Tang and colleagues compared MPS responses between equal amounts of whey, casein, and soy protein (~20 g, with each providing ~10 g of EAAs) during recovery from resistance exercise in healthy young males [15]. Interestingly, although whey protein induced the greatest rise in MPS, the ingestion of soy protein induced a greater increase in MPS compared to the animal-derived casein protein. Another notable finding from this study was that the ingestion of whey and soy protein, both of which are acid soluble, which is important for their digestion in the acidic environment of the stomach, resulted in a more rapid appearance of leucine in the blood than with the ingestion of casein protein. In older adults aged in their early 70s, whey protein isolate in 20 g and 40 g amounts similarly induced significantly higher MPS responses compared to the same respective amounts of soy protein isolate in both a rested state and after a single bout of resistance exercise [54]. However, not all studies have reported lower MPS responses with soy protein compared to animal-based protein sources. A study in younger adults aged in their early 20s observed no differences in MPS responses when 45 g of carbohydrate was co-ingested with either 20 g of protein from whey, soy, or a leucine-enriched soy beverage following a single session of combined resistance and endurance exercise [67]. As this study was conducted in younger adults, it is possible that differences in soy and animal-based protein beverages may only be apparent in older adults as a result of “anabolic resistance” to generate similar MPS responses to equal amounts of protein compared to younger adults [55,74].
More recently, research led by the van Loon group has focused on the effects of other plant-based protein sources for stimulating MPS responses, such as potato, wheat, corn, and pea. Potato protein contains a relatively high level of EAAs (in particular, leucine) and, therefore, represents a promising type of plant-based protein to enhance MPS responses. In support, the ingestion of 30 g of either potato protein or milk protein increased MPS responses following a single bout of resistance exercise, with no differences in the magnitude of response between the potato- or milk-protein groups [57]. This amount of potato protein provided similar amounts of EAAs (~10.5 g), leucine (2.6 g), lysine (~2 g), and methionine (0.6 g) as the equivalent amount of milk protein, showing the intake of potato protein post-exercise can be an efficacious alternative to animal-based protein beverages. Thirty-gram amounts of either pea, wheat, or corn protein were similarly shown to induce comparable rates of MPS as 30 g of milk protein [14], as well as a blend of milk and wheat protein [13] or a blend of milk and corn protein [62] in healthy young males. Additionally, comparable increases in the post-exercise rates of myofibrillar protein synthesis were observed in young healthy adults between a 0.33 g/kg dose of fava bean protein and an EAA-free control beverage [59]. Interestingly, in this study, there were no increases in resting (i.e., non-exercise stimulated) rates of MPS with either beverage; thus, it is possible that the magnitude of post-exercise increase in MPS could have been significantly higher in the control group compared to the fava bean group if the control beverage contained adequate amounts of EAAs. The ingestion of different plant-based protein blends on rates of MPS has also been investigated. Pinckaers and colleagues showed that consuming 30 g of a plant blend protein (15 g wheat + 7.5 g corn + 7.5 g pea protein) in 24 healthy, young, recreationally active males induced comparable rates of MPS to an equivalent amount of milk protein despite an attenuated postprandial rise in circulating plasma essential amino acid concentrations (i.e., isoleucine, threonine, and valine) [60]. In contrast, higher rates of MPS were observed following the ingestion of 25 g whey protein compared to the same amount of a plant-based protein blend composed of 88% pea protein (contains a moderate amount of leucine and lysine but low amounts of methionine) and 12% canola protein (contains higher amounts of methionine) [71]. Notably, in this work, when the plant-based blend was enriched with an additional 1.5 g of leucine, there were no differences in MPS responses. While these findings collectively highlight the importance of an adequate EAA profile in a plant-based protein supplement/beverage for stimulating MPS responses, a caveat to the discussed studies is that they were all undertaken in young and healthy adults.
Older adults have been shown to present with a blunted postprandial MPS response to amino acid/protein intake, coined “anabolic resistance”, compared to younger adults, with the bulk of this work incorporating animal-based protein sources [74,75,76,77]. Thus, to address the capacity for anabolic resistance with plant-based/vegan protein sources, Gorissen and co-workers compared MPS responses to different amounts of wheat hydrolysate, casein, and whey protein in older adults aged in their early 70s [55]. The results showed a 35 g amount of wheat protein hydrolysate did not induce any increase in MPS despite containing 2.5 g of leucine. In contrast, a 60 g dose of wheat protein hydrolysate did significantly increase MPS, and this response was greater than a 35 g dose of whey protein (albeit not statistically significant), in which both of these beverages contained 4.4 g of leucine [55]. Circulating leucine concentrations were substantially lower when consuming only 35 g of wheat protein compared to a 60 g amount of wheat protein. Moreover, although plasma leucine concentrations were still lower after 60 g of wheat protein when compared to 35 g of whey protein (despite being matched for leucine content), it did appear to be substantially higher than when consuming a 35 g dose and was evidently enough to stimulate MPS to a similar degree to the whey protein group. While the 35 g dose of wheat contained 2.5 g of leucine (generally considered enough to maximally stimulate MPS), it is likely that this is insufficient to maximally stimulate MPS in older individuals, possibly due to decreased digestion and absorption of plant-based proteins in elderly individuals; therefore, they may require greater doses (e.g., 3–4 g) [78]. This demonstrates that older adults may require greater amounts of leucine to optimally stimulate MPS; therefore, a higher amount of overall plant-based protein, in this case wheat, may help facilitate more sustained availability of amino acids essential for maximizing MPS responses in older adults. More research on older adults with different types of plant-based proteins is required to support this contention.
More recently, investigations of MPS responses using non-animal protein sources have extended to fungi, in particular, mycoprotein, which is produced by continuous flow fermentation of the filamentous fungi Fusarium venenatum. Mycoprotein is a high-protein meat substitute derived from the fungus Fusarium venenatum (PTA 2684), and 100 g of its dry matter typically contains 45 g protein [79]. Initial work showed that a 70 g bolus of mycoprotein containing 31.5 g protein stimulated a greater increase in MPS than a leucine-matched (2.5 g) bolus of milk protein in young resistance-trained adults [63]. While the mycoprotein beverage in this study contained twice the amount of energy as the milk protein beverage, both beverages still provided adequate levels of protein previously shown to maximize MPS responses, thus canceling out protein quantity being a contributing factor to the results observed. The same group has also reported lower amounts of mycoprotein (~25–40 g), either as a protein concentrate [69] or as part of a blend with pea protein [68], to both significantly increase rates of MPS and to levels similar to the ingestion of isonitrogenous amounts of either 25 g protein from spirulina (blue-green alga) or chlorella (microalga) with exercise in healthy younger adults.
3.2. Effects of Whole-Food Protein Sources on Muscle Protein Synthesis Responses Between Vegan and Omnivorous Diets
It is critical to note that few studies have investigated MPS responses following the consumption of whole-food vegan sources. This certainly limits our understanding of whether the well-established higher fiber content and presence of anti-nutritional compounds in plant-based whole foods that can constitute a large part of vegan diets can compromise protein digestion and amino acid absorption kinetics and subsequently reduce MPS responses. Two studies from the Wall group incorporated mycoprotein as part of a whole-food vegan diet and compared the effects on MPS responses to an animal-based (whole-food) diet [65,66]. In the first of these two studies, 19 healthy female and male older adults were randomly assigned to either an omnivorous (consuming milk protein) or vegan (mycoprotein supplement) condition for three days, with both groups concomitantly performing resistance exercise [65]. The results showed both supplements significantly increased MPS in the exercised and resting legs, with no differences in the daily MPS responses observed between groups. In the second study, which builds upon the first one, 16 young adults in their early 20s were recruited. Participants adhered to a similar diet but with a higher protein content (1.8 g/kg) derived primarily from animal and mycoprotein sources. Concurrently with their daily unilateral leg resistance exercise, the participants proceeded to phase 2 of the study. During this phase, their protein intake increased to approximately 2 g/kg/d for 10 weeks, along with a progressive, high-volume (5 days/week) resistance-training program. The results of this study were consistent with the first one, showing a significant increase in daily myofibrillar protein synthesis rates, lean mass, thigh muscle volume, muscle fiber cross-sectional areas, and 1 RM strength, with no notable differences between groups [66].
To further determine the capacity for whole-food vegan protein sources to modulate MPS responses, Pinckaers and colleagues employed a cross-over design to investigate acute MPS responses to either 0.45 g of protein per kg body mass of either lean ground beef (with potatoes and string beans) or a plant-based meal (with a composition of quinoa, soybeans, chickpeas, and broad beans) in older (65–85 years) females and males [61]. Their results showed that ingestion of the whole-food meal containing beef induced a more rapid and greater rise in circulating plasma essential amino acids compared to the ingestion of an isonitrogenous and isocaloric whole-food meal containing only plant-based food sources (i.e., vegan) [61]. Furthermore, the greater postprandial amino acid availability following ingestion of the omnivorous meal resulted in ~47% higher muscle protein synthesis rates when compared to the ingestion of the vegan meal. The authors theorize that the disparate postprandial plasma amino acid profile following ingestion of both these meals can be attributed to differences in food digestibility [57], protein digestion and amino acid absorption [80], and splanchnic amino acid sequestration [81] following ingestion of the plant- versus animal-based protein sources. The significance of these findings is that they provide evidence to suggest that the food matrix specific to plant-based protein sources, including anti-nutritional factors (e.g., dietary fiber, trypsin inhibitors, and phytates), is significant enough to attenuate acute MPS responses. However, these differences in MPS responses were most recently shown to be absent when the dietary intervention was extended to a 10-day period [73]. Specifically, comparable rates of cumulative MPS responses were observed between isocaloric and isonitrogenous vegan and omnivorous diets providing 1.3 g/kg/day protein in community-dwelling older adults [73]. Such findings highlight the importance of incorporating free-living basal and postprandial MPS rates over an extended period of time with diet and physical activity. In this regard, while no specific exercise component was included in this study, participants were highly physically active, as evidenced by an average step count of ~12500 steps per day. Whether similar responses between vegan and omnivorous diets would be apparent if participants undertook resistance training (i.e., a more established anabolic stimulus that may increase dietary protein needs to facilitate higher MPS responses) remains an area for future investigation.
3.3. Limitations of Current Evidence Comparing Muscle Protein Synthesis Responses Between Vegan and Omnivorous Diets
Despite an increasing number of studies investigating the anabolic capacity of plant-based proteins and, by extension, whole-food diets on MPS responses, several methodological and technical restrictions still exist that limit current knowledge in this area. Firstly, comparatively fewer studies have measured cumulative MPS responses following strict vegan-based diets (and subsequent comparisons to omnivorous diets) over several days and weeks as opposed to acute MPS responses with single-source, supplemental proteins, predominantly powdered forms. In this regard, traditional acute measures of MPS in human skeletal muscle are largely confined to sterile (and costly) continuous intravenous infusions of amino acid tracers (i.e., phenylalanine) and serial blood sample collections. Such methods are further narrowed to short investigation periods (typically < 12 h) under tightly controlled laboratory conditions that not only prevent changes in protein turnover over days to weeks to be measured but also fail to capture the inherent effects of sleep patterns, medication, habitual or incidental forms of physical activity (and inactivity), and the cumulative effects of repeated exercise bouts that can all influence MPS responses [82,83]. Such limitations can be circumvented, to a large extent, by using/ingesting deuterium oxide labeling to quantify the cumulative rates of MPS over days and weeks. Indeed, the three studies to date that have compared the effects of vegan and omnivorous whole-food diets on MPS responses have utilized this methodological advance. However, outside of mycoprotein as a supplemental vegan protein source, there is a paucity of knowledge regarding the long-term effects (i.e., over weeks to months) of other supplemental vegan protein sources that can help form part of a whole-food vegan diet, such as, but not limited to, potato, pea, or corn protein, with multiple bouts of resistance exercise on MPS responses. Such knowledge would uncover whether vegan/plant protein sources (as part of a vegan diet) can be used as an effective means to build new muscle tissue at levels comparable to omnivorous diets in conjunction with resistance training across a human’s lifespan. It would also be interesting to determine how vegan and omnivorous diets alter the turnover rates of individual contractile, metabolic, and mitochondrial muscle proteins with exercise. Utilizing the proteomics-based Dynamic Proteome Profiling technique (with deuterium oxide labeling), we previously showed that short-term resistance exercise (three sessions over nine days) while consuming a high-fat, low-carbohydrate diet increased the synthesis of select myofibrillar and sarcoplasmic proteins compared to non-exercising participants consuming the same diet [84]. Whether vegan and omnivorous diets differentially alter specific muscle proteins with exercise to impact the magnitude of overall fractional and mixed MPS responses remains an area for future research.
4. The Gut–Muscle Axis: A Mechanism for Altering Muscle Anabolism Between Vegan and Omnivorous Diets?
Considering the inherent differences in quantities of macro- and micronutrients, dietary fiber, and presence of anti-nutritional factors between “typical” vegan and omnivorous diets, it stands to reason that some of the reported differences (or even similarities) in MPS responses between these dietary patterns may be mediated via the microbiota–gut–muscle axis. Diet-induced changes in the gut microbiome through carbohydrate and protein fermentation and intestinal inflammation [39,85] typically manifest within 24 h [86]. As such, given the changes are in alignment with the time course of diet-induced changes in MPS responses, alterations in microbiome responses may impact the magnitude of MPS responses with diet and/or exercise. As no study to date has investigated and compared the effects of omnivorous and vegan diets on concomitant MPS and microbiome responses, the following discussion provides a theoretical mechanistic basis for how each dietary pattern may influence the gut–muscle axis.
Perhaps the most logical starting point is the different macronutrients and other dietary components that vary between typical vegan and omnivorous dietary patterns. Western-style omnivorous diets tend to be higher in saturated and overall fat, protein, and red meat, all of which have been associated with compromised gut barrier function and/or elevated inflammatory markers [87,88]. High intakes of saturated and overall fat have been shown to disrupt tight junction proteins in the intestinal epithelium in mice [89], while increased bile secretion in response to high-fat diets may influence intestinal barrier function through receptor-dependent and receptor-independent mechanisms, increase intestinal inflammation, and cause shifts in gut microbiota composition detrimental to the gut barrier [87]. A higher intake of heme iron, common in red meat, may similarly lead to shifts in the gut microbiota that promote degradation of the mucin-2 network and, subsequently, the mucus bi-layer in the colon, also increasing gut permeability [90]. Each of these serves to promote the translocation of microbial products, such as lipopolysaccharides from Gram-negative bacteria into the systemic circulation, which leads to low-grade inflammation [91]. Moreover, a higher protein intake has been shown to favor a pro-inflammatory microbiota profile, as well as compromise the colonic epithelium structure, likely due to increased fermentation products resulting from the microbial metabolization of aromatic and sulfur-containing amino acids (though this may be offset if there is increased consumption of dietary fiber) [92]. The integrity of the gut barrier and the inflammatory nature of these dietary components is important to consider, as systemic inflammation as a result of this may interfere with insulin sensitivity [93] and the mechanistic target of rapamycin complex 1 (mTORC1) activation [18]. Nonetheless, the higher overall protein and essential amino acid content typical of omnivorous diets may mitigate many of these adverse effects, ultimately supporting MPS despite the potential suboptimal microbiota responses discussed.
Conversely, omnivorous diets are typically lower in fiber when compared with vegan diets, which may reduce intestinal SCFA production. Mechanistically, this can increase both Forkhead box O (FOXO) and nuclear factor–kappa B (NF-κB) signaling pathways (Figure 1) and, subsequently, muscle protein breakdown via the regulation of E3 ubiquitin ligases and specific autophagy factors [10]. However, while the higher fiber content of vegan diets may enhance intestinal SCFA production and subsequently reduce FOXO and NF-κB signaling, higher levels of SCFAs may also activate AMP-activated protein kinase (AMPK) signaling. AMPK is a cellular energy sensor that is activated when cellular energy levels are low and subsequently restores energy balance by both promoting catabolic processes that generate ATP and inhibiting anabolic processes that consume ATP. One mechanism by which AMPK mediates this role is through inhibiting mTORC1 signaling through direct and indirect phosphorylation events [94]. The phosphorylation and activation of mTORC1 through exercise and nutrition is a key determinant for increasing rates of MPS by regulating translation initiation via the phosphorylation of downstream ribosomal protein S6 kinase (S6K) and eukaryotic translation initiation factor 4E-binding proteins (4E-BPs) [95]. Although moderate AMPK activation generally benefits overall metabolic health and does not necessarily negate robust anabolic stimuli, sustained or excessive AMPK activity could blunt the phosphorylation of mTORC1’s downstream targets and thus hinder maximal rates of MPS. Further to this, vegan dietary patterns can also present challenges with respect to lower intakes of certain essential amino acids that are crucial for the robust stimulation of mTORC1. This may be compounded by reductions in protein digestibility, absorption, and bioavailability due to the presence of insoluble fiber and anti-nutrient factors, such as phytates and oxalates.
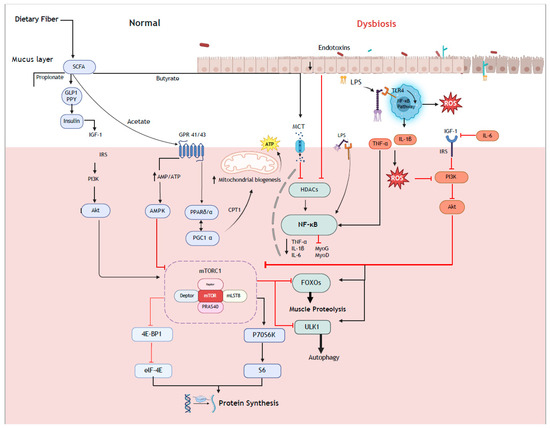
Figure 1.
Putative cellular mechanism that may regulate the muscle protein synthesis responses via the gut–muscle axis. The higher dietary fiber intake typically associated with vegan diets may mediate increases in the production of short-chain fatty acids that, through activation of the IGF-mTORC1 signaling axis, can stimulate muscle protein synthesis responses.
The production of SCFAs from dietary fiber fermentation is proposed as a key mechanism of action to influence muscle mass and function via the microbiota–gut–muscle axis. The metabolite butyrate appears to be one of the main intermediaries of gut and muscle through its direct (i.e., regulation of anabolic pathways) and indirect actions (i.e., anti-inflammatory processes, immune regulation, and homeostasis maintenance). Key bacteria that degrade carbohydrates into SCFAs belong mostly to Firmicutes phylum, including Bacteroides spp., Prevotella spp., Ruminococcus spp., Bifidobacterium spp., Clostridium spp., and Akkermansia muciniphila. Compared to omnivorous diets, both short- and long-term vegan diets resulted in higher levels of Faecalibacterium prausnitzii, Roseburia spp., Clostridium spp., and Anaerostipes spp., which are well-known SCFA producers, especially butyrate. However, given only a small proportion of SCFAs are present within a fecal sample, it is often difficult to confirm this relationship via fecal analysis. Notwithstanding, the creation of next-generation probiotics that are known butyrate and other SCFA producers might be promising not only as an ergogenic aid in exercise-induced muscle adaptation but may also mitigate the onset and progression of diseases characterized by muscle loss, such as sarcopenia and cancer cachexia.
A less-explored mechanism by which microbes might interact with muscle health is through their role in direct protein metabolism, particularly in plant-based diets. Long-term adherence to plant-based diets could conceivably promote microbial adaptations that influence colonic protein metabolism, although the extent to which this meaningfully contributes to amino acid availability remains speculative. While protein digestion primarily occurs in the small intestine, plant proteins are generally less digestible than animal proteins, which may lead to a greater delivery of undigested protein to the colon when consumed in large amounts. Over time, microbial shifts in response to plant-based diets may facilitate colonic amino acid and peptide absorption, potentially compensating for lower small intestinal digestibility. However, as discussed earlier, colonic protein fermentation also produces potentially harmful byproducts, such as p-cresol, ammonia, and hydrogen sulfide, which may have negative implications for gut and systemic health. Thus, while microbial adaptations could enhance colonic amino acid salvage, an increased delivery of plant-based protein to the colon may not always be favorable.
Beyond the role of the gut microbiota, dietary microbes present in fermented foods or probiotics may also influence protein metabolism and muscle health. Although not traditionally considered part of the gut–muscle axis, microbial fermentation of plant-based proteins prior to ingestion or during early digestion may improve their availability by reducing anti-nutritional factors and enhancing protein hydrolysis, thereby increasing upper-intestinal amino acid absorption. Studies have shown that probiotic supplementation alongside plant-based proteins can enhance amino acid bioavailability in the small intestine and lead to improvements in body composition over time [96,97]. Given that these effects were observed after two weeks of supplementation, it remains unclear whether this represents an adaptive response to microbial colonization or an acute enhancement of early protein metabolism, though in vitro models suggest the latter is more likely [98]. Future research could explore how the microbial fermentation of foods before ingestion or inclusion of probiotics in supplements—by increasing protein solubility and hydrolysis—may serve as a practical strategy to optimize muscle health in plant-based diets.
In practice, both vegan and omnivorous diets can support muscle health when care is taken to optimize dietary quality. Excessive consumption of red and processed meats may raise the inflammatory burden and disrupt gut function, negating some of the potential benefits of complete proteins and a higher leucine content. Well-planned omnivorous diets, such as Mediterranean-style eating patterns that emphasize lean proteins, fiber-rich plant-based foods, and healthy fats, can mitigate these risks by reducing inflammation and promoting a favorable gut microbiome. Meanwhile, carefully designed vegan diets with diverse protein sources, adequate total protein intake, and methods to enhance nutrient bioavailability can leverage the anti-inflammatory potential of fiber and phytonutrients. Striking the right balance between nutrient supply, gut health, and metabolic signaling ultimately appears central to maximizing muscle protein synthesis and preserving skeletal muscle function.
5. Conclusions and Future Directions
The mechanisms linking the gut–muscle axis to dietary patterns remain poorly understood in humans, with much of the current evidence extrapolated from animal models or theoretical pathways. While SCFAs, systemic inflammation, and signaling pathways, like mTORC1 and AMPK, are promising areas of focus, there is a need for human studies that directly connect microbiota-derived metabolites to muscle protein synthesis and breakdown. Advanced techniques, such as isotope tracer studies and metabolomics, could provide insight into these mechanisms. Additionally, the role of exercise as a modulator of gut microbiota and its interaction with diet is underexplored. The current evidence indicates that vegan diets can effectively stimulate rates of MPS to levels comparable to omnivorous-based supplements and whole-food diets. However, further interrogation of chronic MPS responses (i.e., over multiple weeks to months) with increasingly popular plant-based protein supplements (that form part of a balanced, whole-food vegan diet), such as potato, corn, and oats, are still required. Whole-food diets, with their complex food matrices and bioactive compounds, may exert unique effects on gut microbiota, nutrient bioavailability, and MPS that cannot be captured by studying isolated proteins. Future research should explore the effects of complete dietary patterns, particularly in older adults and athletes with higher protein demands. Additionally, whether specially formulated hydrolyzed or fermented versions of plant-based sources to improve protein digestibility and absorption can induce higher rates of MPS represents a further area for research. Future research should also prioritize multi-center, long-term studies across diverse populations to account for geographic variations in dietary cuisines and cooking practices, particularly when it comes to the preparation of different plant-based meals that form meal components of vegan diets, to help improve the broader relevance of its findings. Studies examining how resistance (and endurance) exercise influence gut microbiota composition and function and whether these changes synergize with dietary interventions to enhance muscle adaptations are crucial. Finally, lifestyle factors, such as stress, sleep, and medication use, which can significantly impact gut microbiota composition, are rarely considered in the current research. Addressing these variables could provide a more holistic understanding of how diet and lifestyle collectively influence the gut–muscle axis. By integrating these approaches with long-term dietary interventions, future research could develop tailored strategies to improve muscle outcomes across diverse populations and life stages and help bridge critical gaps in our understanding of the gut–muscle axis, guiding evidence-based dietary recommendations for health and performance.
Author Contributions
Conceptualization, M.B.C. and D.M.C.; methodology, M.B.C., D.M.C. and W.A.-R.; investigation, M.B.C., D.M.C. and W.A.-R.; data curation, M.B.C., D.M.C. and W.A.-R.; writing—original draft preparation, M.B.C., D.M.C., W.A.-R. and S.K.; writing—review and editing, M.B.C., D.M.C., W.A.-R. and S.K.; supervision, M.B.C., D.M.C. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan diet health benefits in metabolic syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Losno, E.A.; Sieferle, K.; Perez-Cueto, F.J.A.; Ritz, C. Vegan diet and the gut microbiota composition in healthy adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Wilson, P.B. Nutrition behaviors, perceptions, and beliefs of recent marathon finishers. Phys. Sportsmed. 2016, 44, 242–251. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Moore, W.J.; Barr-Anderson, D. The interconnectedness of diet choice and distance running: Results of the research understanding the nutrition of endurance runners (RUNNER) study. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 205–211. [Google Scholar] [CrossRef]
- Pohl, A.; Schünemann, F.; Bersiner, K.; Gehlert, S. The impact of vegan and vegetarian diets on physical performance and molecular signaling in skeletal muscle. Nutrients 2021, 13, 3884. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and nutrient intake and nutritional status of finnish vegans and non-vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Kouw, I.W.K.; Hendriks, F.K.; van Kranenburg, J.M.X.; de Groot, L.; Verdijk, L.B.; Snijders, T.; van Loon, L.J.C. No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br. J. Nutr. 2021, 126, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Smeets, J.S.J.; Kouw, I.W.K.; Goessens, J.P.B.; Gijsen, A.P.B.; de Groot, L.; Verdijk, L.B.; van Loon, L.J.C.; Snijders, T. Post-prandial muscle protein synthesis rates following the ingestion of pea-derived protein do not differ from ingesting an equivalent amount of milk-derived protein in healthy, young males. Eur. J. Nutr. 2024, 63, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Siddharth, J.; Chakrabarti, A.; Pannérec, A.; Karaz, S.; Morin-Rivron, D.; Masoodi, M.; Feige, J.N.; Parkinson, S.J. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017, 9, 1698–1720. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, J.K.; Hu, Y.M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res. Rev. 2022, 81, 101739. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Przysławski, J. Non-pharmacological treatments for insulin resistance: Effective intervention of plant-based diets-a critical review. Nutrients 2022, 14, 1400. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Lee, K.A.; Björk, J.R.; Leeming, E.R.; Campmans-Kuijpers, M.J.E.; de Haan, J.J.; Vila, A.V.; Maltez-Thomas, A.; Segata, N.; Board, R.; et al. Association of a mediterranean diet with outcomes for patients treated with immune checkpoint blockade for advanced melanoma. JAMA Oncol. 2023, 9, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Weisburger, J.H.; Wynder, E.L. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J. Nutr. 1975, 105, 878–884. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Ramakrishna, B.S. Faecal microbiota composition in vegetarians: Comparison with omnivores in a cohort of young women in southern India. Br. J. Nutr. 2012, 108, 953–957. [Google Scholar] [CrossRef]
- Matijašić, B.B.; Obermajer, T.; Lipoglavšek, L.; Grabnar, I.; Avguštin, G.; Rogelj, I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur. J. Nutr. 2014, 53, 1051–1064. [Google Scholar] [CrossRef]
- Ferrocino, I.; Di Cagno, R.; De Angelis, M.; Turroni, S.; Vannini, L.; Bancalari, E.; Rantsiou, K.; Cardinali, G.; Neviani, E.; Cocolin, L. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: Culturable populations and rRNA DGGE profiling. PLoS ONE 2015, 10, e0128669. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Laghi, L.; Gobbetti, M.; Ercolini, D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome 2016, 4, 57. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the influence of vegan, vegetarian and omnivore oriented westernized dietary styles on human gut microbiota: A cross sectional study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Trefflich, I.; Dietrich, S.; Braune, A.; Abraham, K.; Weikert, C. Short- and branched-chain fatty acids as fecal markers for microbiota activity in vegans and omnivores. Nutrients 2021, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, M.; Budinska, E.; Kuzma, M.; Pelantova, H.; Hradecky, J.; Heczkova, M.; Daskova, N.; Bratova, M.; Modos, I.; Videnska, P.; et al. Vegan diet is associated with favorable effects on the metabolic performance of intestinal microbiota: A cross-sectional multi-omics study. Front. Nutr. 2021, 8, 783302. [Google Scholar] [CrossRef] [PubMed]
- Stege, P.B.; Hordijk, J.; Shetty, S.A.; Visser, M.; Viveen, M.C.; Rogers, M.R.C.; Gijsbers, E.; Dierikx, C.M.; van der Plaats, R.Q.J.; van Duijkeren, E.; et al. Impact of long-term dietary habits on the human gut resistome in the Dutch population. Sci. Rep. 2022, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, S.; Ferrero, G.; De Filippis, F.; Francavilla, A.; Pasolli, E.; Panero, V.; Cordero, F.; Segata, N.; Grioni, S.; Pensa, R.G.; et al. Stool microRNA profiles reflect different dietary and gut microbiome patterns in healthy individuals. Gut 2022, 71, 1302–1314. [Google Scholar] [CrossRef]
- Seel, W.; Reiners, S.; Kipp, K.; Simon, M.C.; Dawczynski, C. Role of dietary fiber and energy intake on gut microbiome in vegans, vegetarians, and flexitarians in comparison to omnivores-insights from the nutritional evaluation (NuEva) study. Nutrients 2023, 15, 1914. [Google Scholar] [CrossRef]
- Fackelmann, G.; Manghi, P.; Carlino, N.; Heidrich, V.; Piccinno, G.; Ricci, L.; Piperni, E.; Arrè, A.; Bakker, E.; Creedon, A.C.; et al. Gut microbiome signatures of vegan, vegetarian and omnivore diets and associated health outcomes across 21,561 individuals. Nat. Microbiol. 2025, 10, 41–52. [Google Scholar] [CrossRef]
- van Faassen, A.; Bol, J.; van Dokkum, W.; Pikaar, N.A.; Ockhuizen, T.; Hermus, R.J. Bile acids, neutral steroids, and bacteria in feces as affected by a mixed, a lacto-ovovegetarian, and a vegan diet. Am. J. Clin. Nutr. 1987, 46, 962–967. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Zhang, C.; Björkman, A.; Cai, K.; Liu, G.; Wang, C.; Li, Y.; Xia, H.; Sun, L.; Kristiansen, K.; Wang, J.; et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front. Immunol. 2018, 9, 908. [Google Scholar] [CrossRef]
- Kohnert, E.; Kreutz, C.; Binder, N.; Hannibal, L.; Gorkiewicz, G.; Müller, A.; Storz, M.A.; Huber, R.; Lederer, A.K. Changes in gut microbiota after a four-week intervention with vegan vs. meat-rich diets in healthy participants: A randomized controlled trial. Microorganisms 2021, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Data, O.W.I. Per-Capita-Milk-Consumption. Available online: https://ourworldindata.org/grapher/per-capita-milk-consumption (accessed on 17 July 2023).
- Chen, P.Y.; Li, S.; Koh, Y.C.; Wu, J.C.; Yang, M.J.; Ho, C.T.; Pan, M.H. Oolong tea extract and citrus peel polymethoxyflavones reduce transformation of l-carnitine to trimethylamine-n-oxide and decrease vascular inflammation in l-carnitine feeding mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Toribio-Mateas, M.A.; Bester, A.; Klimenko, N. Impact of plant-based meat alternatives on the gut microbiota of consumers: A real-world study. Foods 2021, 10, 2040. [Google Scholar] [CrossRef]
- Slimani, N.; Fahey, M.; Welch, A.A.; Wirfält, E.; Stripp, C.; Bergström, E.; Linseisen, J.; Schulze, M.B.; Bamia, C.; Chloptsios, Y.; et al. Diversity of dietary patterns observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr. 2002, 5, 1311–1328. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in meat consumption in the USA. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Guenther, P.M.; Subar, A.F.; Kirkpatrick, S.I.; Dodd, K.W. Americans do not meet federal dietary recommendations. J. Nutr. 2010, 140, 1832–1838. [Google Scholar] [CrossRef]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; van Loon, L.J. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Bahniwal, R.; Holloway, T.M.; Lim, C.; McLeod, J.C.; McGlory, C.; Phillips, S.M. Potato protein isolate stimulates muscle protein synthesis at rest and with resistance exercise in young women. Nutrients 2020, 12, 1235. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Hendriks, F.K.; Hermans, W.J.H.; Goessens, J.P.B.; Senden, J.M.; JMX, V.A.N.K.; Wodzig, W.; Snijders, T.; LJC, V.A.N.L. Potato protein ingestion increases muscle protein synthesis rates at rest and during recovery from exercise in humans. Med. Sci. Sports Exerc. 2022, 54, 1572–1581. [Google Scholar] [CrossRef]
- Kouw, I.W.; Pinckaers, P.J.; Le Bourgot, C.; van Kranenburg, J.M.; Zorenc, A.H.; de Groot, L.C.; van Loon, L.J. Ingestion of an ample amount of meat substitute based on a lysine-enriched, plant-based protein blend stimulates postprandial muscle protein synthesis to a similar extent as an isonitrogenous amount of chicken in healthy, young men. Br. J. Nutr. 2022, 128, 1955–1965. [Google Scholar] [CrossRef]
- Davies, R.W.; Kozior, M.; Lynch, A.E.; Bass, J.J.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The effect of fava bean (Vicia faba L.) protein ingestion on myofibrillar protein synthesis at rest and after resistance exercise in healthy, young men and women: A randomised control trial. Nutrients 2022, 14, 3688. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Kouw, I.W.K.; Gorissen, S.H.M.; Houben, L.H.P.; Senden, J.M.; Wodzig, W.; de Groot, L.; Verdijk, L.B.; Snijders, T.; van Loon, L.J.C. The muscle protein synthetic response to the ingestion of a plant-derived protein blend does not differ from an equivalent amount of milk protein in healthy young males. J. Nutr. 2023, 152, 2734–2743. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Domić, J.; Petrick, H.L.; Holwerda, A.M.; Trommelen, J.; Hendriks, F.K.; Houben, L.H.; Goessens, J.P.; van Kranenburg, J.M.; Senden, J.M.; et al. Higher muscle protein synthesis rates following ingestion of an omnivorous meal compared with an isocaloric and isonitrogenous vegan meal in healthy, older adults. J. Nutr. 2024, 154, 2120–2132. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Weijzen, M.E.G.; Houben, L.H.P.; Zorenc, A.H.; Kouw, I.W.K.; de Groot, L.; Verdijk, L.B.; Snijders, T.; van Loon, L.J.C. The muscle protein synthetic response following corn protein ingestion does not differ from milk protein in healthy, young adults. Amino Acids 2024, 56, 8. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Coelho, M.O.C.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.O.; Jackman, S.R.; Blackwell, J.R.; Finnigan, T.J.A.; Stephens, F.B.; Dirks, M.L.; et al. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Coelho, M.O.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.; Finnigan, T.J.; Wall, B.T. Branched-chain amino acid fortification does not restore muscle protein synthesis rates following ingestion of lower-compared with higher-dose mycoprotein. J. Nutr. 2020, 150, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Dunlop, M.V.; Machin, D.J.; Coelho, M.O.C.; Pavis, G.F.; Porter, C.; Murton, A.J.; Abdelrahman, D.R.; Dirks, M.L.; Stephens, F.B.; et al. A mycoprotein-based high-protein vegan diet supports equivalent daily myofibrillar protein synthesis rates compared with an isonitrogenous omnivorous diet in older adults: A randomised controlled trial. Br. J. Nutr. 2021, 126, 674–684. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Coelho, M.O.C.; Murton, A.J.; Abdelrahman, D.R.; Blackwell, J.R.; Koscien, C.P.; Knapp, K.M.; Fulford, J.; Finnigan, T.J.A.; Dirks, M.L.; et al. Vegan and omnivorous high protein diets support comparable daily myofibrillar protein synthesis rates and skeletal muscle hypertrophy in young adults. J. Nutr. 2023, 153, 1680–1695. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Peeters, W.M.; Zorenc, A.H.; Schierbeek, H.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with whey, soy, or leucine-enriched soy protein after concurrent resistance- and endurance-type exercise. J. Nutr. 2019, 149, 210–220. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; van der Heijden, I.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Ingestion of mycoprotein, pea protein, and their blend support comparable postexercise myofibrillar protein synthesis rates in resistance-trained individuals. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E267–E279. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Blackwell, J.R.; Stephens, F.B.; Wall, B.T. Mycoprotein ingestion within or without its wholefood matrix results in equivalent stimulation of myofibrillar protein synthesis rates in resting and exercised muscle of young men. Br. J. Nutr. 2023, 130, 20–32. [Google Scholar] [CrossRef]
- van der Heijden, I.; West, S.; Monteyne, A.J.; Finnigan, T.J.; Abdelrahman, D.R.; Murton, A.J.; Wall, B.T. Algae ingestion increases resting and exercised myofibrillar protein synthesis rates to a similar extent as mycoprotein in young adults. J. Nutr. 2023, 153, 3406–3417. [Google Scholar] [CrossRef]
- Lim, C.; Janssen, T.A.; Currier, B.S.; Paramanantharajah, N.; McKendry, J.; Abou Sawan, S.; Phillips, S.M. Muscle protein synthesis in response to plant-based protein isolates with and without added leucine versus whey protein in young men and women. Curr. Dev. Nutr. 2024, 8, 103769. [Google Scholar] [CrossRef]
- van der Heijden, I.; Monteyne, A.J.; West, S.; Morton, J.P.; Langan-Evans, C.; Hearris, M.A.; Wall, B.T. Plant protein blend ingestion stimulates post-exercise myofibrillar protein synthesis rates equivalently to whey in resistance-trained adults. Med. Sci. Sports Exerc. 2024, 56, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Domić, J.; Pinckaers, P.J.; Grootswagers, P.; Siebelink, E.; Gerdessen, J.C.; van Loon, L.J.; de Groot, L.C. A well-balanced vegan diet does not compromise daily mixed muscle protein synthesis rates when compared with an omnivorous diet in active older adults: A randomized controlled cross-over trial. J. Nutr. 2024. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef]
- Shad, B.J.; Thompson, J.L.; Breen, L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E803–E817. [Google Scholar] [CrossRef]
- Wilkinson, K.; Koscien, C.P.; Monteyne, A.J.; Wall, B.T.; Stephens, F.B. Association of postprandial postexercise muscle protein synthesis rates with dietary leucine: A systematic review. Physiol. Rep. 2023, 11, e15775. [Google Scholar] [CrossRef]
- Finnigan, T.; Needham, L.; Abbott, C. Chapter 19—Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 305–325. [Google Scholar] [CrossRef]
- Borack, M.S.; Reidy, P.T.; Husaini, S.H.; Markofski, M.M.; Deer, R.R.; Richison, A.B.; Lambert, B.S.; Cope, M.B.; Mukherjea, R.; Jennings, K.; et al. Soy-dairy protein blend or whey protein isolate ingestion induces similar postexercise muscle mechanistic target of rapamycin complex 1 signaling and protein synthesis responses in older men. J. Nutr. 2016, 146, 2468–2475. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J. Appl. Physiol. 2014, 116, 1353–1364. [Google Scholar] [CrossRef]
- Camera, D.M.; Smiles, W.J.; Hawley, J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol. Med. 2016, 98, 131–143. [Google Scholar] [CrossRef]
- Trommelen, J.; van Loon, L.J.C. Quantification and interpretation of postprandial whole-body protein metabolism using stable isotope methodology: A narrative review. Front. Nutr. 2024, 11, 1391750. [Google Scholar] [CrossRef] [PubMed]
- Camera, D.M.; Burniston, J.G.; Pogson, M.A.; Smiles, W.J.; Hawley, J.A. Dynamic proteome profiling of individual proteins in human skeletal muscle after a high-fat diet and resistance exercise. FASEB J. 2017, 31, 5478–5494. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Camilleri, M.; Lyle, B.J.; Madsen, K.L.; Sonnenburg, J.; Verbeke, K.; Wu, G.D. Role for diet in normal gut barrier function: Developing guidance within the framework of food-labeling regulations. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G17–G39. [Google Scholar] [CrossRef]
- Myles, I.A. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef]
- Rodrigues, P.B.; Dátilo, M.N.; Sant’Ana, M.R.; Nogueira, G.; Marin, R.M.; Nakandakari, S.; de Moura, L.P.; da Silva, A.S.R.; Ropelle, E.R.; Pauli, J.R.; et al. The early impact of diets enriched with saturated and unsaturated fatty acids on intestinal inflammation and tight junctions. J. Nutr. Biochem. 2023, 119, 109410. [Google Scholar] [CrossRef]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Camera, D.M.; Edge, J.; Short, M.J.; Hawley, J.A.; Coffey, V.G. Early time course of Akt phosphorylation after endurance and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Fritz, P.; Fritz, R.; Bóday, P.; Bóday, Á.; Bató, E.; Kesserű, P.; Oláh, C. Gut microbiome composition: Link between sports performance and protein absorption? J. Int. Soc. Sports Nutr. 2024, 21, 2297992. [Google Scholar] [CrossRef]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic administration increases amino acid absorption from plant protein: A placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef]
- Keller, D.; Van Dinter, R.; Cash, H.; Farmer, S.; Venema, K. Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1). Benef. Microbes 2017, 8, 491–496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).