Bifidogenic Effect of 2′-Fucosyllactose (2′-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial
Highlights
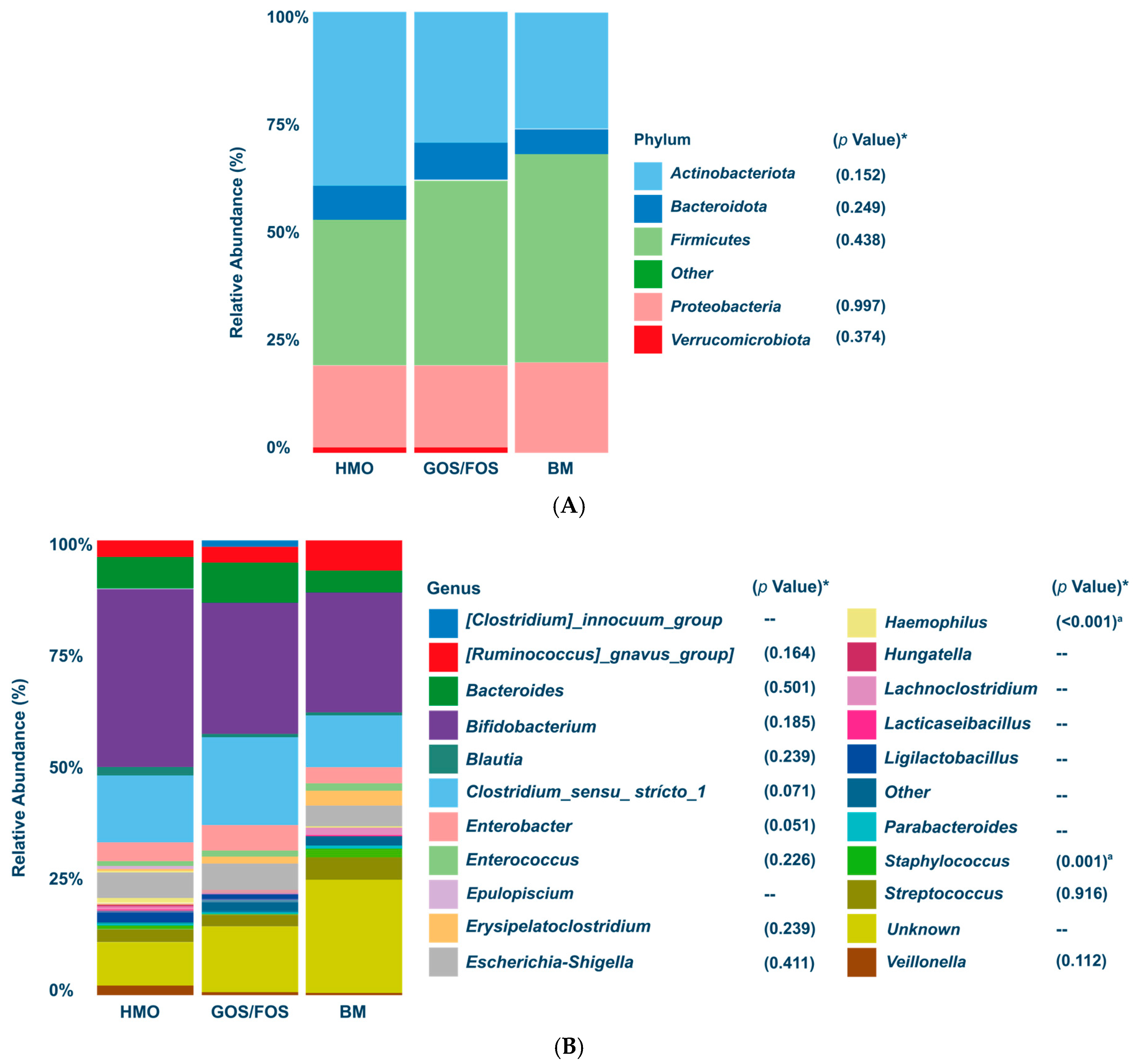
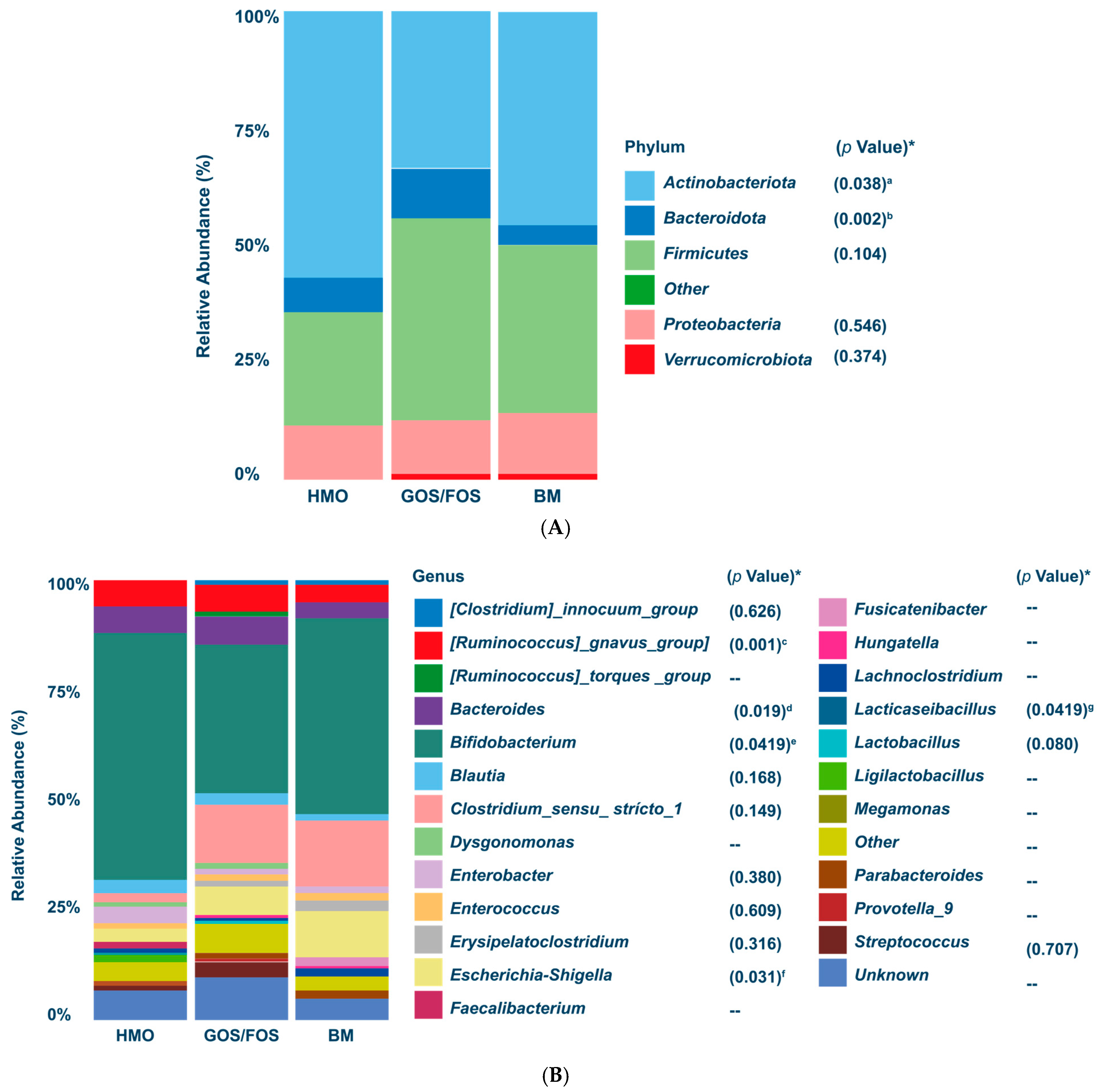
- Dominance of Actinobacteriota and Bifidogenic Effect: After the intervention, Actinobacteriota became the predominant phylum in both the HMO and BM groups, with the Bifidobacterium genus showing similar relative abundance in the HMO formula-fed and BM groups (p > 0.05).
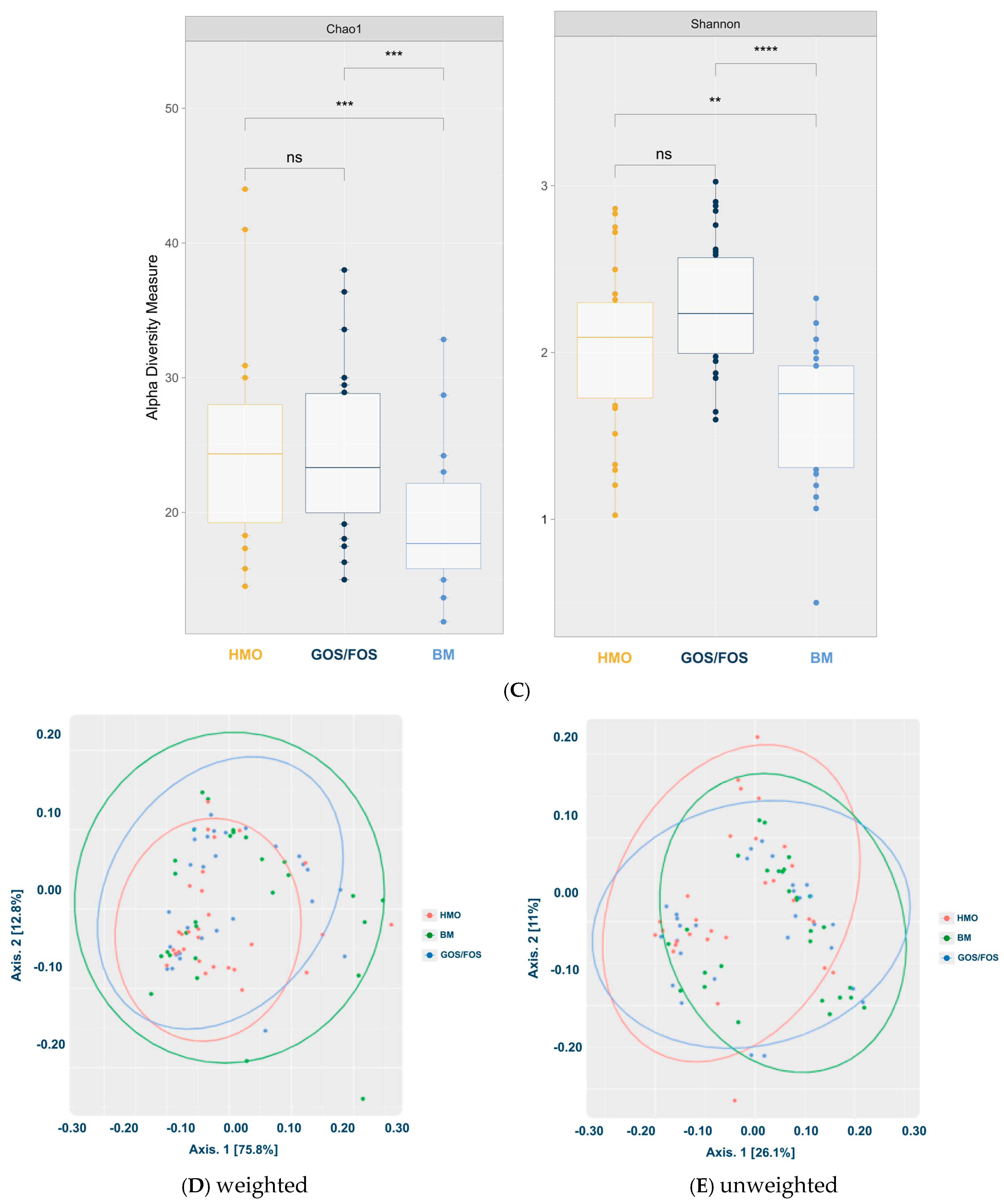
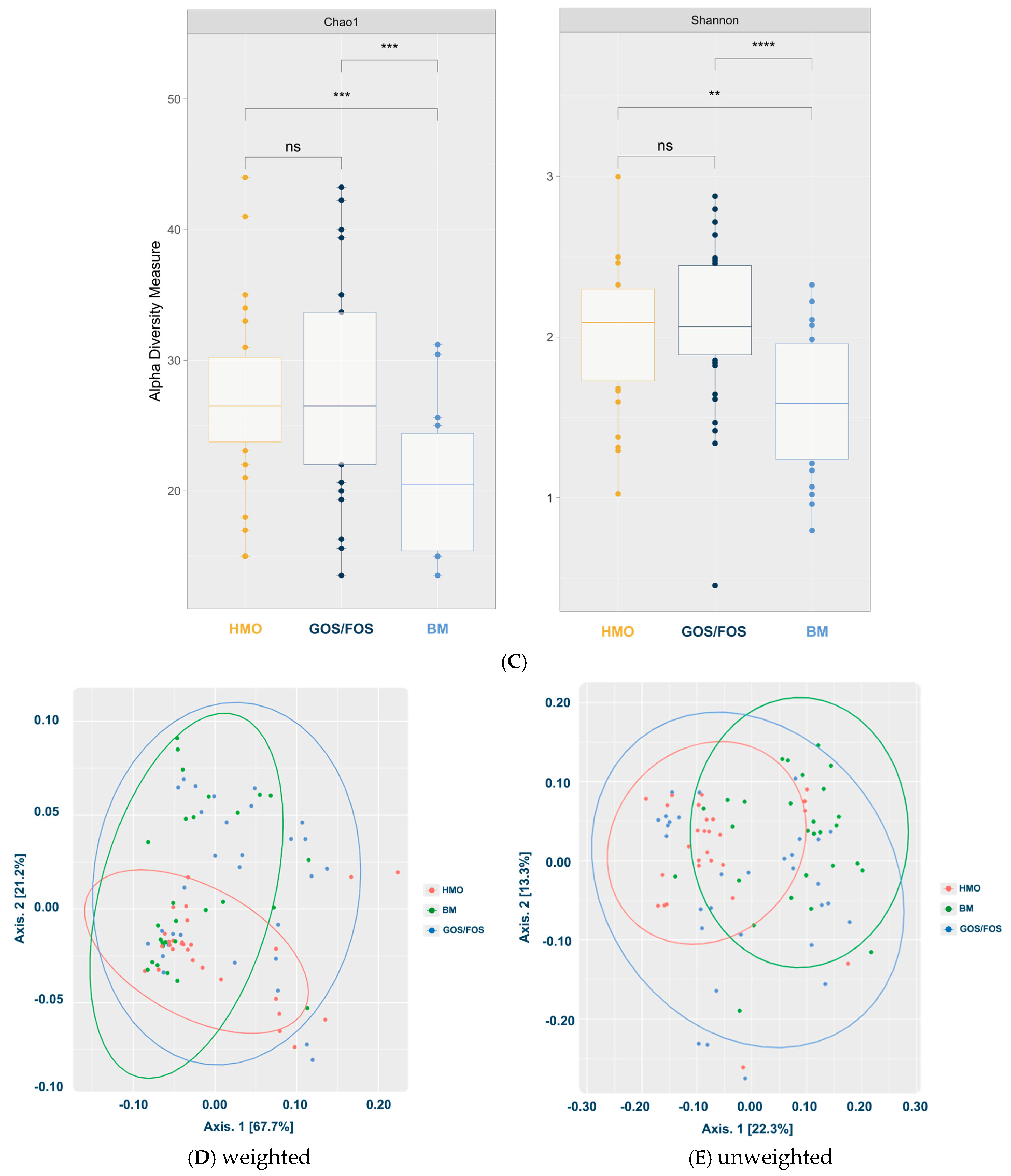
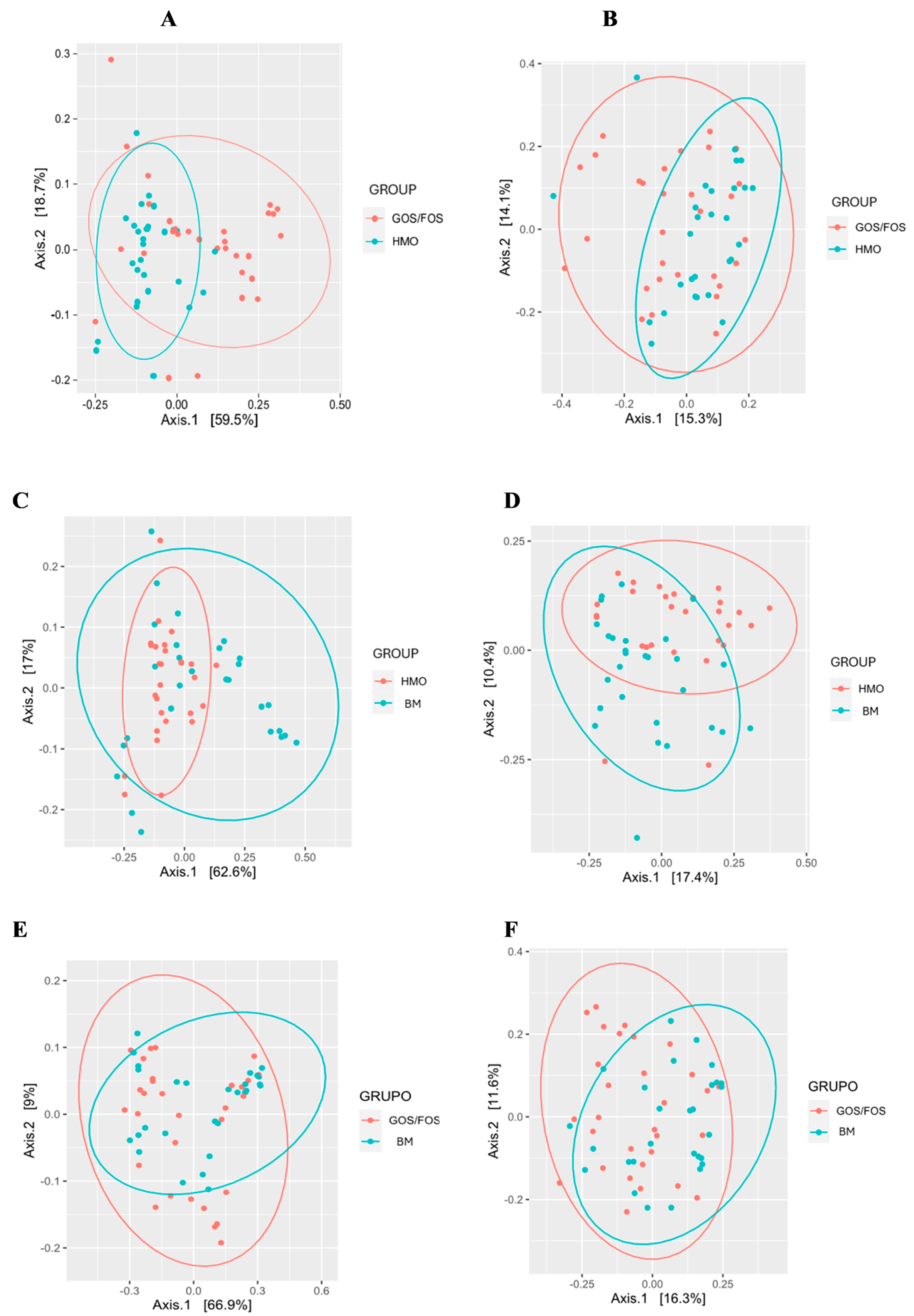
- Microbiota Diversity Differences: Formula-fed infants exhibited higher alpha-diversity of gut microbiota compared to breastfed infants (p < 0.05), and beta-diversity significantly varied across feeding groups.
- Enhanced Bifidogenic Impact: The addition of 2′-FL to a prebiotic-containing formula (GOS + FOS) strengthened its bifidogenic effect, further supporting its safety, tolerability, and positive impact on gut microbiota development.
Abstract
1. Introduction
2. Materials and Methods
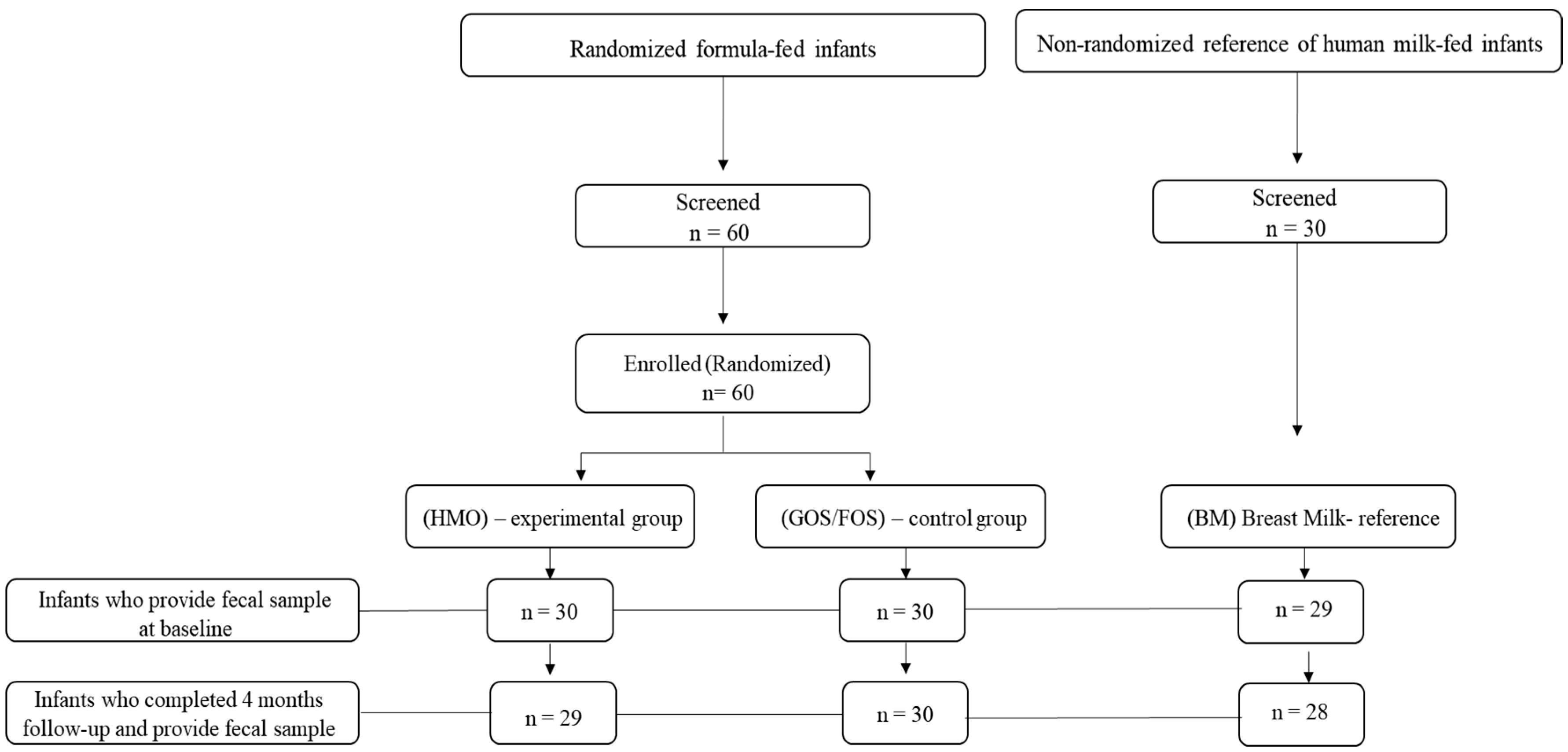
2.1. Study Design
2.2. Study Population
2.3. Gut Microbiota Analysis
2.3.1. Collection and Storage
2.3.2. Microbiota Analysis
2.3.3. Statistical Analysis and Bioinformatics
3. Results
3.1. Demographic and Anthropometric Characteristics and Fecal Microbiota Profile of Infants at Study Enrollment
3.2. Dietary Intervention and Anthropometric Changes
3.3. Fecal Microbiota Profile After Intervention
3.3.1. Relative Abundance of Bacterial Phyla and Genera
3.3.2. Alpha-Diversity and Beta-Diversity in the Final Collection
3.3.3. Beta-Diversity: Pairwise Comparison Between Groups After Intervention
3.3.4. Gastrointestinal Symptoms and Bowel Habits of Infants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-FL | 2-Fucosyllactose |
| FOS | Fructo-oligosaccharides |
| GOS | Galacto-oligosaccharides |
| GOS/FOS group | Control group |
| HMO | Human milk oligosaccharides |
| HMO group | Experimental group |
| BM group | Breast milk group (reference) |
References
- World Health Organization, United Nations Children’s Fund. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- BRASIL; Ministério da Saúde; Secretaria de Atenção Primaria à Saúde; Departamento de Promoção da Saúde. Guia Alimentar Para Crianças Brasileiras Menores De 2 Anos; Secretaria de Atenção Primaria à Saúde; Departamento de Promoção da Saúde; Ministério da Saúde: Brasília, Brazil, 2019. [Google Scholar]
- Sociedade Brasileira de Pediatria (SBP); Weffort, V.R.S. Manual De Alimentação: Orientações Para Alimentação Do Lactente Ao Adolescente, Na Escola, Na Gestante, Na Prevenção De Doenças E Segurança Alimentar; Sociedade Brasileira de Pediatria, Departamento Científico de Nutrologia; SBP: São Paulo, Brazil, 2018. [Google Scholar]
- ILSI-Brasil. Dinâmica da Composição Do Leite Humano E Suas Implicações Clínicas; ILSI Brasil (International Life Sciences Institute do Brasil): São Paulo, Brazil, 2018. [Google Scholar]
- Weffort, V.R.S.; Lamounier, J.A. Nutrição Em Pediatria: Da Neonatologia À Adolescência, 3rd ed.; Manole: São Paulo, Brazil, 2024; pp. 156–185. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.C.; Mello, C.S. Aleitamento Materno: Tópicos Atuais E Evidências Clínicas, 1st ed.; Rubio: Rio de Janeiro, Brazil, 2022; pp. 13–38. [Google Scholar]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Yu, Z.T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship Between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Borewicz, K.; Gu, F.; Saccenti, E.; Arts, I.C.W.; Penders, J.; Thijs, C.; van Leeuwen, S.S.; Lindner, C.; Nauta, A.; van Leusen, E.; et al. Correlating Infant Fecal Microbiota Composition and Human Milk Oligosaccharide Consumption by Microbiota of 1-Month-Old Breastfed Infants. Mol. Nutr. Food Res. 2019, 63, e1801214. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First Foods and Gut Microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Thomson, P.; Medina, D.A.; Garrido, D. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiol. 2018, 75, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2′-fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Ruiz-Moyano, S.; Lemay, D.G.; Sela, D.A.; German, J.B.; Mills, D.A. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci. Rep. 2015, 5, 13517, Erratum in Sci Rep. 2015, 5, 15311. [Google Scholar] [CrossRef]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula With 2′FL Show Growth and 2′FL Uptake Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kajzer, J.; Oliver, J.; Marriage, B.J. Gastrointestinal Tolerance of Formula Supplemented with Oligosaccharides. FASEB J. 2016, 30, 671–674. [Google Scholar] [CrossRef]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio 2020, 11, e03196-19. [Google Scholar] [CrossRef]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Task Force. Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants Elk Grove Village; US Food and Drug Administration: Silver Spring, MD, USA, 1988. Available online: http://www.fda.gov (accessed on 4 March 2025).
- WHO Multicenter Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; Volume 14. [Google Scholar]
- Mason, C.E. Microbiomics 2019–2020, by Zimo Catalog. Available online: https://zymoresearch.eu/pages/literature (accessed on 4 March 2025).
- Cruz, G.N.F.; Christoff, A.P.; de Oliveira, L.F.V. Equivolumetric Protocol Generates Library Sizes Proportional to Total Microbial Load in 16S Amplicon Sequencing. Front. Microbiol. 2021, 12, 638231. [Google Scholar] [CrossRef]
- Sakanaka, M.; Gotoh, A.; Yoshida, K.; Odamaki, T.; Koguchi, H.; Xiao, J.Z.; Kitaoka, M.; Katayama, T. Varied Pathways of Infant Gut-Associated Bifidobacterium to Assimilate Human Milk Oligosaccharides: Prevalence of the Gene Set and Its Correlation with Bifidobacteria-Rich Microbiota Formation. Nutrients 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- De Leoz, M.L.; Kalanetra, K.M.; Bokulich, N.A.; Strum, J.S.; Underwood, M.A.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk glycomics and gut microbial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: A proof-of-concept study. J. Proteome Res. 2015, 14, 491–502. [Google Scholar] [CrossRef]
- Hu, M.; Li, M.; Li, C.; Miao, M.; Zhang, T. Effects of Human Milk Oligosaccharides in Infant Health Based on Gut Microbiota Alteration. J. Agric. Food Chem. 2023, 71, 994–1001. [Google Scholar] [CrossRef]
- Chichlowski, M.; van Diepen, J.A.; Prodan, A.; Olga, L.; Ong, K.K.; Kortman, G.A.M.; Dunger, D.B.; Gross, G. Early development of infant gut microbiota in relation to breastfeeding and human milk oligosaccharides. Front. Nutr. 2023, 10, 1003032. [Google Scholar] [CrossRef]
- Lawson, M.A.E.; O’Neill, I.J.; Kujawska, M.; Gowrinadh Javvadi, S.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef]
- Lou, Y.C.; Rubin, B.E.; Schoelmerich, M.C.; DiMarco, K.S.; Borges, A.L.; Rovinsky, R.; Song, L.; Doudna, J.A.; Banfield, J.F. Infant microbiome cultivation and metagenomic analysis reveal Bifidobacterium 2′-fucosyllactose utilization can be facilitated by coexisting species. Nat. Commun. 2023, 14, 7417. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Sprenger, N.; Ghyselinck, J.; Marsaux, B.; Marzorati, M.; Rochat, F. A Comparison of the In Vitro Effects of 2′Fucosyllactose and Lactose on the Composition and Activity of Gut Microbiota from Infants and Toddlers. Nutrients 2021, 13, 726. [Google Scholar] [CrossRef]
- Natividad, J.M.; Marsaux, B.; Rodenas, C.L.G.; Rytz, A.; Vandevijver, G.; Marzorati, M.; Van den Abbeele, P.; Calatayud, M.; Rochat, F. Human Milk Oligosaccharides and Lactose Differentially Affect Infant Gut Microbiota and Intestinal Barrier In Vitro. Nutrients 2022, 14, 2546. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, L.Q.; Liu, F.; Wu, J.Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef]
- Estorninos, E.; Lawenko, R.B.; Palestroque, E.; Sprenger, N.; Benyacoub, J.; Kortman, G.A.M.; Boekhorst, J.; Bettler, J.; Cercamondi, C.I.; Berger, B. Term infant formula supplemented with milk-derived oligosaccharides shifts the gut microbiota closer to that of human milk-fed infants and improves intestinal immune defense: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 142–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brasil. Resolução RDC no 43, de 19 de Setembro de 2011. Aprova o Regulamento Técnico que Estabelece os Requisitos Mínimos de Identidade, Composição, Qualidade e Segurança a que Devem Obedecer às fórmulas Infantis para Lactentes. DOU no 182, de 21 de Setembro de 2011 vol. 2011. 2011. Available online: https://www.ibfan.org.br/site/wp-content/uploads/2014/06/Resolucao_RDC_n_43_de_19_de_setembro_de_2011.pdf (accessed on 4 March 2025).
- Brasil. Resolução RDC no 44, de 19 de setembro de 2011. Aprova o Regulamento técnico que Estabelece os Requisitos mínimos de Identidade, Composição, Qualidade e Segurança a que devem Obedecer às fórmulas Infantis de Seguimento para Lactentes e Crianças de Primeira Infância. DOU no 182, de 21 de Setembro de 2011 vol. 2011. 2011. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0044_19_09_2011.html (accessed on 4 March 2025).
- Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients. Available online: http://data.europa.eu/eli/reg/1997/258/oj (accessed on 4 March 2025).
- International Committee of Medical Journal Editors. Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication [Internet]; ICMJE Secretariat Office, American College of Physicians: Philadelphia, PA, USA, 2008; Available online: http://www.icmje.org (accessed on 30 March 2021).
| Characteristics | GROUP | p 1,2 | ||
|---|---|---|---|---|
| HMO (n = 29) | GOS/FOS (n = 30) | BM (n = 29) | ||
| Infant age (days) | ||||
| Enrollment | 16.9 ± 4.62 | 16.7 ± 6.21 | 17.1 ± 5.25 | 0.953 |
| Sex | 0.81 | |||
| Female | 15 (51.7%) | 13 (43.3%) | 14 (48.3%) | |
| Male | 14 (48.3%) | 17 (56.7%) | 15 (51.7%) | |
| Gestational age (weeks) | 38.7 ± 1.10 | 38.6 ± 0.95 | 39.0 ± 1.07 | 0.316 |
| Delivery mode | 0.28 | |||
| Vaginal | 11 (37.9%) | 11 (36.7%) | 16 (55.2%) | |
| C-Section | 18 (62.1%) | 19 (63.3%) | 13 (44.8%) | |
| Weight (grams) | ||||
| Birth | 3155.10 ± 318.24 | 3178.80 ± 342.59 | 3140.2 ± 353.40 | 0.907 |
| Enrollment | 3253.40 ± 376.40 | 3309.00 ± 367.04 | 3191.2 ± 387.34 | 0.489 |
| Length (cm) | ||||
| Birth | 49.1 ± 1.78 | 49.1 ± 1.59 | 48.7 ± 1.26 | 0.624 |
| Enrollment | 50.1 ± 1.87 | 50.3 ± 1.58 | 49.9 ± 1.35 | 0.595 |
| Head circumference (cm) | ||||
| Birth | 34.1 ± 0,88 | 34.5 ± 0.98 | 34.3 ± 1.09 | 0.292 |
| Enrollment | 35.0 ± 0.91 | 35.5 ± 1.07 | 35.1 ± 1.01 | 0.132 |
| Body Mass Index (BMI) | ||||
| Birth | 13.0 ± 0.71 | 13.1 ± 0.82 | 13.2 ± 1.04 | 0.854 |
| Enrollment | 12.9 ± 0.77 | 13.0 ± 0.95 | 12.8 ± 1.01 | 0.596 |
| Characteristics | GROUP | p 1 | ||
|---|---|---|---|---|
| HMO (n = 29) | GOS/FOS (n = 30) | BM (n = 29) | ||
| Infant age (days) | ||||
| Final | 111.8 ± 6.20 | 111.0 ± 6.44 | 112.1 ± 6.61 | 0.786 |
| Interval duration (days) | 94.9 ± 5.35 | 94.3 ± 6.64 | 94.9 ± 7.09 | 0.899 |
| (Minimum and maximum) | 85–105 | 85–110 | 85–109 | |
| Weight (grams) | ||||
| Final | 6190.30 ± 311.56 | 6068.80 ± 458.68 | 6000.70 ± 514.27 | 0.251 |
| Incremental 2 | 2936.90 ± 363.09 | 2759.80 ± 352.60 | 2809.60 ± 570.33 | 0.287 |
| Length (cm) | ||||
| Final | 61.10 ± 1.50 | 61.00 ± 1.50 | 60.80 ± 1.46 | 0.798 |
| Incremental 2 | 10.99 ± 1.51 | 10.68 ± 1.70 | 10.94 ± 1.80 | 0.745 |
| Head Circumference (cm) | ||||
| Final | 40.3 ± 0.92 | 40.5 ± 0.97 | 40.4 ± 1.11 | 0.824 |
| Incremental 2 | 5.37 ± 0.84 | 5.02 ± 1.09 | 5.32 ± 1.09 | 0.364 |
| Body Mass Index (BMI) | ||||
| Final | 16.6 ± 0.50 | 16.2 ± 0.95 | 16.2 ± 1.03 | 0.194 |
| Incremental 2 | 3.66 ± 1.03 | 3.22 ± 1.23 | 3.42 ± 1.20 | 0.351 |
| Symptoms/ Behavior 1 (per Day) | GROUP | p 3,4 | ||
|---|---|---|---|---|
| HMO (n = 29) | GOS/FOS (n = 30) | BM (n = 29) | ||
| Regurgitation | 0.082 | |||
| No | 17 (58.6%) | 16 (53.3%) | 09 (31%) | |
| Yes | 12 (41.4%) | 14 (46.7%) | 20 (69%) | |
| Number of times/day 2 | 0.0 (0.0–2.0) | 0.0 (0,0–3.0) | 1.0 (0,0–3.0) | 0.003 a |
| Volume (mL) 2 | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | 15.0 (5.0–30.0) | <0.001 b |
| Flatulence | 0.218 | |||
| No | 18 (62.1%) | 18 (60%) | 12 (41.4%) | |
| Yes | 11 (37.9%) | 12 (40%) | 17 (58.6%) | |
| Number of times/day 2 | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.016 c |
| Excessive Crying | 0.108 | |||
| No | 19 (65.5%) | 16 (53.3%) | 11 (37.9%) | |
| Yes | 10 (34.5%) | 14 (46.7%) | 18 (62.1%) | |
| Time (minutes) 2 | 0.0 (0.0–10.0) | 0.0 (0.0–15.0) | 0.0 (0.0–15.0) | 0.047 d |
| Irritation/Agitated | 0.125 | |||
| No | 23 (79.3%) | 18 (60%) | 16 (55.2%) | |
| Yes | 06 (20.7%) | 12 (40%) | 13 (44.8%) | |
| Time (minutes) 2 | 0.0 (0.0–10.0) | 0.0 (0.0–20.0) | 0.0 (0.0–15.0) | <0.001 e |
| Sleep | ||||
| Hours/night 2 | 15.0 (11.0–17.0) | 12.0 (4.0–15.0) | 12.0 (10.0–15.0) | <0.001 f |
| Manifestations 1 | GROUP | p 2,4 | ||
|---|---|---|---|---|
| HMO (n = 29) | GOS/FOS (n = 30) | BM (n = 30) | ||
| Defecation | 0.861 | |||
| No | 03 (10.3%) | 02 (6.7%) | 03 (10%) | |
| Yes | 26 (89.7%) | 28 (93.3%) | 27 (90%) | |
| Frequency 3 | ||||
| Number of times/day | 2.0 (2.0–3.0) | 3.0 (1.0–4.2) | 3.0 (1.0–5.0) | 0.455 |
| Straining during defecation | 0.81 | |||
| No | 22 (84.6%) | 25 (90.1%) | 24 (90.5%) | |
| Yes | 04 (15.4%) | 03 (9.9%) | 03 (9.5%) | |
| Fecal Consistency 5 | 0.262 | |||
| Unformed | 23 (88.5%) | 22 (79.1%) | 22 (82.1%) | |
| Formed | 03 (11.5%) | 06 (20.9%) | 17 (17.9%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarini, T.; Tonon, K.M.; Araujo Filho, H.B.d.; Morais, M.B.d. Bifidogenic Effect of 2′-Fucosyllactose (2′-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial. Nutrients 2025, 17, 973. https://doi.org/10.3390/nu17060973
Lazarini T, Tonon KM, Araujo Filho HBd, Morais MBd. Bifidogenic Effect of 2′-Fucosyllactose (2′-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial. Nutrients. 2025; 17(6):973. https://doi.org/10.3390/nu17060973
Chicago/Turabian StyleLazarini, Tamara, Karina Merini Tonon, Humberto Bezerra de Araujo Filho, and Mauro Batista de Morais. 2025. "Bifidogenic Effect of 2′-Fucosyllactose (2′-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial" Nutrients 17, no. 6: 973. https://doi.org/10.3390/nu17060973
APA StyleLazarini, T., Tonon, K. M., Araujo Filho, H. B. d., & Morais, M. B. d. (2025). Bifidogenic Effect of 2′-Fucosyllactose (2′-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial. Nutrients, 17(6), 973. https://doi.org/10.3390/nu17060973






