Aerial Yam Bulbils Protect Against APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Mitochondrial Dysfunction Through Nrf2 Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. AYB Extract
2.2. Cell Culture and AYB Extract, Silymarin and APAP Treatments
2.3. Cell Viability Assay
2.4. Hoechst 33342 Staining
2.5. Western Blot Analysis
2.6. Measurement of Intracellular ROS
2.7. Measurement of Mitochondrial Membrane Potential
2.8. Measurement of Mitochondrial ROS Production
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Nuclear Fractionation of TIB-73 Cells
2.11. Determination of Diosgenin Content
2.12. Statistical Analysis
3. Results
3.1. AYB Extract Protects TIB-73 Cells from APAP-Induced Hepatotoxicity
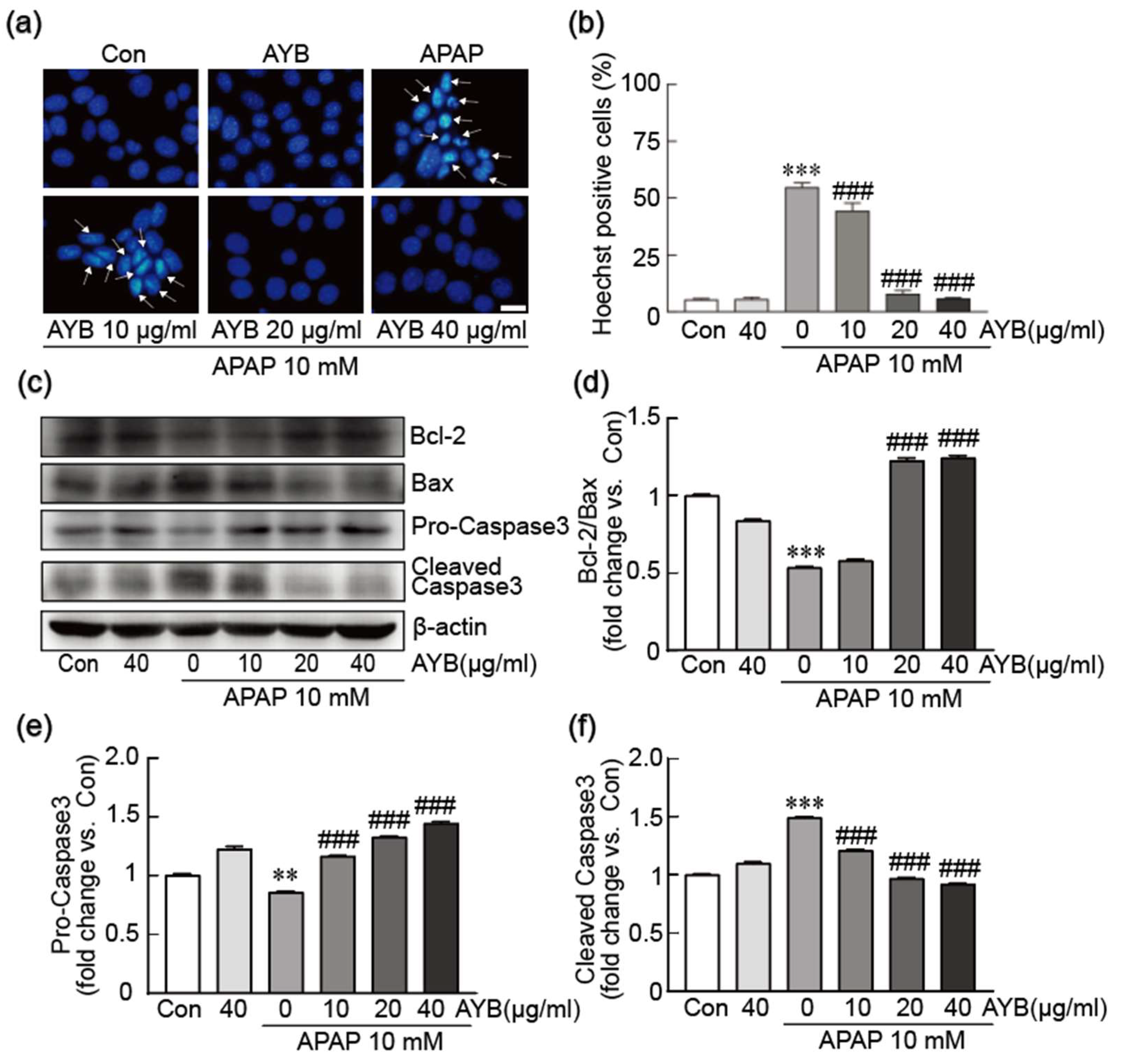
3.2. AYB Extract Inhibits TIB-73 Cells from APAP-Induced Apoptosis
3.3. AYB Extract Blocks APAP-Induced Oxidative Stress in TIB-73 Cells
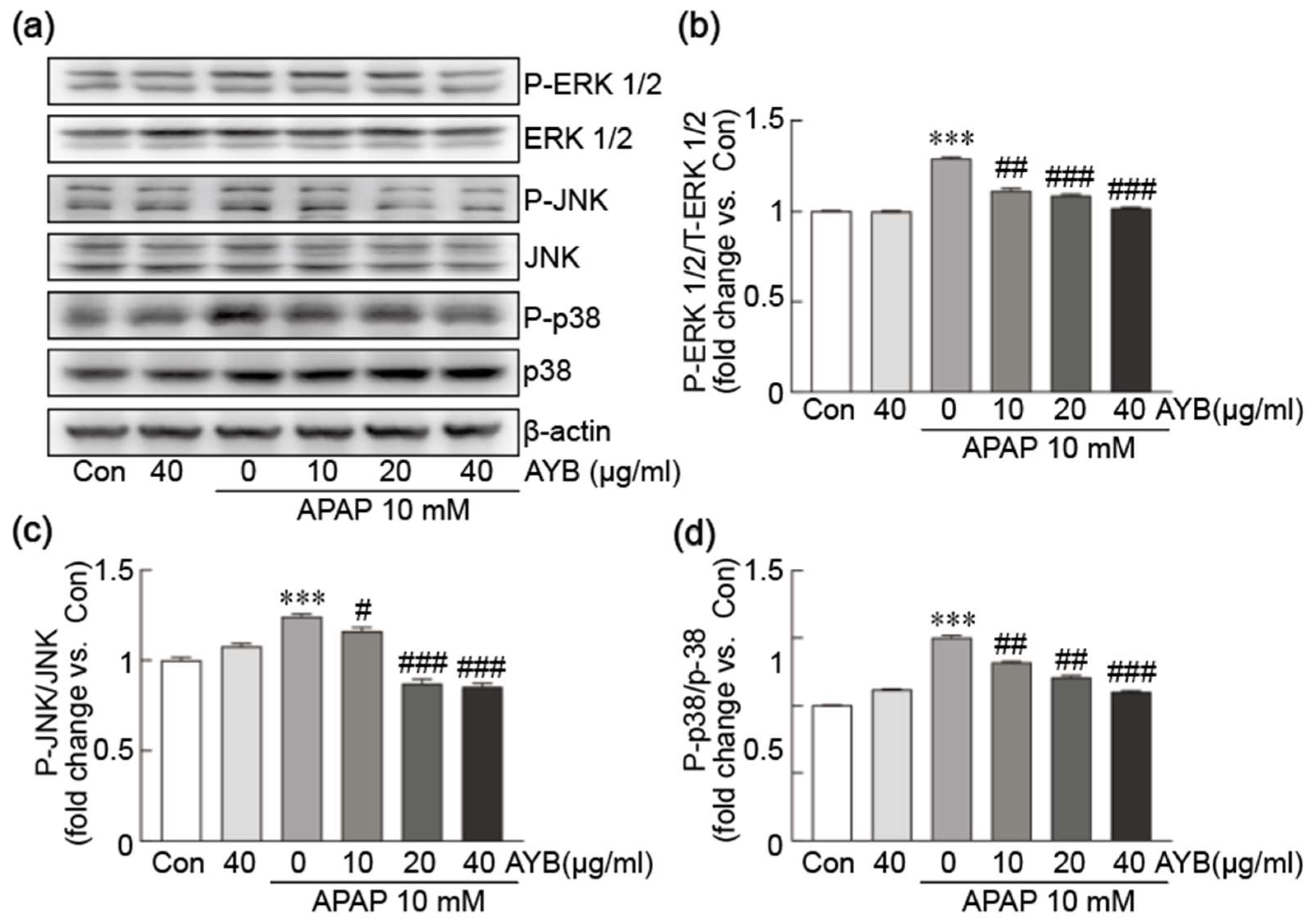
3.4. AYB Extract Suppresses APAP-Induced Activation of the MAPK Signaling Pathway in TIB-73 Cells
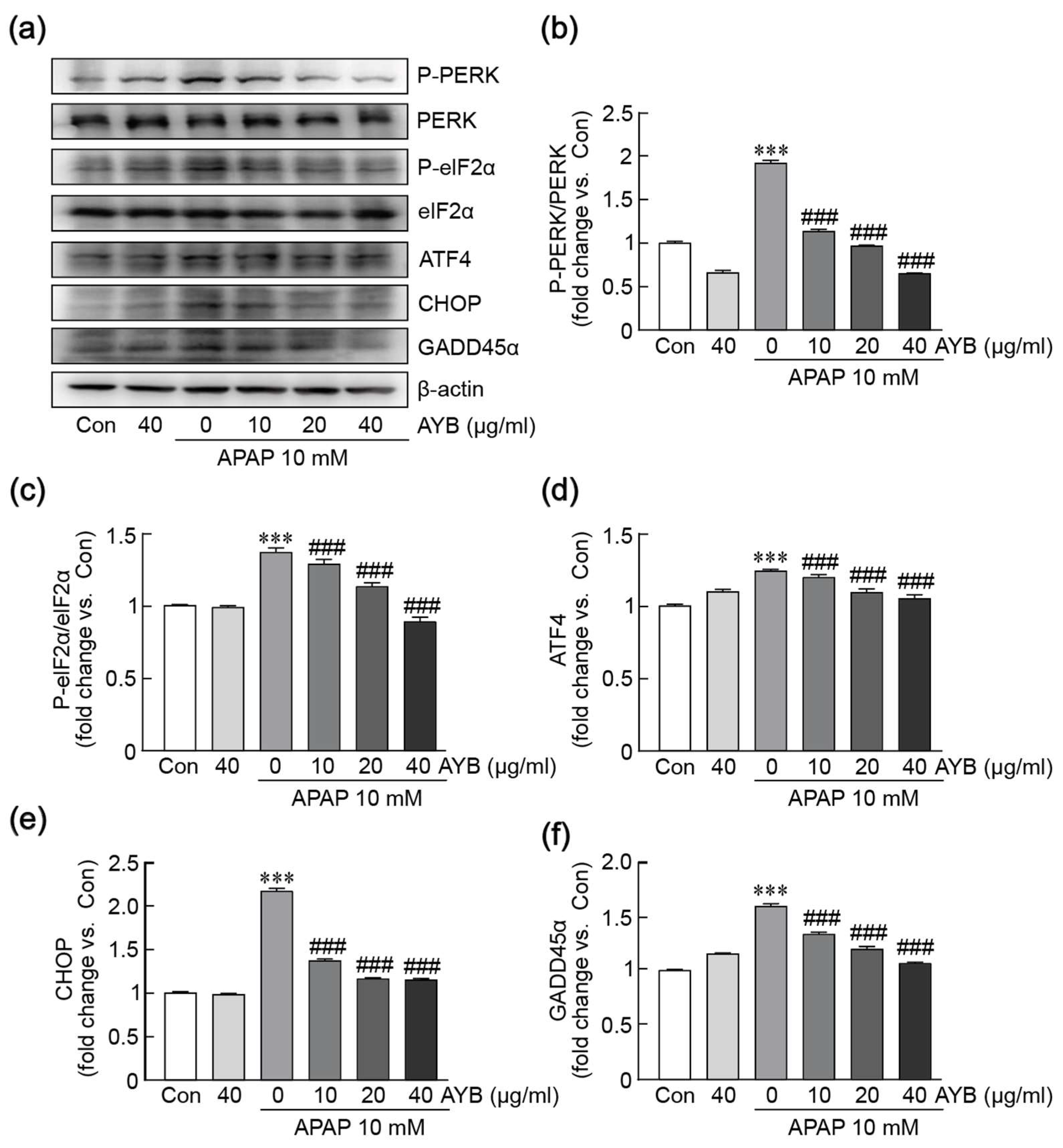
3.5. AYB Extract Mitigates APAP-Induced ER Stress in TIB-73 Cells
3.6. AYB Extract Protects Against APAP-Induced Mitochondrial Dysfunction in TIB-73 Cells
3.7. AYB Extract Mitigates APAP-Induced Oxidative Stress in TIB-73 Cells via Activation of the Nrf2 Pathway
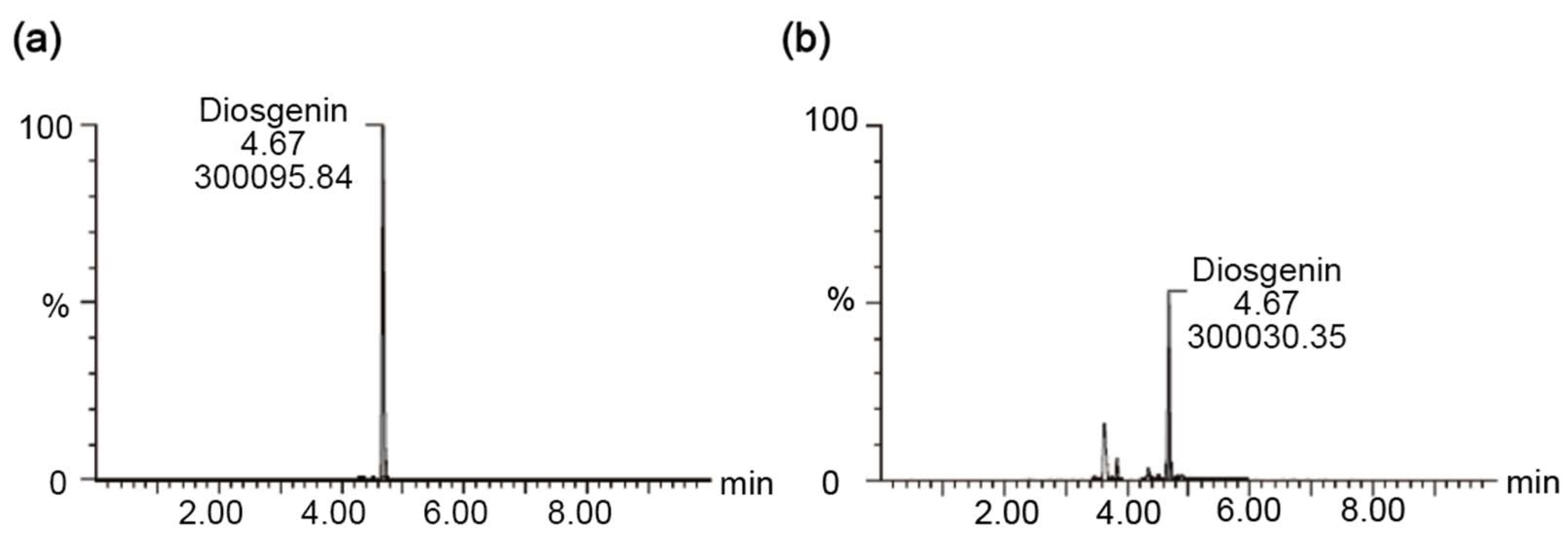
3.8. UPLC-MS Analysis of Diosgenin Content in AYB Extracts
3.9. The Protective Effect of AYB Extract Is Similar to Those of Silymarin on APAP-Induced Hepatotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Liangpunsakul, S.; Chalasani, N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am. J. Gastroenterol. 2007, 102, 558–562, quiz 693. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, T.H.; Yu, H.N.; Hu, B.H.; Hu, B.; Ma, P.; Yang, Y.; Yang, N.D.; Hu, J.; Cao, T.T.; et al. Autophagy impairment as a key feature for acetaminophen-induced ototoxicity. Cell Death Dis. 2021, 12, 3. [Google Scholar] [CrossRef]
- Jiang, Z.T.; Yang, X.; Han, Y.; Li, J.; Hu, C.; Liu, C.D.; Xiao, W. Sarmentosin promotes USP17 and regulates Nrf2-mediated mitophagy and cellular oxidative stress to alleviate APAP-induced acute liver failure. Phytomedicine 2022, 104, 154337. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Miwa, G.T.; Lu, A.Y.; Nelson, S.D. N-acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA 1984, 81, 1327–1331. [Google Scholar] [CrossRef]
- Xie, Y.; McGill, M.R.; Du, K.; Dorko, K.; Kumer, S.C.; Schmitt, T.M.; Ding, W.X.; Jaeschke, H. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2015, 289, 213–222. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Alamri, Z.Z. The role of liver in metabolism: An updated review with physiological emphasis. Int. J. Basic Clin. Pharmacol. 2018, 7, 2271–2276. [Google Scholar] [CrossRef]
- Bovi, A.P.D.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Dornas, W.; Schuppan, D. Mitochondrial oxidative injury: A key player in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G400–G411. [Google Scholar] [CrossRef]
- Amorim, R.; Magalhaes, C.C.; Borges, F.; Oliveira, P.J.; Teixeira, J. From Non-Alcoholic Fatty Liver to Hepatocellular Carcinoma: A Story of (Mal)Adapted Mitochondria. Biology 2023, 12, 595. [Google Scholar] [CrossRef]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop-Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Jiao, R.; Wu, Y.; Xu, F.; Li, Y.; Mao, L. Physicochemical properties and antioxidant activity of polysaccharides from Chinese yam (Dioscorea opposita Thunb.). JSFA Rep. 2023, 3, 137–145. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Ma, F.; Wang, R.; Zhang, Y.; Bai, J.; Fang, H.; Ma, W.; Liu, W.; Li, Q.; Liu, X. Polysaccharides from Dioscorea opposita Thunb.: Isolation, structural characterization, and anti-inflammatory and anti-tumor effects against hepatocellular carcinoma. Chem. Biol. Technol. Agric. 2023, 10, 43. [Google Scholar] [CrossRef]
- Singh, R.; Patade, V.Y.; Sanchita; Singh, A. Antimicrobial potential of silver nanoparticles biosynthesized using aerial yam bulbils for control of selected phytopathogens. Arch. Phytopathol. Plant Prot. 2021, 54, 2275–2293. [Google Scholar] [CrossRef]
- Zhou, H.X.; Zhang, X.; Huang, R.G.; Su, T.C. Antifatigue effects and antioxidant activity in polysaccharide fractions from Chinese yam bulbils. Food Sci. Nutr. 2024, 12, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Tungalag, T.; Kang, H.S. Bulbils of Aerial Yam Attenuate Ethanol-Induced Hepatotoxicity in HepG2 Cells through Inhibition of Oxidative Stress by Activation of the Nuclear Factor Erythroid-2-Related Factor 2 Signaling Pathway. Nutrients 2024, 16, 542. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Xu, W.; Hellerbrand, C.; Kohler, U.A.; Bugnon, P.; Kan, Y.W.; Werner, S.; Beyer, T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Invest. 2008, 88, 1068–1078. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Chan, Y.C.; Chang, S.C.; Liu, S.Y.; Yang, H.L.; Hseu, Y.C.; Liao, J.W. Beneficial effects of yam on carbon tetrachloride-induced hepatic fibrosis in rats. J. Sci. Food Agric. 2010, 90, 161–167. [Google Scholar] [CrossRef]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Ku, B.; Liang, C.Y.; Jung, J.U.; Oh, B.H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, X.; Zhao, S.; Zou, K.; Xie, J.; Wang, X.; Liu, C.; Wang, J.; Wang, Y. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine 2018, 38, 90–97. [Google Scholar] [CrossRef]
- Jaeschke, H.; Knight, T.R.; Bajt, M.L. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003, 144, 279–288. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide dismutase administration: A review of proposed human uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Kim, Y.S.; Vallur, P.G.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Moshal, K.S.; Metreveli, N.; Frank, I.; Tyagi, S.C. Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia. Curr. Vasc. Pharmacol. 2008, 6, 84–92. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-l.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Choi, Y.H. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2021, 43, 303–312. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lee, C.-H.; Chen, C.-M.; Cheng, C.-W.; Chen, P.-N.; Ying, T.-H.; Hsieh, Y.-H. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell. Physiol. Biochem. 2018, 46, 322–334. [Google Scholar] [CrossRef]
- Pihán, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017, 24, 1478–1487. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Liu, H.; Wang, C.; Xu, S.; Xu, B.; Deng, Y.; Yang, T.; Liu, W. Exploring the molecular mechanisms underlie the endoplasmic reticulum stress-mediated methylmercury-induced neuronal developmental damage. Ecotoxicol. Environ. Saf. 2022, 245, 114099. [Google Scholar] [CrossRef]
- Levings, D.C.; Wang, X.; Kohlhase, D.; Bell, D.A.; Slattery, M. A distinct class of antioxidant response elements is consistently activated in tumors with NRF2 mutations. Redox Biol. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Gambhir, L.; Tyagi, G.; Bhardwaj, R.; Kapoor, N.; Sharma, G. Perturbation of cellular redox status: Role of Nrf2, a master regulator of cellular redox. React. Oxyg. Species 2022, 181, 102319. [Google Scholar] [CrossRef]
- Šuk, J. Induction of Heme Oxygenase and Biological Role of Its Metabolic Products. Ph.D. Thesis, Univerzita Karlova, Prague, Czech Republic, 2019. [Google Scholar]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, V.V.; Shelke, R.P.; Kadu, U.E.; Gaikwad, P.M. Protective effect of diosgenin against carbon tetrachloride and cisplatin induced hepatotoxicity in rats. Int. J. Biomed. Res. 2018, 4, 50–56. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Cong, X.; Zheng, L.; Xu, L.; Yin, L.; Peng, J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol. Lett. 2012, 214, 69–80. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, S.; Yang, D.K. Aerial Yam Bulbils Protect Against APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Mitochondrial Dysfunction Through Nrf2 Activation. Nutrients 2025, 17, 966. https://doi.org/10.3390/nu17060966
Xiang S, Yang DK. Aerial Yam Bulbils Protect Against APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Mitochondrial Dysfunction Through Nrf2 Activation. Nutrients. 2025; 17(6):966. https://doi.org/10.3390/nu17060966
Chicago/Turabian StyleXiang, Siyu, and Dong Kwon Yang. 2025. "Aerial Yam Bulbils Protect Against APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Mitochondrial Dysfunction Through Nrf2 Activation" Nutrients 17, no. 6: 966. https://doi.org/10.3390/nu17060966
APA StyleXiang, S., & Yang, D. K. (2025). Aerial Yam Bulbils Protect Against APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Mitochondrial Dysfunction Through Nrf2 Activation. Nutrients, 17(6), 966. https://doi.org/10.3390/nu17060966






