Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy
Abstract
1. Introduction
2. Materials and Methods
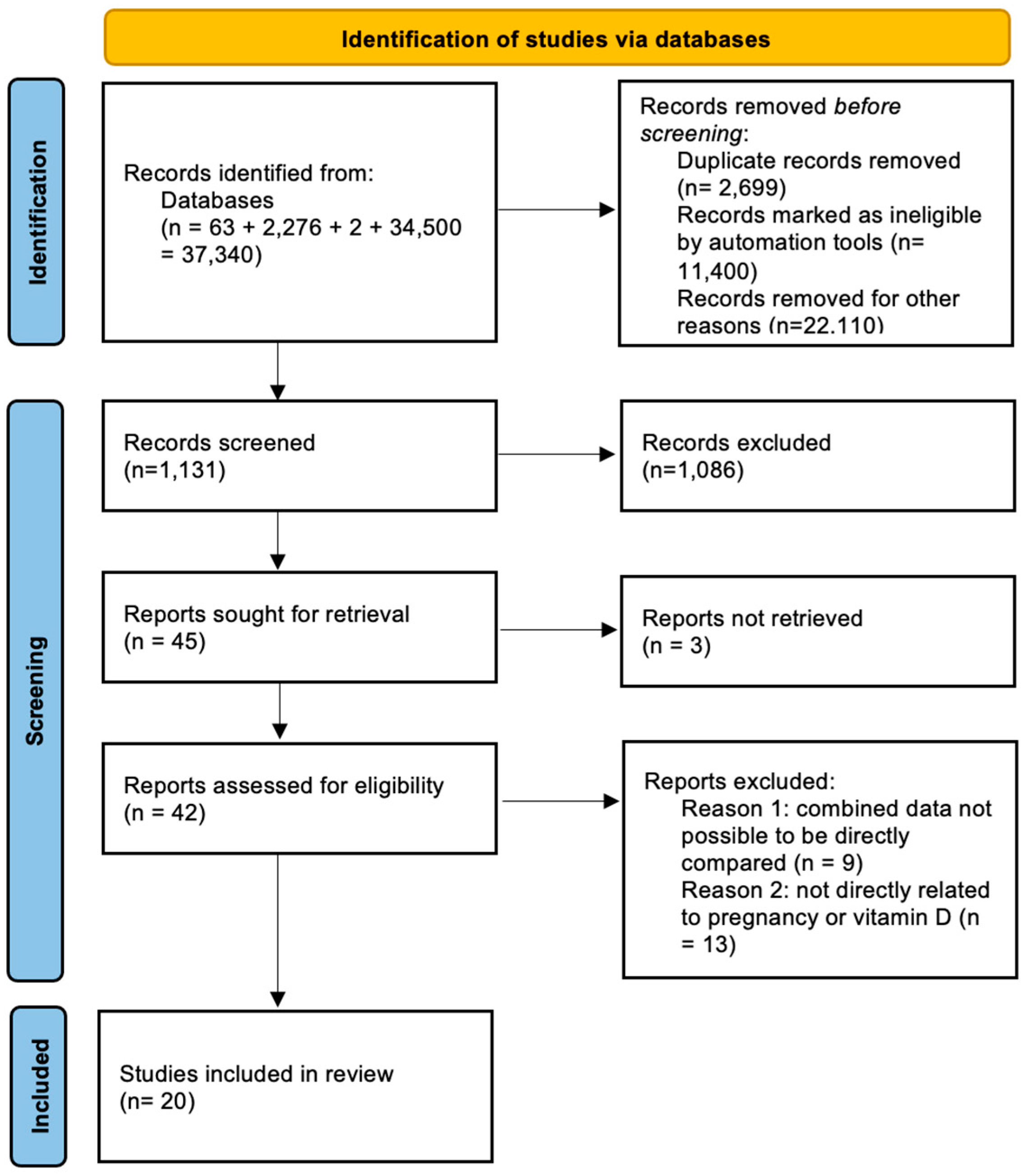
2.1. Eligibility Criteria, Information Sources, and Search Strategy
2.2. Study Selection
2.3. Data Extraction
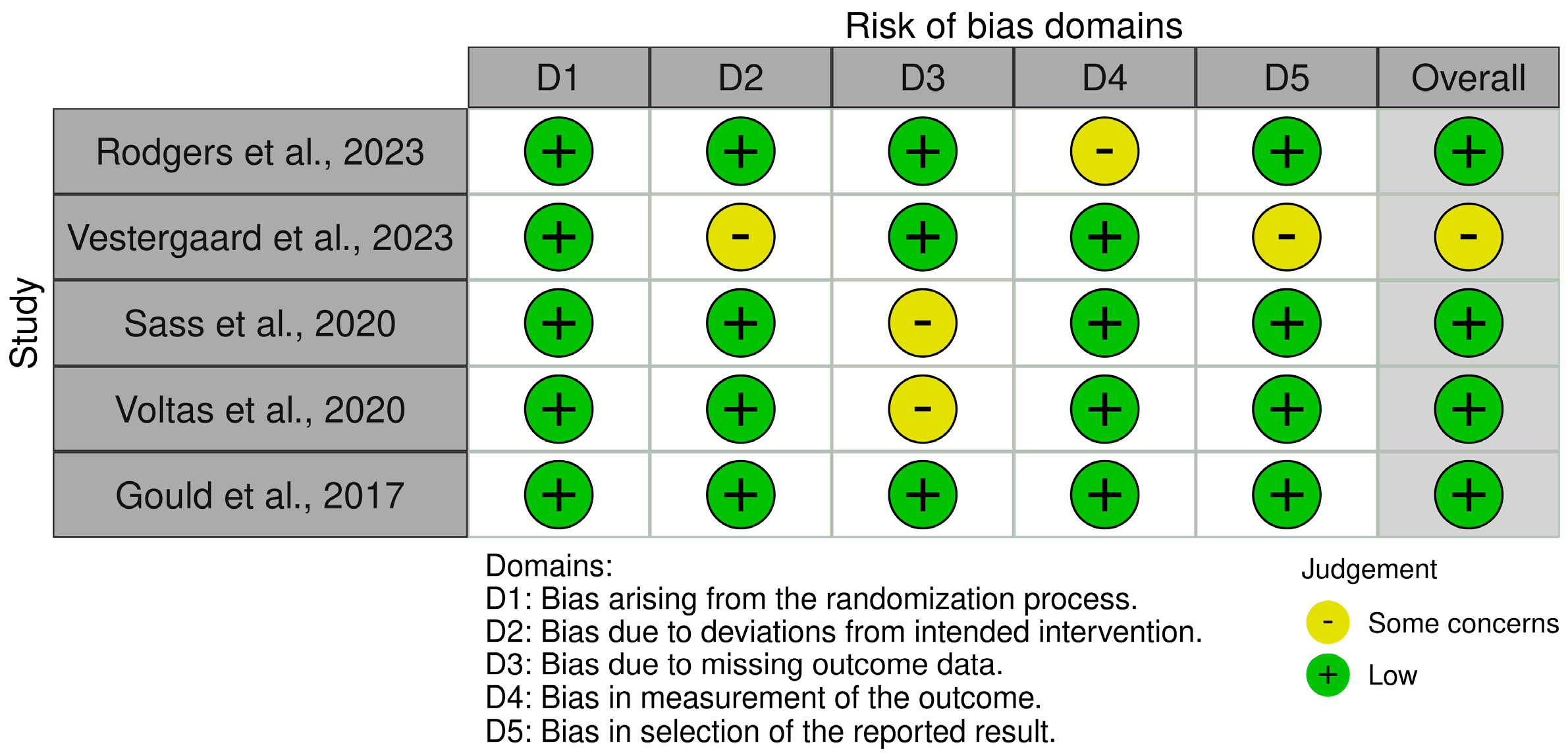
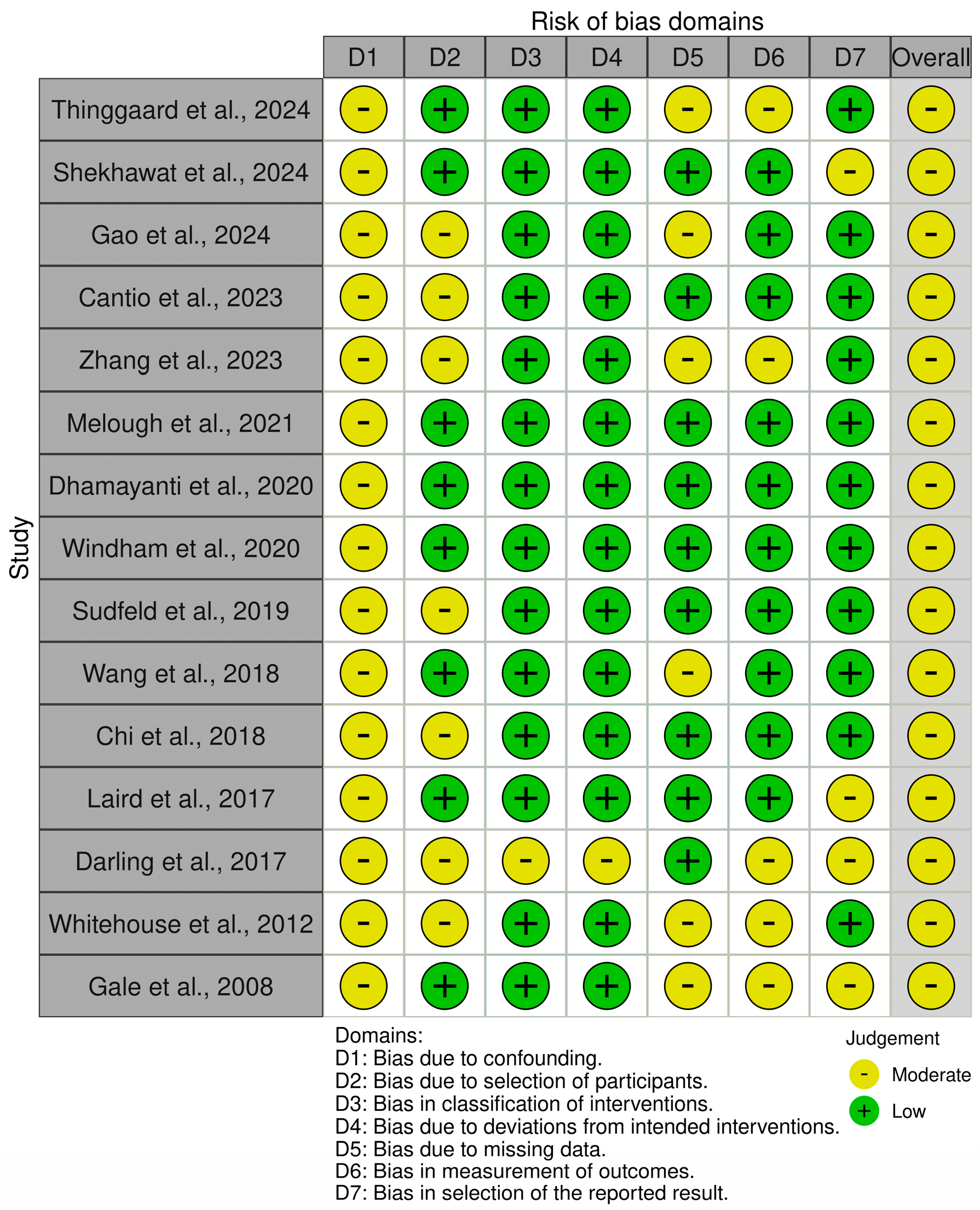
2.4. Assessment of Risk of Bias
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wimalawansa, S.J. Physiology of Vitamin D—Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-C.; Huang, C.-Y.; Wang, J.-H.; Shih, C.-L.; Wu, P. Effects of vitamin D in pregnancy on maternal and offspring health-related outcomes: An umbrella review of systematic review and meta-analyses. Nutr. Diabetes 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am. J. Physiol.-Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C.; The Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin d3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Becker, A.; Eyles, D.W.; McGrath, J.J.; Grecksch, G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav. Brain Res. 2005, 161, 306–312. [Google Scholar] [CrossRef]
- Tuovinen, S.; Räikkönen, K.; Holmlund-Suila, E.; Hauta-alus, H.; Helve, O.; Rosendahl, J.; Enlund-Cerullo, M.; Kajantie, E.; Valkama, S.; Viljakainen, H.; et al. Effect of High-Dose vs Standard-Dose Vitamin D Supplementation on Neurodevelopment of Healthy Term Infants: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2124493. [Google Scholar] [CrossRef]
- Sass, L.; Vinding, R.K.; Stokholm, J.; Bjarnadóttir, E.; Noergaard, S.; Thorsen, J.; Sunde, R.B.; McGrath, J.; Bønnelykke, K.; Chawes, B.; et al. High-Dose Vitamin D Supplementation in Pregnancy and Neurodevelopment in Childhood: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2026018. [Google Scholar] [CrossRef]
- García-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 2468–2481. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Koutra, K.; Chalkiadaki, G.; Katrinaki, M.; Kyriklaki, A.; Kampouri, M.; Margetaki, K.; Vafeiadi, M.; Papavasiliou, S.; et al. High maternal vitamin D levels in early pregnancy may protect against behavioral difficulties at preschool age: The Rhea mother–child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 2018, 27, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A. Unraveling mechanisms of human brain evolution. Cell 2024, 187, 5838–5857. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Luo, J.; Mao, D.; Lei, X.; Liu, C.; Zhang, S.; Yao, Q.; Li, J.; Zhang, J.; et al. Effect modification by maternal vitamin D status in the association between prenatal exposure to per- and polyfluoroalkyl substances and neurodevelopment in 2-year-old children. Environ. Int. 2024, 185, 108563. [Google Scholar] [CrossRef]
- Shekhawat, D.S.; Singh, K.; Singh, P.; Vyas, V.; Varthya, S.B.; Sharma, P. Prenatal vitamin D levels and infant cognitive, motor, language and social-emotional development at 6 and 9 months of age. Nutr. Neurosci. 2024, 28, 263–272. [Google Scholar] [CrossRef]
- Thinggaard, C.M.; Dalgård, C.; Möller, S.; Christesen, H.B.T.; Bilenberg, N. Vitamin D status in pregnancy and cord blood is associated with symptoms of attention-deficit hyperactivity disorder at age 5 years: Results from Odense Child Cohort. Aust. N. Z. J. Psychiatry 2024, 58, 1090–1102. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.-Y.; Wang, X.-R.; Jiao, X.-T.; Zhang, J.; Tian, Y.; Li, L.-L.; Chen, C.; Yu, X.-D. Maternal and neonatal blood vitamin D status and neurodevelopment at 24 months of age: A prospective birth cohort study. World J. Pediatr. 2023, 19, 883–893. [Google Scholar] [CrossRef]
- Cantio, E.; Bilenberg, N.; Nørgaard, S.M.; Beck, I.H.; Möller, S.; Cantio, C.; Jensen, T.K.; Mortensen, N.B.; Rasmussen, A.; Christesen, H.B.T. Vitamin D status in pregnancy and childhood associates with intelligence quotient at age 7 years: An Odense child cohort study. Aust. N. Z. J. Psychiatry 2023, 57, 1062–1072. [Google Scholar] [CrossRef]
- Melough, M.M.; Murphy, L.E.; Graff, J.C.; Derefinko, K.J.; LeWinn, K.Z.; Bush, N.R.; Enquobahrie, D.A.; Loftus, C.T.; Kocak, M.; Sathyanarayana, S.; et al. Maternal Plasma 25-Hydroxyvitamin D during Gestation Is Positively Associated with Neurocognitive Development in Offspring at Age 4–6 Years. J. Nutr. 2021, 151, 132–139. [Google Scholar] [CrossRef]
- Dhamayanti, M.; Noviandhari, A.; Supriadi, S.; Judistiani, R.T.; Setiabudiawan, B. Association of maternal vitamin D deficiency and infants’ neurodevelopmental status: A cohort study on vitamin D and its impact during pregnancy and childhood in Indonesia. J. Paediatr. Child Health 2020, 56, 16–21. [Google Scholar] [CrossRef]
- Windham, G.C.; Pearl, M.; Poon, V.; Berger, K.; Soriano, J.W.; Eyles, D.; Lyall, K.; Kharrazi, M.; Croen, L.A. Maternal Vitamin D Levels During Pregnancy in Association With Autism Spectrum Disorders (ASD) or Intellectual Disability (ID) in Offspring; Exploring Non-linear Patterns and Demographic Sub-groups. Autism Res. 2020, 13, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; Jacobson, D.L.; Rueda, N.M.; Neri, D.; Mendez, A.J.; Butler, L.; Siminski, S.; Hendricks, K.M.; Mellins, C.A.; Duggan, C.P.; et al. Third Trimester Vitamin D Status Is Associated With Birth Outcomes and Linear Growth of HIV-Exposed Uninfected Infants in the United States. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 81, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.-Z.; Zhu, L.; Zhang, Z.-L.; Jin, F.-F.; Shao, H.-R.; Zheng, J.-Y.; Wu, C.; Hu, G.-Q. The Relationship between Maternal Serum Vitamin D Levels and Infant Neurodevelopment and Anthropometry: A Prospective Observational Study. J. Nutr. Sci. Vitaminol. 2018, 64, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Rayman, M.P.; Steer, C.D.; Golding, J.; Lanham-New, S.A.; Bath, S.C. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2017, 117, 1682–1692. [Google Scholar] [CrossRef]
- Laird, E.; Thurston, S.; Van Wijngaarden, E.; Shamlaye, C.; Myers, G.; Davidson, P.; Watson, G.; McSorley, E.; Mulhern, M.; Yeates, A.; et al. Maternal Vitamin D Status and the Relationship with Neonatal Anthropometric and Childhood Neurodevelopmental Outcomes: Results from the Seychelles Child Development Nutrition Study. Nutrients 2017, 9, 1235. [Google Scholar] [CrossRef]
- Whitehouse, A.J.O.; Holt, B.J.; Serralha, M.; Holt, P.G.; Kusel, M.M.H.; Hart, P.H. Maternal Serum Vitamin D Levels During Pregnancy and Offspring Neurocognitive Development. Pediatrics 2012, 129, 485–493. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.D.; Huang, L.S.; Chen, Q.; Ouyang, F.X.; Wang, X.; Zhang, J. Fetal vitamin D concentration and growth, adiposity and neurodevelopment during infancy. Eur. J. Clin. Nutr. 2018, 72, 1396–1403. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Voltas, N.; Canals, J.; Hernández-Martínez, C.; Serrat, N.; Basora, J.; Arija, V. Effect of Vitamin D Status during Pregnancy on Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2020, 12, 3196. [Google Scholar] [CrossRef]
- Rodgers, M.D.; Mead, M.J.; McWhorter, C.A.; Ebeling, M.D.; Shary, J.R.; Newton, D.A.; Baatz, J.E.; Gregoski, M.J.; Hollis, B.W.; Wagner, C.L. Vitamin D and Child Neurodevelopment—A Post Hoc Analysis. Nutrients 2023, 15, 4250. [Google Scholar] [CrossRef]
- Vestergaard, A.L.; Andersen, M.K.; Olesen, R.V.; Bor, P.; Larsen, A. High-Dose Vitamin D Supplementation Significantly Affects the Placental Transcriptome. Nutrients 2023, 15, 5032. [Google Scholar] [CrossRef]
- Gould, J.F.; Anderson, A.J.; Yelland, L.N.; Smithers, L.G.; Skeaff, C.M.; Zhou, S.J.; Gibson, R.A.; Makrides, M. Association of cord blood vitamin D with early childhood growth and neurodevelopment. J. Paediatr. Child Health 2017, 53, 75–83. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14, 4230. [Google Scholar] [CrossRef]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Gáll, Z.; Székely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef]
- Sailike, B.; Onzhanova, Z.; Akbay, B.; Tokay, T.; Molnár, F. Vitamin D in Central Nervous System: Implications for Neurological Disorders. Int. J. Mol. Sci. 2024, 25, 7809. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, R.; Wang, J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 86. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clin. Nutr. 2022, 41, 1532–1540. [Google Scholar] [CrossRef]
- Snellman, G.; Melhus, H.; Gedeborg, R.; Byberg, L.; Berglund, L.; Wernroth, L.; Michaëlsson, K. Determining Vitamin D Status: A Comparison between Commercially Available Assays. PLoS ONE 2010, 5, e11555. [Google Scholar] [CrossRef]
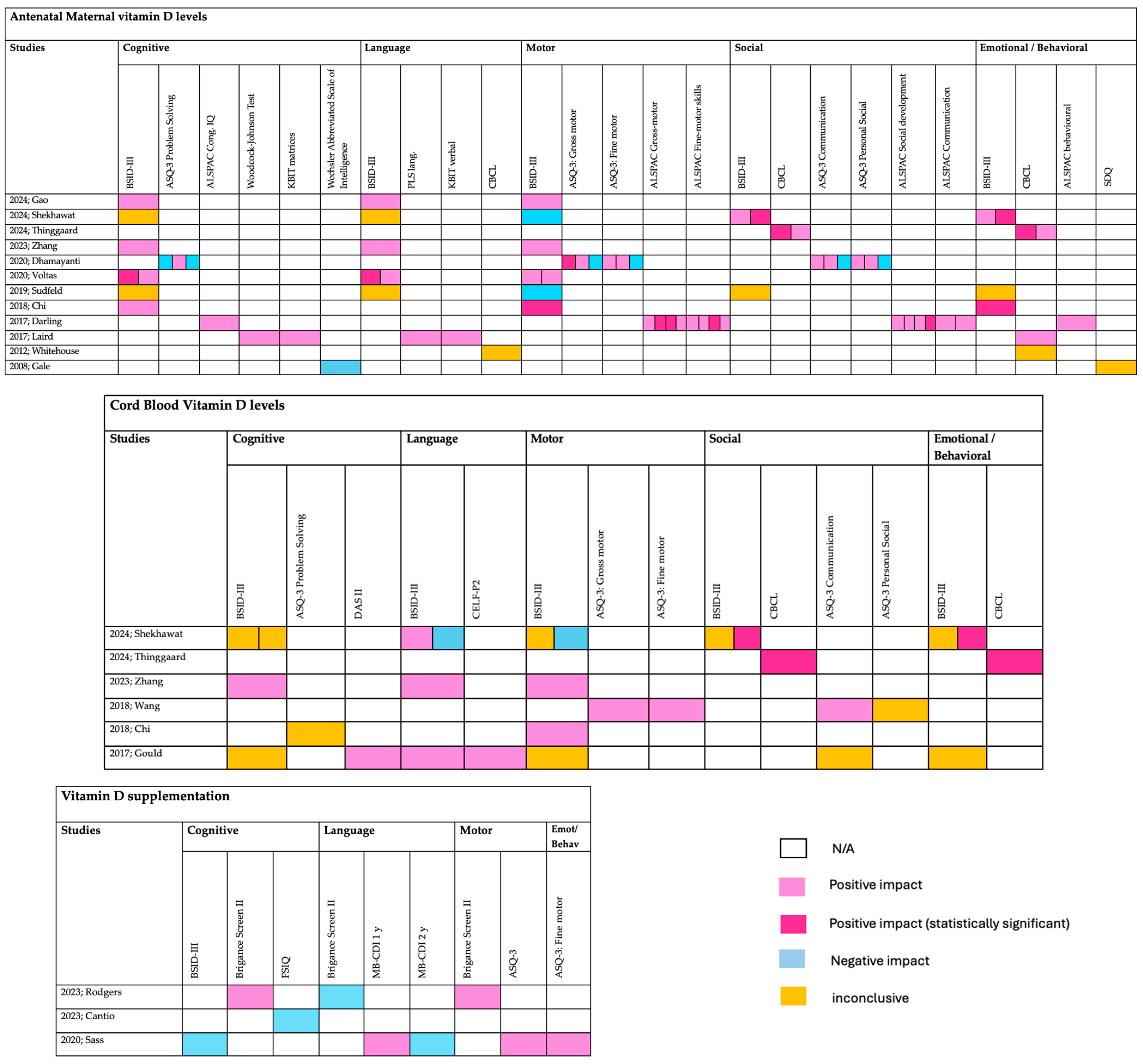
| Antenatal maternal Vitamin D levels | ||||||
| Year; Author | Study type | Patient number | Timing | Outcome | Outcome measures | Assessment scale |
| 2024; Gao [14] | Prospective | 746 mother–child pairs | second trimester | 24 months | Cognitive, Language, Motor | BSID-III |
| 2024; Shekhawat [15] | Prospective | 175 mother–child pairs | Intrapartum period (36.02–40.4 weeks of gestation | 6 and 9 months of age | cognitive, motor, language and social-emotional | BSID-III |
| 2024; Thinggaard [16] | Prospective | 944 mother–child pairs | GA < 20 weeks, GA ≥ 20 weeks and umbilical cord at delivery | 5 years | ADHD symptoms (emotional, behavioral and social problems) | CBCL/1½-5 |
| 2023; Zhang [17] | Prospective | 649 mother–infant pairs | in three trimesters, and cord blood at delivery | 24 months | cognition, language, motor, social-emotional, and adaptive behaviour | BSID-III |
| 2023; Cantio [18] | Prospective | 1404 mother–child pairs | GA < 20 weeks or GA ≥ 20 weeks, from the umbilical cord | 7 years | cognitive functions | WISC-V FSIQ |
| 2021; Melough [19] | Prospective | 1019 eligible dyads | 16 and 28 weeks | 4–6 Years | IQ (Neurocognitive Development) | SB5 VIQ NVIQ FSIQ |
| 2020; Voltas [29] | Randomized | 422 mother–infant pairs | 12th and 36th weeks of gestation | 0 to 42 months | -cognitive, language, and motor skills | BSID-III |
| 2020; Dhamayanti [20] | Prospective | 141 mother–infant pairs | 10 and 14 weeks of gestational age | 3, 6 and 12 months | gross motor, fine motor, communication, problem solving and personal–social domains | ASQ-3 |
| 2020; Windham [21] | Retrospective | 534 ASD, 181 ID, 421 controls | mid-pregnancy | n/a | Autism or low IQ | -DSM-IV-TR -composite developmental/cognitive score < 70 |
| 2019; Sudfeld [22] | Prospective | 257 HIV–infected mothers and their HIV–exposed uninfected infants | Third trimester | 12 months +/− 3 months | neurodevelopment (cognitive, motor, and language) | BSID-III |
| 2018; Chi [23] | Prospective | 160 mother–child pairs | Over 28 weeks | 6 months | -cognitive, motor, and language -socioemotional and adaptive behavior scores | -BSID-III -MDI -PDI |
| 2017; Darling [24] | Prospective | 7065 mother–child pairs | During pregnancy (Mean 23.4 weeks) | 6 months–9 years | -behavioral development (81 months of age) -IQ (8 years of age) | -SDQ -Wechsler Intelligence Scale for Children |
| 2017; Laird [25] | Prospective | 202 healthy pregnant women | One day after delivery | 5 years | birth weight and head circumference, neurocognitive function | FT, PLS-TL, PLS-VA, PLS-AC, WJSAT, CBCL, KBIT-VK, KBIT-M |
| 2012; Whitehouse [26] | Prospective | 743 women | 18 weeks | 2-, 5-, 8-, 10-, 14-, 17-years | neurodevelopment behavioral, emotional, and language | CBCL |
| 2008; Gale [4] | Prospective | 596 women | Third trimester | 9 months, 9 years | -Cognitive function -Psychological health | -WASI -SDQ |
| Vitamin D supplementation | ||||||
| 2023; Rodgers [30] | Randomized | 350 women | From 12–16 weeks of gestation until delivery | 3–5 years | language, motor, and academic | Brigance Screen II |
| 2023; Vestergaard [31] | Randomized | 70 pregnancies | late first trimester to delivery | time of delivery | genes involved in pathways linked to autism spectrum disorders, ADHD, schizophrenia | next-generation RNA sequencing |
| 2020; Sass [9] | Randomized | 623 women | From 24 weeks until 1 week postpartum | 6 years | -Cognitive at 2.5 y.o. -motor milestone achievement -language at age 3 y.o. -emotional/behavioural at 6 y.o. | BSID-III, Denver Developmental Index, WHO milestone registration, MacArthur-Bates Communicative Development Inventories, ASQ, SDQ |
| Fetal Vitamin D and neurodevelopment | ||||||
| 2018; Wang [27] | Prospective | 1244 infants | At delivery | 6 months, 1 and 2 years | communication, gross motor, fine motor, problem-solving skills, and personal social) | ASQ |
| 2017; Gould [32] | Randomized | 337 infants | At delivery | −18 months −4 years | cognitive, language and motor | -BSID-III -DAS II and CELF-P2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varthaliti, A.; Rodolaki, K.; Lygizos, V.; Vlachos, D.E.; Thomakos, N.; Sioutis, D.; Daskalakis, G.; Pergialiotis, V. Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy. Nutrients 2025, 17, 978. https://doi.org/10.3390/nu17060978
Varthaliti A, Rodolaki K, Lygizos V, Vlachos DE, Thomakos N, Sioutis D, Daskalakis G, Pergialiotis V. Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy. Nutrients. 2025; 17(6):978. https://doi.org/10.3390/nu17060978
Chicago/Turabian StyleVarthaliti, Antonia, Kalliopi Rodolaki, Vasilios Lygizos, Dimitrios Efthymios Vlachos, Nikolaos Thomakos, Dimos Sioutis, George Daskalakis, and Vasilios Pergialiotis. 2025. "Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy" Nutrients 17, no. 6: 978. https://doi.org/10.3390/nu17060978
APA StyleVarthaliti, A., Rodolaki, K., Lygizos, V., Vlachos, D. E., Thomakos, N., Sioutis, D., Daskalakis, G., & Pergialiotis, V. (2025). Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy. Nutrients, 17(6), 978. https://doi.org/10.3390/nu17060978