A Narrative Review of Quercetin’s Role as a Bioactive Compound in Female Reproductive Disorders
Abstract
1. Introduction
2. Methods
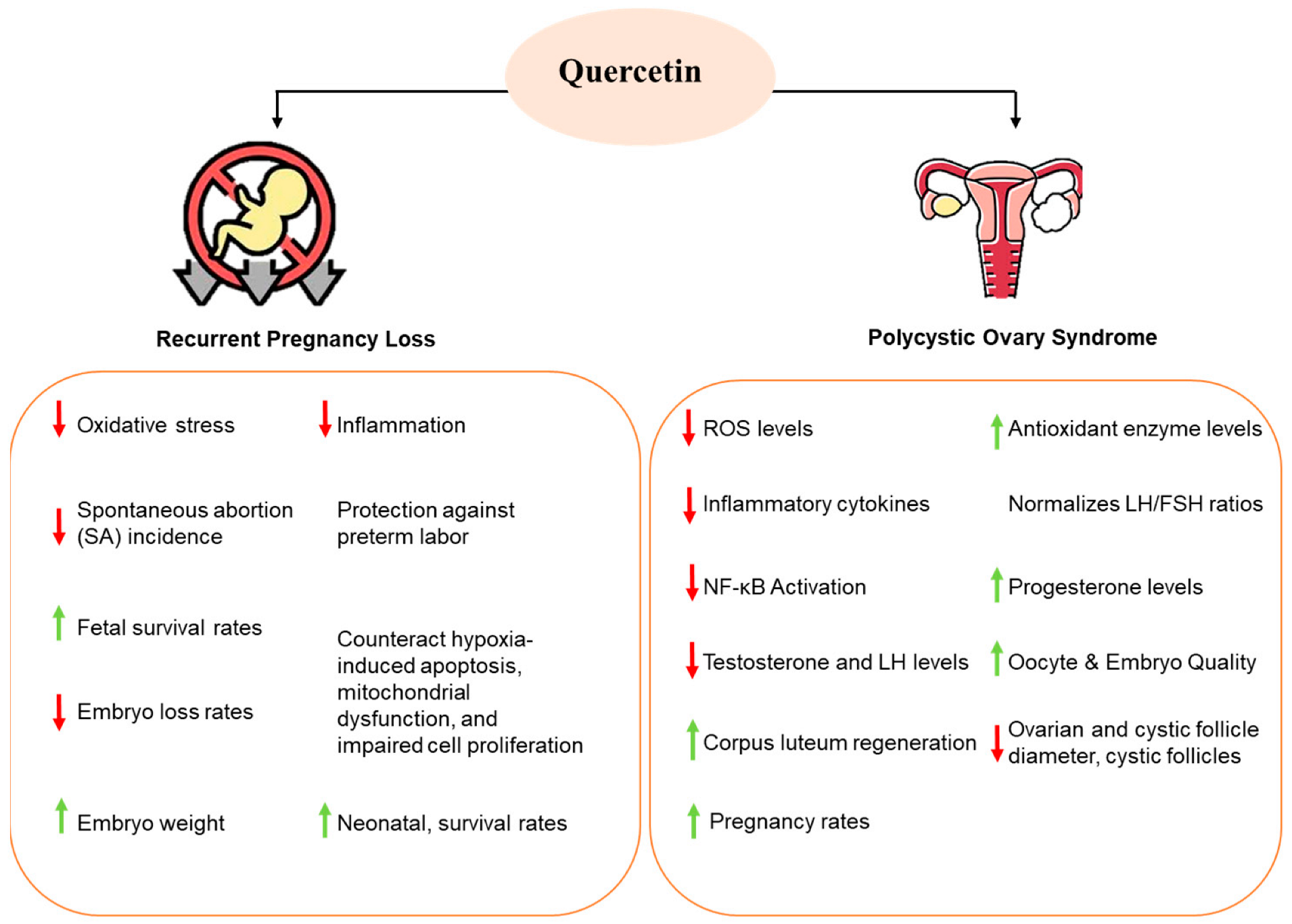
3. Effect of Quercetin on Pregnancy and Miscarriage
4. Effect of Quercetin on Polycystic Ovary Syndrome
5. Effect of Quercetin on Gynecological Cancers
5.1. Cervical Cancer
5.2. Ovarian Cancer
5.3. Endometrial Cancer
6. Limitations and Challenges of Quercetin Usage
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szilágyi, A.; Szabó, I. Endocrine Characteristics of Polycystic Ovary Syndrome (PCOS); NISCAIR-CSIR: New Delhi, India, 2003. [Google Scholar]
- Keyvani, V.; Kheradmand, N.; Navaei, Z.N.; Mollazadeh, S.; Esmaeili, S.-A. Epidemiological trends and risk factors of gynecological cancers: An update. Med. Oncol. 2023, 40, 93. [Google Scholar]
- Laijawala, R.A. Recurrent Pregnancy Loss: Immunological aetiologies and associations with mental health. Brain Behav. Immun. -Health 2024, 41, 100868. [Google Scholar]
- Madigan, M.; Karhu, E. The role of plant-based nutrition in cancer prevention. J. Unexplored Med. Data 2018, 3, 9. [Google Scholar]
- DeClercq, V.; Nearing, J.T.; Sweeney, E. Plant-Based Diets and Cancer Risk: What is the Evidence? Curr. Nutr. Rep. 2022, 11, 354–369. [Google Scholar] [CrossRef]
- Pourteymour Fard Tabrizi, F.; Hajizadeh-Sharafabad, F.; Vaezi, M.; Jafari-Vayghan, H.; Alizadeh, M.; Maleki, V. Quercetin and polycystic ovary syndrome, current evidence and future directions: A systematic review. J. Ovarian Res. 2020, 13, 11. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Vašková, J.; Klepcová, Z.; Špaková, I.; Urdzík, P.; Štofilová, J.; Bertková, I.; Kľoc, M.; Rabajdová, M. The importance of natural antioxidants in female reproduction. Antioxidants 2023, 12, 907. [Google Scholar] [CrossRef]
- Valentová, K.; Šíma, P.; Rybková, Z.; Křížan, J.; Malachová, K.; Křen, V. (Anti)mutagenic and immunomodulatory properties of quercetin glycosides. J. Sci. Food Agric. 2016, 96, 1492–1499. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Ding, J.; Mei, S.; Cai, M.; Zhang, D.; Yu, J. Integrated network pharmacology and clinical study to reveal the effects and mechanisms of bushen huoxue huatan decoction on polycystic ovary syndrome. Evid. -Based Complement. Altern. Med. 2022, 2022, 2635375. [Google Scholar]
- Sharma, H.; Sen, S.; Singh, N. Molecular pathways in the chemosensitization of cisplatin by quercetin in human head and neck cancer. Cancer Biol. Ther. 2005, 4, 949–955. [Google Scholar] [CrossRef]
- Li, J.; Long, H.; Cong, Y.; Gao, H.; Lyu, Q.; Yu, S.; Kuang, Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod. Biol. Endocrinol. 2021, 19, 63. [Google Scholar]
- Lai, W.-F.; Wong, W.-T. Design and optimization of quercetin-based functional foods. Crit. Rev. Food Sci. Nutr. 2022, 62, 7319–7335. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Lupattelli, A.; Koren, G.; Nordeng, H. Herbal medicine use in pregnancy: Results of a multinational study. BMC Complement. Altern. Med. 2013, 13, 355. [Google Scholar]
- Ożarowski, M.; Mikołajczak, P.Ł.; Kujawski, R.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. Pharmacological effect of quercetin in hypertension and its potential application in pregnancy-induced hypertension: Review of in vitro, in vivo, and clinical studies. Evid. -Based Complement. Altern. Med. 2018, 2018, 7421489. [Google Scholar]
- Tomkiewicz, J.; Darmochwał-Kolarz, D. The diagnostics and treatment of recurrent pregnancy loss. J. Clin. Med. 2023, 12, 4768. [Google Scholar] [CrossRef]
- Melo, P.; Dhillon-Smith, R.; Islam, M.A.; Devall, A.; Coomarasamy, A. Genetic causes of sporadic and recurrent miscarriage. Fertil. Steril. 2023, 120, 940–944. [Google Scholar]
- Carbonnel, M.; Pirtea, P.; de Ziegler, D.; Ayoubi, J.M. Uterine factors in recurrent pregnancy losses. Fertil. Steril. 2021, 115, 538–545. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar]
- Hempstock, J.; Jauniaux, E.; Greenwold, N.; Burton, G.J. The contribution of placental oxidative stress to early pregnancy failure. Hum. Pathol. 2003, 34, 1265–1275. [Google Scholar]
- Obeagu, E.; Obeagu, G. Enhancing maternal and fetal well-being: The role of antioxidants in pregnancy. Elite J. Med. Sci. 2024, 2, 76–87. [Google Scholar]
- Araújo, J.R.; Correia-Branco, A.; Pereira, A.C.; Pinho, M.J.; Keating, E.; Martel, F. Oxidative stress decreases uptake of neutral amino acids in a human placental cell line (BeWo cells). Reprod. Toxicol. 2013, 40, 76–81. [Google Scholar] [CrossRef]
- Bartho, L.A.; Holland, O.J.; Moritz, K.M.; Perkins, A.V.; Cuffe, J.S. Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. J. Physiol. 2019, 597, 3053–3067. [Google Scholar] [CrossRef]
- Umekawa, T.; Sugiyama, T.; Kihira, T.; Murabayashi, N.; Zhang, L.; Nagao, K.; Kamimoto, Y.; Ma, N.; Yodoi, J.; Sagawa, N. Overexpression of thioredoxin-1 reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth via glucose metabolism. Endocrinology 2008, 149, 3980–3988. [Google Scholar] [CrossRef]
- Webster, J.; Miller, M.; Vemulapalli, R. Encephalitozoon cuniculi-associated placentitis and perinatal death in an alpaca (Lama pacos). Vet. Pathol. 2008, 45, 255–258. [Google Scholar] [CrossRef]
- Safronova, V.; Matveeva, N.; Avkhacheva, N.; Sidel’nikova, V.; Van’ko, L.; Sukhikh, G. Changes in regulation of oxidase activity of peripheral blood granulocytes in women with habitual abortions. Bull. Exp. Biol. Med. 2003, 136, 257–260. [Google Scholar] [CrossRef]
- Yoshida, K.; Kusama, K.; Shinohara, G.; Sato, S.; Yoshie, M.; Tamura, K. Quercetin stimulates trophoblast fusion via the mitochondrial function. Sci. Rep. 2024, 14, 287. [Google Scholar] [CrossRef]
- Takashima, M.; Tanaka, W.; Matsuyama, H.; Tajiri, H.; Sakakibara, H. Maternal quercetin consumption during pregnancy may help regulate total cholesterol/HDL-cholesterol ratio without effect on cholesterol levels in male progeny consuming high-fat diet. Nutrients 2021, 13, 1242. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Li, Y.; Wang, R.; Wang, C.; Li, Y. New molecular mechanisms of quercetin in improving recurrent spontaneous abortion based on in-depth network pharmacology and molecular docking. Front. Chem. 2024, 12, 1407667. [Google Scholar] [CrossRef]
- Wu, S.; Tian, Y.; Zhang, Q.; Fu, Z.; Lan, H.; Zhou, X.; Ma, L.; Lou, Y. Protective effect of quercetin on lipopolysaccharide-induced miscarriage based on animal experiments and network pharmacology. Mol. Med. Rep. 2024, 29, 99. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Q.; Zhang, J.; Li, X.; Huang, J.; Duan, S.; Zhang, W. Quercetin prevents lipopolysaccharide-induced experimental preterm labor in mice and increases offspring survival rate. Reprod. Sci. 2020, 27, 1047–1057. [Google Scholar] [CrossRef]
- Dong, J.; Young, P.J.; Se, T.; Yahweh; Hwi, L.M. Quercetin inhibits NF-κB and AP-1 activation through induction of heme oxygenase-1 and attenuation of Src mediated PI3K/Akt, p38 and c-jun N-terminal protein kinase phosphorylations in raw 264.7 cells. In Proceedings of the 2012 Winter Symposium of the Korean Society for Laboratory Animal Science, Jeju, Republic of Korea, 28–30 June 2012; p. 97. [Google Scholar]
- Liu, W.; Zhang, M.; Feng, J.; Fan, A.; Zhou, Y.; Xu, Y. The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. Int. J. Environ. Res. Public Health 2017, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Ziaei, S.; Parsay, S. Association between nutritional status with spontaneous abortion. Int. J. Fertil. Steril. 2016, 10, 337. [Google Scholar]
- Zhou, J.; Li, L.; Pan, X.; Wang, J.; Qi, Q.; Sun, H.; Li, C.; Wang, L. The effect of a traditional Chinese quadri-combination therapy and its component quercetin on recurrent spontaneous abortion: A clinical trial, network pharmacology and experiments-based study. Front. Pharmacol. 2022, 13, 965694. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Kang, W.; Huang, L.; Gong, S.; Zhang, T.; Huang, X.; He, F.; Ye, Y.; Tang, Y. miR-34a/DRP-1-mediated mitophagy participated in cisplatin-induced ototoxicity via increasing oxidative stress. BMC Pharmacol. Toxicol. 2023, 24, 16. [Google Scholar] [CrossRef]
- Yu, S.; Long, H.; Lyu, Q.F.; Zhang, Q.H.; Yan, Z.G.; Liang, H.X.; Chai, W.R.; Yan, Z.; Kuang, Y.P.; Qi, C. Protective effect of quercetin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. PLoS ONE 2014, 9, e89520. [Google Scholar] [CrossRef]
- Vanhees, K.; Godschalk, R.W.; Sanders, A.; van Waalwijk van Doorn-Khosrovani, S.B.; van Schooten, F.J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 2011, 290, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S.; Hegyi, P.; Nyirády, P.; Ács, N. Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2023, 21, 10. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes 2021, 70, 627–637. [Google Scholar] [CrossRef]
- Shrivastava, S.; Conigliaro, R.L. Polycystic ovarian syndrome. Med. Clin. N. Am. 2023, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zehravi, M.; Maqbool, M.; Ara, I. Polycystic ovary syndrome and infertility: An update. Int. J. Adolesc. Med. Health 2022, 34, 1–9. [Google Scholar] [CrossRef]
- Tousizadeh, S.; Mohammadi-Moghadam, F.; Mohammadian-Hafshejani, A.; Sadeghi, R. Comparison of zinc levels in mothers with and without abortion: A systematic review and meta-analysiss. Heliyon 2024, 10, e30605. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a syndrome: Genomic insights into PCOS causal mechanisms and classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Yang, Q.; Zhou, Y.; Liu, M.; Shan, H. Oxidative stress and antioxidant imbalance in ovulation disorder in patients with polycystic ovary syndrome. Front. Nutr. 2022, 9, 1018674. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef]
- Mazloomi, S.; Sheikh, N.; Sanoee Farimani, M.; Pilehvari, S. Association of Prx4, total oxidant status, and inflammatory factors with insulin resistance in polycystic ovary syndrome. Int. J. Endocrinol. 2021, 2021, 9949753. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y. Signaling pathways and targeted therapeutic strategies for polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1191759. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Ma, C.; Xiang, Q.; Song, G.; Wang, X. Quercetin and polycystic ovary syndrome. Front. Pharmacol. 2022, 13, 1006678. [Google Scholar] [CrossRef]
- Khadrawy, O.Z.S. Modulation of Nrf2-Mediated Oxidative Stress Response in Bovine Granulosa Cells and Preimplantation Embryos; Universitäts-und Landesbibliothek Bonn: Bonn, Germany, 2019. [Google Scholar]
- Davoodian, N.; Kadivar, A.; Davoodian, N.; Ahmadi, E.; Nazari, H.; Mehrban, H. The effect of quercetin in the maturation media on cumulus-granulosa cells and the developmental competence of bovine oocytes. Theriogenology 2022, 189, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, O.T.; Bamidele, O.; Adetunji, C.O.; Priscilla, B.; Femi, A.; Ayobami, D.; Okotie, G.; Oluwaseun, I.; Olugbenga, E.; Mali, P.C. Quercetin modulates granulosa cell mRNA androgen receptor gene expression in dehydroepiandrosterone-induced polycystic ovary in Wistar rats via metabolic and hormonal pathways. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 4. [Google Scholar]
- Jahan, S.; Abid, A.; Khalid, S.; Afsar, T.; Shaheen, G.; Almajwal, A.; Razak, S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J. Ovarian Res. 2018, 11, 26. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elfiky, A.M.; Abo-Zeid, F.S. The anti-androgenic effect of quercetin on hyperandrogenism and ovarian dysfunction induced in a dehydroepiandrosterone rat model of polycystic ovary syndrome. Steroids 2022, 177, 108936. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.Z.u.h.; Shrivastva, V.k.; Mir, M.A.; Sheikh, W.M.; Ganie, M.A.; Rather, G.A.; Shafi, M.; Bashir, S.M.; Ansari, M.A.; Al-Jafary, M.A. Effect of quercetin on steroidogenesis and folliculogenesis in ovary of mice with experimentally-induced polycystic ovarian syndrome. Front. Endocrinol. 2023, 14, 1153289. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, Y.; Ma, M.; Li, M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J. Formos. Med. Assoc. 2022, 121, 1081–1092. [Google Scholar] [CrossRef]
- Hussain, L.; Aamir, N.; Hussain, M.; Asif, M.; Chauhdary, Z.; Manzoor, F.; Siddique, R.; Riaz, M. Therapeutic investigation of standardized aqueous methanolic extract of bitter melon (Momordica charantia L.) for its potential against polycystic ovarian syndrome in experimental animals’ model: In vitro and in vivo studies. Evid. -Based Complement. Altern. Med. 2022, 2022, 5143653. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar]
- Younas, A.; Hussain, L.; Shabbir, A.; Asif, M.; Hussain, M.; Manzoor, F. Effects of fagonia indica on letrozole-induced polycystic ovarian syndrome (PCOS) in young adult female rats. Evid. -Based Complement. Altern. Med. 2022, 2022, 1397060. [Google Scholar] [CrossRef]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Moini, A.; Alipoor, E.; Rezvan, N.; Gorgani-Firuzjaee, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother. Res. 2018, 32, 2282–2289. [Google Scholar] [CrossRef]
- Vaez, S.; Parivr, K.; Amidi, F.; Rudbari, N.H.; Moini, A.; Amini, N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am. J. Reprod. Immunol. 2023, 89, e13644. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-L.; Wu, F. Effect of Bushen Huatan Recipe on the Akt signal pathway in polycystic ovarian syndrome model rats with insulin resistance: An experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi = Chin. J. Integr. Tradit. West. Med. 2014, 34, 230–234. [Google Scholar]
- Lin, Y.; Xiang, L.; Li, X.; Tang, Q.; Meng, F.; Chen, W. Exploring the mechanism of Yi-Jing decoction in treating polycystic ovary syndrome by using network pharmacology. Curr. Med. Chem. 2023, 30, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Z.; Tang, L.; Shen, M.; Zhou, Z.; Wei, Y.; Zhao, Y.; Bai, S.; Song, L. Associations of Dietary Intakes with Gynecological Cancers: Findings from a Cross-Sectional Study. Nutrients 2022, 14, 5026. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Tiwary, S.; Hussain, M. Functional foods for prevention and treatment of cancer. Asian. J. Pharm. Clin. Res. 2021, 14, 4–10. [Google Scholar] [CrossRef]
- Serna-Thomé, G.; Castro-Eguiluz, D.; Fuchs-Tarlovsky, V.; Sánchez-López, M.; Delgado-Olivares, L.; Coronel-Martínez, J.; Molina-Trinidad, E.M.; de la Torre, M.; Cetina-Pérez, L. Use of functional foods and oral supplements as adjuvants in cancer treatment. Rev. Investig. Clin. 2018, 70, 136–146. [Google Scholar] [CrossRef]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Chemotherapy and dietary phytochemical agesnts. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef]
- Chen, X.; Xu, P.; Zhang, H.; Su, X.; Guo, L.; Zhou, X.; Wang, J.; Huang, P.; Zhang, Q.; Sun, R. EGFR and ERK activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro. Oncol. Lett. 2021, 22, 754. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, X.; Li, J.; Shen, K.; Bai, Y.; Li, Y.; Luan, H.; Tuo, S. The effect of quercetin on cervical cancer cells as determined by inducing tumor endoplasmic reticulum stress and apoptosis and its mechanism of action. Am. J. Transl. Res. 2021, 13, 5240–5247. [Google Scholar]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Wei, W.; Liu, T.; Ding, B.; Cao, S. Study on relationship of quercetin on cervical carcinoma in nude mice model of tumor growth and the expression of heparanase. Chin. J. Biochem. Pharm. 2015, 26–29, 32. [Google Scholar]
- Li, J.; Li, Z.; Gao, Y.; Liu, S.; Li, K.; Wang, S.; Gao, L.; Shi, M.; Liu, Z.; Han, Z.; et al. Effect of a Drug Delivery System Made of Quercetin Formulated into PEGylation Liposomes on Cervical Carcinoma In Vitro and In Vivo. J. Nanomater. 2021, 2021, 9389934. [Google Scholar] [CrossRef]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. -Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, J.; Ding, C.; Chen, G. Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145. Mol. Med. Rep. 2015, 12, 3127–3131. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.-H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677–25695. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.S.; Kalinina, E.V.; Tatarskiy, V.V.; Volodina, Y.L.; Petrova, A.S.; Novichkova, M.D.; Zhdanov, D.D.; Shtil, A.A. Suppression of the Antioxidant System and PI3K/Akt/mTOR Signaling Pathway in Cisplatin-Resistant Cancer Cells by Quercetin. Bull. Exp. Biol. Med. 2022, 173, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Kalinina, E.; Nuzhina, J.; Volodina, Y.; Shtil, A.; Tatarskiy, V. Potentiation of Cisplatin Cytotoxicity in Resistant Ovarian Cancer SKOV3/Cisplatin Cells by Quercetin Pre-Treatment. Int. J. Mol. Sci. 2023, 24, 10960. [Google Scholar] [CrossRef] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; Bonanno, G.; De Vincenzo, R.; Ferrandina, G.; Maggiano, N.; Capelli, A.; Mancuso, S. Inhibitory effect of quercetin on primary ovarian and endometrial cancers and synergistic activity with cis-diamminedichloroplatinum(II). Gynecol. Oncol. 1992, 45, 13–19. [Google Scholar] [CrossRef]
- Yang, L.; Ma, H. Quercetin inhibits the biological activity of endometrial cancer by regulating autophagy: A network pharmacology analysis and cellular experimental validation. J. Funct. Foods 2025, 125, 106657. [Google Scholar] [CrossRef]
- Zhang, L.; Mohankumar, K.; Martin, G.; Mariyam, F.; Park, Y.; Han, S.J.; Safe, S. Flavonoids Quercetin and Kaempferol Are NR4A1 Antagonists and Suppress Endometriosis in Female Mice. Endocrinology 2023, 164, bqad133. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.; Ma, M.; Guo, H. Quercetin inhibits the progression of endometrial HEC-1-A cells by regulating ferroptosis—A preliminary study. Eur. J. Med. Res. 2022, 27, 292. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Franciosi, M.L.M.; Cardoso, A.M. Oxidative damage and antioxidants in cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, N.; Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef]
- Holub, K.; Biete, A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin. Transl. Oncol. 2019, 21, 836–844. [Google Scholar] [CrossRef]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and Inflammation: Inseparable Actors of Cancer Progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: The role of chronic inflammation and oxidative stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef]
- Alrawaiq, N.S.; Abdullah, A. A review of flavonoid quercetin: Metabolism, bioactivity and antioxidant properties. Int. J. Pharm.Tech. Res. 2014, 6, 933–941. [Google Scholar]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef]
- Deng, S.; Yuan, P.; Sun, J. The role of NF-κB in carcinogenesis of cervical cancer: Opportunities and challenges. Mol. Biol. Rep. 2024, 51, 538. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.; Mohapatra, S.; Sahoo, A.; Baral, B.; Debata, P.R. Shifting of cell cycle arrest from the S-phase to G2/M phase and downregulation of EGFR expression by phytochemical combinations in HeLa cervical cancer cells. J. Biochem. Mol. Toxicol. 2022, 36, e22947. [Google Scholar] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Nandi, S.; Sikder, R.; Rapior, S.; Arnould, S.; Simal-Gandara, J.; Acharya, K. A review for cancer treatment with mushroom metabolites through targeting mitochondrial signaling pathway: In vitro and in vivo evaluations, clinical studies and future prospects for mycomedicine. Fitoterapia 2024, 172, 105681. [Google Scholar] [PubMed]
- Islam, S.M.R.; Siddiqua, T.J. 20—Functional foods in cancer prevention and therapy: Recent epidemiological findings. In Functional Foods in Cancer Prevention and Therapy; Kabir, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 405–433. [Google Scholar] [CrossRef]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, Z.; Chen, C.; Xu, W.; Liu, T.; Dong, Y.; Wang, J.; Wang, H.; Zhu, X. The impact of quercetin and paclitaxel combination on ovarian cancer cells. iScience 2024, 27, 110434. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch. Biochem. Biophys. 2021, 701, 108795. [Google Scholar] [PubMed]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, G.; Weng, D. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS ONE 2014, 9, e100314. [Google Scholar]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Peeri, N.C.; Bertrand, K.A.; Na, R.; De Vivo, I.; Setiawan, V.W.; Seshan, V.E.; Alemany, L.; Chen, Y.; Clarke, M.A.; Clendenen, T. Understanding risk factors for endometrial cancer in young women. JNCI J. Natl. Cancer Inst. 2025, 117, 76–88. [Google Scholar]
- Park, S.L.; Goodman, M.T.; Zhang, Z.F.; Kolonel, L.N.; Henderson, B.E.; Setiawan, V.W. Body size, adult BMI gain and endometrial cancer risk: The multiethnic cohort. Int. J. Cancer 2010, 126, 490–499. [Google Scholar]
- Kedzia, M.; Basta, P.; Czajkowski, K.; Gogacz, M.; Spaczynski, R.; Mroczkowska, B.; Stojko, R.; Szaflik, T.; Szubert, M.; Szyllo, K. Guidelines of the Polish Society of Gynecologists and Obstetricians on the management of women with endometriosis. Ginekol. Pol. 2024, 95, 729–758. [Google Scholar] [CrossRef] [PubMed]
- de Neufville Lucas, A.R. Lifestyle Interventions for Endometrial Cancer Survivors: Feasibility and Efficacy of a Novel Mindfulness and Dietary Counseling Program. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2014. [Google Scholar]
- Slighoua, M.; Amrati, F.E.; Chebaibi, M.; Mahdi, I.; Al Kamaly, O.; El Ouahdani, K.; Drioiche, A.; Saleh, A.; Bousta, D. Quercetin and Ferulic Acid Elicit Estrogenic Activities In Vivo and In Silico. Molecules 2023, 28, 5112. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cha, Y.-J.; Lee, K.-H.; Yim, J.-E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef]
- Joseph, A.; Shanmughan, P.; Balakrishnan, A.; Maliakel, B. Enhanced Bioavailability and Pharmacokinetics of a Natural Self-Emulsifying Reversible Hybrid-Hydrogel System of Quercetin: A Randomized Double-Blinded Comparative Crossover Study. ACS Omega 2022, 7, 46825–46832. [Google Scholar] [CrossRef]
- Van Zanden, J.J.; van der Woude, H.; Vaessen, J.; Usta, M.; Wortelboer, H.M.; Cnubben, N.H.; Rietjens, I.M. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem. Pharmacol. 2007, 74, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Santos, M.R.; Canário, S.; Borges, C.; Florêncio, M.H.; Mira, L. Plasma quercetin metabolites: Structure-antioxidant activity relationships. Arch Biochem. Biophys 2004, 432, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free. Radic. Res. 2001, 35, 941–952. [Google Scholar] [PubMed]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [PubMed]
- Singh, A.; Kureel, A.K.; Dutta, P.; Kumar, S.; Rai, A.K. Curcumin loaded chitin-glucan quercetin conjugate: Synthesis, characterization, antioxidant, in vitro release study, and anticancer activity. Int. J. Biol. Macromol. 2018, 110, 234–244. [Google Scholar]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 105686. [Google Scholar] [CrossRef]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef]
| Cancer Type | Sample/Model | Effect of Quercetin | Study Type | References |
|---|---|---|---|---|
| Cervical | HeLa, SiHa cells | ↓ cell viability (dose-dependent), G2/M arrest, p53-dependent mitochondrial apoptosis, ↓ NF-κB (p50, p65), ↓ CXCL8, MYC, IL-2, IL-1A | In vitro | [76,77,78] |
| Cervical | Nude mouse model (xenograft) | ↓ tumor growth, alters tumor endothelial ultrastructure, potential anti-angiogenic effects | In vivo | [79] |
| Cervical | U14 tumor-bearing mice + PEG-Que-NLs | Improved cytotoxicity vs. free quercetin; higher tumor inhibition rate with PEGylated liposomes | In vivo | [80] |
| Ovarian | SKOV-3 cells | Dose/time-dependent ↓ in cell proliferation, G0/G1 arrest, ↓ survivin, ↑ apoptosis | In vitro | [81] |
| Ovarian | PA-1 cell line (human metastatic) | ↓ Bcl-2, Bcl-xL; ↑ Bax, caspase-3/9, cytochrome c → promotes mitochondrial apoptosis | In vitro | [82] |
| Ovarian | OVCAR-3 TOV-112D A2780 | Overcomes cisplatin resistance and radiosensitization; pro-apoptotic, anti-proliferative, anti-inflammatory | In vitro | [83,84,85] |
| Ovarian | SKOV-3 /mouse xenograft | Low-dose Quercetin reduces chemo cytotoxicity; high dose is pro-apoptotic (↑ antioxidant enzymes, ↓ oxidative damage) | In vitro In vivo | [83] |
| Ovarian | Cisplatin-resistant SKOV-3/CDDP | Re-sensitizes resistant cells to cisplatin, blocks PI3K/Akt/mTOR, ↓ Nrf2 and SOD2, promotes mitochondrial apoptosis, pro-oxidant effect | In vitro | [86,87] |
| Ovarian | Phase I trial (advanced cancers) | IV quercetin safe, anecdotal ↓ CA-125 in an ovarian cancer patient; no follow-up Phase II specific to OC | Clinical (Phase I) | [88] |
| Endometrial | Tumors | Quercetin (0.01–10 μM) → dose-dependent suppression of colony formation | In vitro | [89] |
| Endometrial | Ishikawa, HEC-1 A cells | ↓ proliferation, invasion, migration; | In vitro | [90] |
| ↑ apoptosis; modulates ATF5/JUN/PI3K/AKT/mTOR, induces autophagy | ||||
| Endometrial | Human endometriotic epithelial and stromal cells) + Animal model (mouse) | ↓ proliferation, suppressed multiple pathways (EGFR/c-Myc/survivin, mTOR), ↓ lesion size in mouse model | In vitro In vivo | [91] |
| Endometrial | HEC-1-A cells | ↓ proliferation, migration; ↑ apoptosis, cell cycle arrest; triggers ferroptosis | In vitro | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamineh, Y.; Ghiasvand, M.; Panahi-Alanagh, S.; Rastegarmand, P.; Zolghadri, S.; Stanek, A. A Narrative Review of Quercetin’s Role as a Bioactive Compound in Female Reproductive Disorders. Nutrients 2025, 17, 1118. https://doi.org/10.3390/nu17071118
Khamineh Y, Ghiasvand M, Panahi-Alanagh S, Rastegarmand P, Zolghadri S, Stanek A. A Narrative Review of Quercetin’s Role as a Bioactive Compound in Female Reproductive Disorders. Nutrients. 2025; 17(7):1118. https://doi.org/10.3390/nu17071118
Chicago/Turabian StyleKhamineh, Yasaman, Mahsa Ghiasvand, Sanaz Panahi-Alanagh, Parisa Rastegarmand, Samaneh Zolghadri, and Agata Stanek. 2025. "A Narrative Review of Quercetin’s Role as a Bioactive Compound in Female Reproductive Disorders" Nutrients 17, no. 7: 1118. https://doi.org/10.3390/nu17071118
APA StyleKhamineh, Y., Ghiasvand, M., Panahi-Alanagh, S., Rastegarmand, P., Zolghadri, S., & Stanek, A. (2025). A Narrative Review of Quercetin’s Role as a Bioactive Compound in Female Reproductive Disorders. Nutrients, 17(7), 1118. https://doi.org/10.3390/nu17071118











