The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery
Abstract
1. Introduction
2. Subjects and Methods
2.1. Clinical Study Design
2.2. Nutritional Assessment
2.3. Physical Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Comparison of Nutritional Assessment Methods
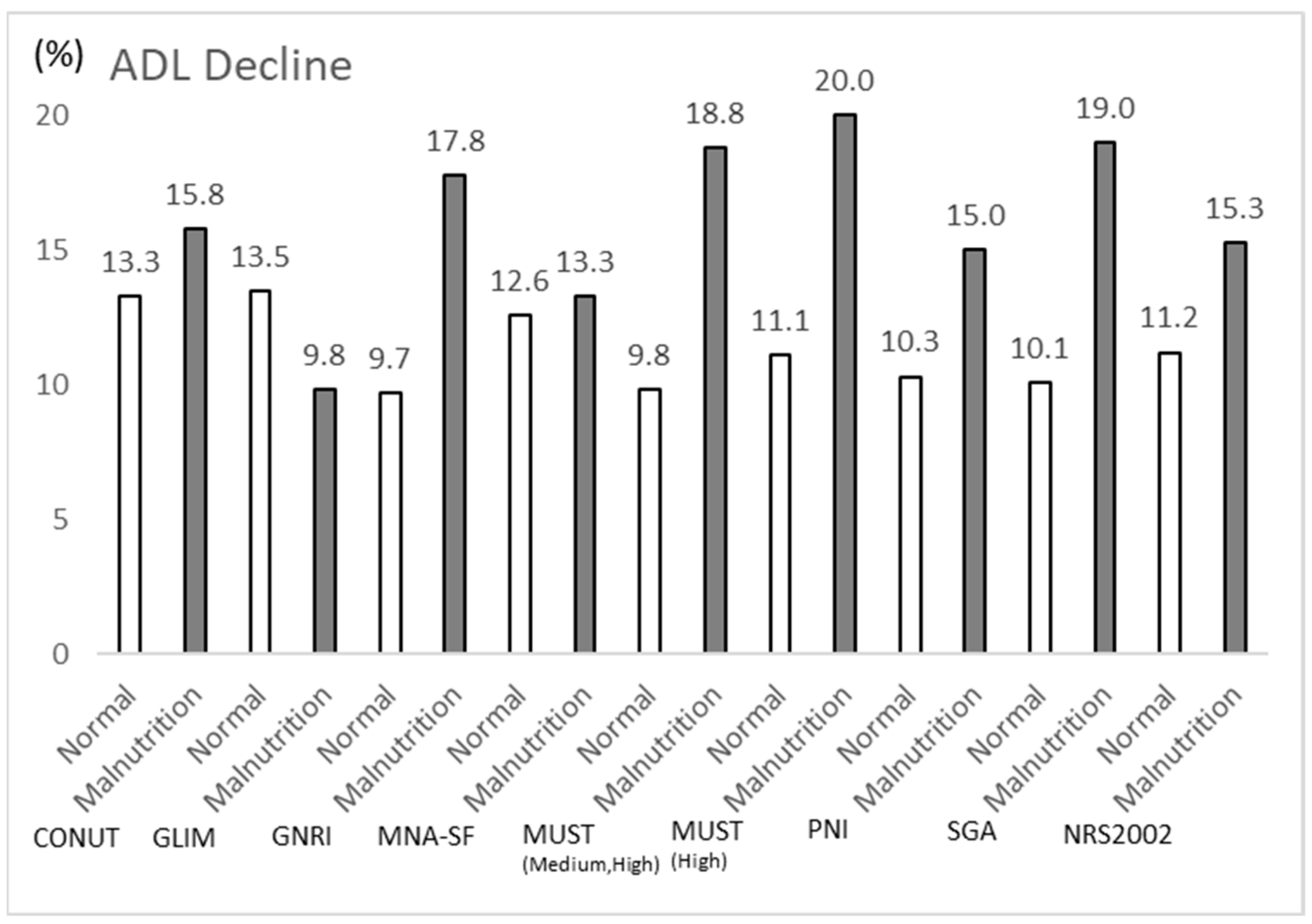
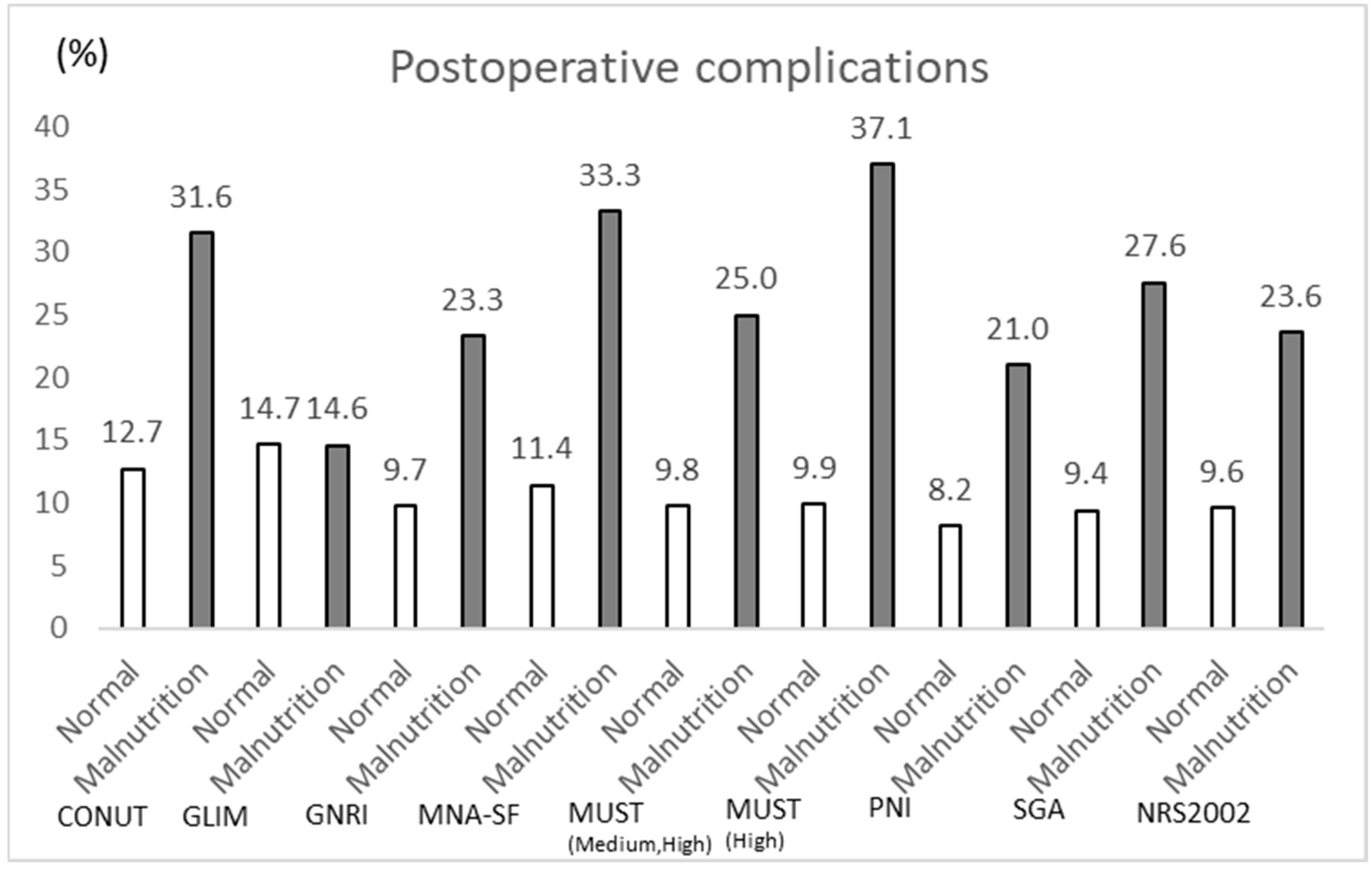
3.3. Comparison of ADL Decline and Occurrence of Severe Complications per Nutritional Assessment of Low Nutrition
3.4. Tool for Predicting ADL Decline and the Occurrence of Severe Complications
3.5. Comparison of Patients with and Without Malnutrition Based on MUST
3.6. Relationship Between MUST Subcomponents and ADL Decline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis: The Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, D.M.; Gelfand, E.V. Valvular heart disease: Classic teaching and emerging paradigms. Am. J. Med. 2013, 126, 1035–1042. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Kim, I. Current Status and Future of Cardiovascular Surgery Treatment. Iwate Med. J. 2020, 71, 215–222. [Google Scholar] [CrossRef]
- Japan Cardiovascular Society. 2022 Report on the Actual Conditions of Cardiovascular Disease Medical Care in Japan, JROAD. Circ. J. 2024, 88, 1234–1256. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Boboshko, V.A.; Nikolaev, D.A.; Vedernikov, P.E. Evaluation of Nutritional Screening Tools for Patients Scheduled for Cardiac Surgery. Nutrition 2013, 29, 436–442. [Google Scholar] [CrossRef]
- Kollef, M.H.; Sharpless, L.; Vlasnik, J.; Pasque, C.; Murphy, D.; Fraser, V.J. The Impact of Nosocomial Infections on Patient Outcomes Following Cardiac Surgery. Chest 1997, 112, 666–675. [Google Scholar] [CrossRef]
- Bove, T.; Calabrò, M.G.; Landoni, G.; Aletti, G.; Marino, G.; Crescenzi, G.; Rosica, C.; Zangrillo, A. The Incidence and Risk of Acute Renal Failure After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 442–445. [Google Scholar] [CrossRef]
- Vosylius, S.; Sipylaite, J.; Ivaskevicius, J. Intensive Care Unit–Acquired Infection: A Prevalence and Impact on Morbidity and Mortality. Acta Anaesthesiol. Scand. 2003, 47, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Compher, C.; Ellen, D.M.A.S.P.E.N. Clinical Guidelines: Nutrition Screening, Assessment, and Intervention in Adults. JPEN J. Parenter. Enteral Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.T.; Yau, D.K.W.; Chiu, L.C.; Wong, M.K.; Yeung, S.S.; Underwood, M.J.; Wong, R.H.; Joynt, G.M.; Lee, A. Effect of Prehabilitation-Related Dietary Protein Intake on Quality of Recovery After Elective Cardiac Surgery (DIETQoR) Study: Protocol of a Randomised Controlled Trial. BMJ Open 2023, 13, e069528. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Goetzenich, A.; Whitman, G.; Ohkuma, R.; Brown, T.; Hatzakorzian, R.; Kristof, A.; Meybohm, P.; Mechanick, J.; Evans, A.; et al. Role of Nutrition Support in Adult Cardiac Surgery: A Consensus Statement from an International Multidisciplinary Expert Group on Nutrition in Cardiac Surgery. Crit. Care 2017, 21, 131. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in Hospital Outpatients and Inpatients: Prevalence, Concurrent Validity and Ease of Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The Mini Nutritional Assessment (MNA) and Its Use in Grading the Nutritional State of Elderly Patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez Salvanés, F.; Fernández, G. CONUT: A tool for controlling nutritional status. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic Nutritional Index in Gastrointestinal Surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A New Index for Evaluating At-Risk Elderly Medical Patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Hiruta, S.; Taniguchi, H.; Konishi, T. Development of a Simple Estimation Formula for the Skeletal Muscle Mass Index (SMI) Using Grip Strength. J-Global 2021, 16, 1. [Google Scholar]
- Juliana, N.; Abd Aziz, N.A.S.; Maluin, S.M.; Abu Yazit, N.A.; Azmani, S.; Kadiman, S.; Hafidz, K.M.; Mohd Fahmi Teng, N.I.; Das, S. Nutritional Status and Post-Cardiac Surgery Outcomes: An Updated Review with Emphasis on Cognitive Function. J. Clin. Med. 2024, 13, 4015. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, L.M.; de Vos, R.; Borgmeijer-Hoelen, M.M.; Haaring, C.; de Mol, B.A. Preoperative unintended weight loss and low body mass index in relation to complications and length of stay after cardiac surgery. Am. J. Clin. Nutr. 2008, 87, 1656–1661. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- van Erck, D.; Dolman, C.D.; Henriques, J.P.; Schoufour, J.D.; Delewi, R.; Scholte op Reimer, W.J.M.; Snaterse, M. Exploring barriers and facilitators of behavioural changes in dietary intake and physical activity: A qualitative study in older adults undergoing transcatheter aortic valve implantation. Eur. Geriatr. Med. 2023, 14, 503–510. [Google Scholar] [CrossRef]
- Cha, J.K.; Kim, H.S.; Kim, E.J.; Lee, E.S.; Lee, J.H.; Song, I.A. Effect of early nutritional support on clinical outcomes of critically ill patients with sepsis and septic shock: A single-center retrospective study. Nutrients 2022, 14, 2318. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Hegerova, P.; Dedkova, Z.; Sobotka, L. Early nutritional support and physiotherapy improved long-term self-sufficiency in acutely ill older patients. Nutrition 2015, 31, 166–170. [Google Scholar] [CrossRef]
- Cheung, H.H.T.; Joynt, G.M.; Lee, A. Diagnostic test accuracy of preoperative nutritional screening tools in adults for malnutrition: A systematic review and network meta-analysis. Int. J. Surg. 2024, 110, 1090–1098. [Google Scholar] [CrossRef]
- Mesnard, T.; Dubosq, M.; Pruvot, L.; Azzaoui, R.; Patterson, B.O.; Sobocinski, J. Benefits of Prehabilitation before Complex Aortic Surgery. J. Clin. Med. 2023, 12, 3691. [Google Scholar] [CrossRef]
- Arthur, H.M.; Daniels, C.; McKelvie, R.; Hirsh, J.; Rush, B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 253–262. [Google Scholar] [CrossRef] [PubMed]
- de Jorge-Huerta, L.; Marco-Alacid, C.; Grande, C.; Velardo Andrés, C. A narrative review of the diagnosis and treatment of sarcopenia and malnutrition in patients with heart failure. Nutrients 2024, 16, 2717. [Google Scholar] [CrossRef] [PubMed]
- Kootaka, Y.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin. Nutr. 2020, 40, 146–152. [Google Scholar] [CrossRef]
- Joaquín, C.; Puig, R.; Gastelurrutia, P.; Lupón, J.; de Antonio, M.; Domingo, M.; Moliner, P.; Zamora, E.; Martin, M.; Alonso, N.; et al. Mini nutritional assessment is a better predictor of mortality than subjective global assessment in heart failure out-patients. Clin. Nutr. 2019, 38, 2740–2746. [Google Scholar] [CrossRef]
- Neelemaat, F.; Meijers, J.; Kruizenga, H.; van Ballegooijen, H.; Schueren, M.v.B.v.d. Comparison of five malnutrition screening tools in one hospital inpatient sample. J. Clin. Nurs. 2011, 20, 2144–2152. [Google Scholar] [CrossRef]
- Raslan, M.; Gonzalez, M.C.; Torrinhas, R.S.; Ravacci, G.R.; Pereira, J.C.; Waitzberg, D.L. Complementarity of subjective global assessment (SGA) and nutritional risk screening 2002 (NRS 2002) for predicting poor clinical outcomes in hospitalized patients. Clin. Nutr. 2011, 30, 49–53. [Google Scholar] [CrossRef]
- Ocón Bretón, M.; Altemir Trallero, J.; Mañas Martínez, A.B.; Sallán Díaz, L.; Aguillo Gutiérrez, E.; Gimeno Orna, J.A. Comparación de dos herramientas de cribado nutricional para predecir la aparición de complicaciones en pacientes hospitalizados. Nutr. Hosp. 2012, 27, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, R.E.; Crawford, T.C.; Brown, P.M.; Grimm, J.C.; Magruder, J.T.; Kilic, A.; Suarez-Pierre, A.; Snyder, S.; Wood, J.D.; Schneider, E.; et al. A Novel Risk Score to Predict the Need for Nutrition Support After Cardiac Surgery. Ann. Thorac. Surg. 2017, 104, 1306–1312. [Google Scholar] [CrossRef][Green Version]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Tanimoto, S.; Okuno, T.; Aoki, J.; Yahagi, K.; Sato, Y.; Tanaka, T.; Koseki, K.; Komiyama, K.; Nakajima, H.; et al. Hemodynamic correlates of nutritional indexes in heart failure. J. Cardiol. 2018, 71, 557–563. [Google Scholar] [CrossRef]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; Committee, A.M. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Al-Najjar, Y.; Clark, A.L. Predicting outcome in patients with left ventricular systolic chronic heart failure using a nutritional risk index. Am. J. Cardiol. 2012, 109, 1315–1320. [Google Scholar]
- Sungurtekin, H.; Sungurtekin, U.; Hanci, V.; Erdem, E. Comparison of two nutrition assessment techniques in hospitalized patients. Nutrition 2004, 20, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Pashmdarfard, M.; Azad, A. Assessment tools to evaluate Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) in older adults: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 33. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, K.; Kitaguchi, S.; Iwatsu, K.; Morikami, Y.; Ichinohe, T.; Yamamoto, T.; Takenaka, K.; Takenaka, H.; Muranaka, H.; Fujita, R.; et al. A decline in activities of daily living due to acute heart failure is an independent risk factor of hospitalization for heart failure and mortality. J. Cardiol. 2019, 73, 522–529. [Google Scholar] [CrossRef]
- Scher, C.; Nepomnyaschy, L.; Amano, T. Comparison of Cognitive and Physical Decline as Predictors of Depression Among Older Adults. J. Appl. Gerontol. 2023, 42, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Soskolne, V.; Kozohovitch, H.; Deviri, E. Holocaust survivors coping with open heart surgery decades later: Posttraumatic symptoms and quality of life. Gen. Hosp. Psychiatry 2004, 26, 443–452. [Google Scholar] [CrossRef]
- Falcoz, P.E.; Chocron, S.; Stoica, L.; Kaili, D.; Puyraveau, M.; Mercier, M.; Etievent, J.P. Open heart surgery: One-year self-assessment of quality of life and functional outcome. Ann. Thorac. Surg. 2003, 76, 1598–1604. [Google Scholar] [CrossRef]
| Factor | N = 197 |
|---|---|
| Age, years (mean ± SD) | 70.4 ± 11.6 |
| Female, n (%) | 61 (31.0%) |
| Length of hospital stay (Days) (mean ± SD) | 21.07 ± 36.10 |
| Surgical procedure | |
| Valve Replacement Surgery | 141 (71.6%) |
| Coronary artery bypass grafting | 56 (28.4%) |
| Biochemical parameters | |
| Albumin, g/dL (mean ± SD) | 3.85 ± 0.54 |
| CRP, g/dL (mean ± SD) | 1.53 ± 3.38 |
| Anthropometric and body composition characteristics | |
| BMI, kg/m2 (mean ± SD) | 22.9 ± 4.1 |
| Barthel Index (mean ± SD) | 75.52 ± 31.9 |
| SMI, kg/m2, male, n = 92 | 6.73 (5.27–8.56) |
| SMI, kg/m2, female, n = 47 | 6.19 (5.16–8.44) |
| Preoperative handgrip strength below average, n (%) | 57 (28.9%) |
| Preoperative handgrip strength, kg, male, n = 92 | 29.4 (9.8–53.6) |
| Preoperative handgrip strength, kg, female, n = 47 | 18.8 (8.8–37.1) |
| Sarcopenia, n (%) | 89 (45.2%) |
| Comorbid diseases | |
| Chronic Kidney Disease, n (%) | 20 (10.2%) |
| Renal function at admission, n (%) | 13 (6.6%) |
| Hemodialysis, n (%) | 22 (11.2%) |
| Diabetes, n (%) | 55 (27.9%) |
| Hypertension, n (%) | 106 (53.8%) |
| Anemia, n (%) | 101 (51.2%) |
| Nutritional Assessment | Nutritional Evaluation | N (%) | Nutritional Assessment | Nutritional Evaluation | N (%) |
|---|---|---|---|---|---|
| MUST | High risk of malnutrition | 35 (17.8%) | SGA | Severely malnourished | 20 (10.2%) |
| Medium risk of malnutrition | 29 (14.7%) | Moderately malnourished or suspected malnutrition | 38 (19.3%) | ||
| Low risk of malnutrition | 133 (67.5%) | Well-nourished | 139 (70.5%) | ||
| GLIM Criteria | Severe malnutrition | 7 (3.5%) | CONUT | Severe malnutrition | 2 (1.1%) |
| Moderate malnutrition | 11 (5.6%) | Moderate malnutrition | 17 (9.2%) | ||
| Mild malnutrition | 23 (11.7%) | Mild malnutrition | 94 (51.1%) | ||
| Normal Status | 156 (79.2%) | Normal nutritional status | 71 (38.6%) | ||
| MNA-SF | Malnutrition | 30 (15.2%) | PNI | PNI ≤ 40 | 97 (49.2%) |
| At risk of malnutrition | 124 (63.0%) | PNI > 40 | 100 (50.8%) | ||
| Malnutrition | 43 (21.8%) | ||||
| NRS-2002 | Severe | 43 (21.8%) | GNRI | Severe risk of malnutrition | 7 (3.6%) |
| Moderate | 26 (13.2%) | Moderate risk of malnutrition | 44 (22.3%) | ||
| Mild | 3 (1.5%) | Mild risk of malnutrition | 22 (11.2%) | ||
| Absent | 125 (63.5%) | Normal nutritional status | 124 (62.9%) |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | p Value | 95% CI | OR | p Value | 95% CI | |||
| MUST (medium and high risk) | 2.13 | 0.123 | 0.25 | 1.08 | 4.75 | 0.014 | 1.37 | 16.50 |
| MUST (high risk) | 2.00 | 0.249 | 0.32 | 1.27 | 2.38 | 0.221 | 0.60 | 9.48 |
| GLIM | 0.69 | 0.711 | 0.50 | 3.80 | 0.53 | 0.450 | 0.10 | 2.74 |
| MNA-SF | 1.07 | 1.000 | 0.35 | 2.55 | 2.88 | 0.192 | 0.59 | 14.10 |
| NRS2002 | 1.43 | 0.545 | 0.35 | 1.53 | 2.08 | 0.240 | 0.61 | 7.05 |
| SGA | 2.09 | 0.140 | 0.26 | 1.10 | 2.03 | 0.309 | 0.52 | 7.94 |
| CONUT | 1.22 | 1.000 | 0.28 | 2.56 | 0.42 | 0.484 | 0.04 | 4.74 |
| PNI | 1.54 | 0.438 | 0.33 | 1.46 | 1.97 | 0.619 | 6.30 | 0.25 |
| GNRI | 2.02 | 0.152 | 0.50 | 3.80 | 1.39 | 0.419 | 4.61 | 0.59 |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | p Value | 95% CI | OR | p Value | 95% CI | |||
| MUST (medium and high Risk) | 3.08 | 0.009 | 0.20 | 0.76 | 2.85 | 0.160 | 0.66 | 12.30 |
| MUST (high risk) | 5.39 | <0.001 | 1.53 | 4.17 | 2.29 | 0.329 | 0.43 | 12.10 |
| GLIM | 0.99 | 1.000 | 0.44 | 2.31 | 0.80 | 0.801 | 0.14 | 4.50 |
| MNA-SF | 3.89 | 0.004 | 0.18 | 0.66 | 2.33 | 0.428 | 0.29 | 18.90 |
| NRS2002 | 2.91 | 0.014 | 0.21 | 0.80 | 2.20 | 0.280 | 0.53 | 9.23 |
| SGA | 3.69 | 0.002 | 0.17 | 0.66 | 0.59 | 0.506 | 0.12 | 2.82 |
| CONUT | 3.16 | 0.063 | 0.19 | 0.87 | 2.29 | 0.460 | 0.25 | 20.60 |
| PNI | 2.96 | 0.020 | 0.18 | 0.84 | 3.72 | 0.066 | 0.92 | 15.10 |
| GNRI | 2.83 | 0.017 | 0.21 | 0.82 | 1.34 | 0.677 | 0.34 | 5.33 |
| MUST (Medium and High Risk) | |||||
|---|---|---|---|---|---|
| Factor | Group | Normal | Malnutrition | p Value | SMD |
| n | 133 | 64 | |||
| Barthel Index Decline, n (%) | 13 (9.8) | 12 (18.8) | 0.123 | 0.259 | |
| Postoperative complications, n (%) | 13 (9.8) | 16 (25.0) | 0.009 | 0.41 | |
| Chronic Kidney Disease, n (%) | 15 (11.3) | 5 (7.8) | 0.615 | 0.118 | |
| Hemodialysis, n (%) | 15 (11.3) | 7 (10.9) | 1 | 0.011 | |
| Diabetes, n (%) | 43 (32.3) | 12 (18.8) | 0.069 | 0.315 | |
| Hypertension, n (%) | 77 (57.9) | 29 (45.3) | 0.132 | 0.254 | |
| Sarcopenia, n (%) | 60 (59.4) | 29 (76.3) | 0.098 | 0.368 | |
| Heart failure n (%) | 25 (18.8) | 16 (25.0) | 0.414 | 0.15 | |
| Anemia, n (%) | 66 (49.6) | 35 (54.7) | 0.607 | 0.101 | |
| Body Mass Index, n (%) | 22.3 or above | 86 (64.7) | 13 (20.3) | <0.001 | 1.004 |
| below 22.3 | 47 (35.3) | 51 (79.7) | |||
| Surgical procedure, n (%) | Valve Replacement Surgery | 94 (70.7) | 47 (73.4) | 0.815 | 0.062 |
| Coronary artery bypass grafting | 39 (29.3) | 17 (26.6) | |||
| Male, n (%) | 96 (72.2) | 40 (62.5) | 0.226 | 0.208 | |
| Age (mean ± SD) | 70.65 (10.45) | 69.92 (13.95) | 0.684 | 0.059 | |
| Length of hospital stay (mean ± SD) | 33.65 (17.78) | 41.22 (26.21) | 0.018 | 0.338 | |
| MUST (High Risk) | |||||
|---|---|---|---|---|---|
| Factor | Group | Normal | Malnutrition | p Value | SMD |
| n | 162 | 35 | |||
| Barthel Index Decline, n (%) | 18 (11.1) | 7 (20.0) | 0.249 | 0.247 | |
| Postoperative complications, n (%) | 16 (9.9) | 13 (37.1) | <0.001 | 0.679 | |
| Chronic Kidney Disease, n (%) | 16 (9.9) | 4 (11.4) | 1 | 0.05 | |
| Hemodialysis, n (%) | 17 (10.5) | 5 (14.3) | 0.726 | 0.115 | |
| Diabetes, n (%) | 46 (28.4) | 9 (25.7) | 0.91 | 0.06 | |
| Hypertension, n (%) | 90 (55.6) | 16 (45.7) | 0.383 | 0.198 | |
| Sarcopenia, n (%) | 75 (62.0) | 14 (77.8) | 0.299 | 0.349 | |
| Heart failure, n (%) | 31 (19.1) | 10 (28.6) | 0.309 | 0.223 | |
| Anemia, n (%) | 80 (49.4) | 21 (60.0) | 0.341 | 0.215 | |
| Body Mass Index, n (%) | 22.3 or above | 93 (57.4) | 6 (17.1) | <0.001 | 0.916 |
| below 22.3 | 69 (42.6) | 29 (82.9) | |||
| Surgical procedure, n (%) | Valve Replacement Surgery | 45 (27.8) | 11 (31.4) | 0.82 | 0.08 |
| Coronary artery bypass grafting | 117 (72.2) | 24 (68.6) | |||
| Male, n (%) | 0.72 (0.45) | 0.54 (0.51) | 0.038 | 0.375 | |
| Age (mean ± SD) | 70.29 (11.31) | 70.97 (13.38) | 0.755 | 0.055 | |
| Length of hospital stay (mean ± SD) | 34.12 (17.89) | 45.31 (30.83) | 0.004 | 0.444 | |
| OR | ||
|---|---|---|
| ADL decline, n (%) | 12 (6.1) | |
| BMI decreased, n (%) | 11 (5.6) | 6.88 |
| Weight loss, n (%) | 3 (1.5) | 0.39 |
| Acute illness and decreased dietary intake, n (%) | 1 (0.5) | 1.09 |
| BMI and weight loss, n (%) | 0 (0) | - |
| BMI and acute illness and decreased dietary intake, n (%) | 0 (0) | - |
| Weight and acute illness and decreased dietary intake, n (%) | 0 (0) | - |
| All (BMI, weight loss, and acute illness and decreased dietary intake), n (%) | 0 (0) | - |
| OR | ||
|---|---|---|
| ADL decline, n (%) | 7 (3.6) | |
| BMI decreased, n (%) | 7 (3.6) | 1.32 |
| Weight loss, n (%) | 2 (1.0) | 1.09 |
| Acute illness and decreased dietary intake, n (%) | 1 (0.5) | 1.00 |
| BMI and weight loss, n (%) | 1 (0.5) | 1.59 |
| BMI and acute illness and decreased dietary intake, n (%) | 1 (0.5) | 2.38 |
| Weight loss and acute illness and decreased dietary intake, n (%) | 0 (0) | 0.76 |
| All (BMI, weight loss and acute illness and decreased dietary intake), n (%) | 1 (0.5) | 2.38 |
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | p Value | 95% CI | OR | p Value | 95% CI | |||
| (Intercept) | 0.06 | 0.095 | 0.00 | 1.63 | 0.04 | 0.060 | 0.00 | 1.15 |
| Length of hospital stay | 1.02 | 0.262 | 0.99 | 1.05 | 1.03 | 0.072 | 1.00 | 1.05 |
| Age | 0.99 | 0.777 | 0.95 | 1.04 | 0.99 | 0.715 | 0.95 | 1.04 |
| BMI | 0.70 | 0.610 | 0.18 | 2.72 | 4.86 | 0.005 | 1.59 | 14.80 |
| Sarcopenia | 1.52 | 0.508 | 0.44 | 5.31 | 1.52 | 0.509 | 0.44 | 5.30 |
| Diabetes | 0.94 | 0.930 | 0.23 | 3.91 | 0.85 | 0.817 | 0.21 | 3.48 |
| Hypertension | 1.01 | 0.989 | 0.32 | 3.19 | 1.13 | 0.835 | 0.35 | 3.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshima, T.; Tsutsumi, R. The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery. Nutrients 2025, 17, 1120. https://doi.org/10.3390/nu17071120
Oshima T, Tsutsumi R. The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery. Nutrients. 2025; 17(7):1120. https://doi.org/10.3390/nu17071120
Chicago/Turabian StyleOshima, Tomomi, and Rie Tsutsumi. 2025. "The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery" Nutrients 17, no. 7: 1120. https://doi.org/10.3390/nu17071120
APA StyleOshima, T., & Tsutsumi, R. (2025). The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery. Nutrients, 17(7), 1120. https://doi.org/10.3390/nu17071120






