Effects of 17,18-Epoxyeicosatetraenoic Acid and 19,20-Epoxydocosapentaenoic Acid Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Brown Adipogenesis and Mitochondrial Respiration
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. Oil Red O Staining
2.4. Western Blot Analysis
2.5. Analysis of Mitochondrial Respiration
2.6. Reporter Gene Assays
2.7. Statistical Analysis
3. Results
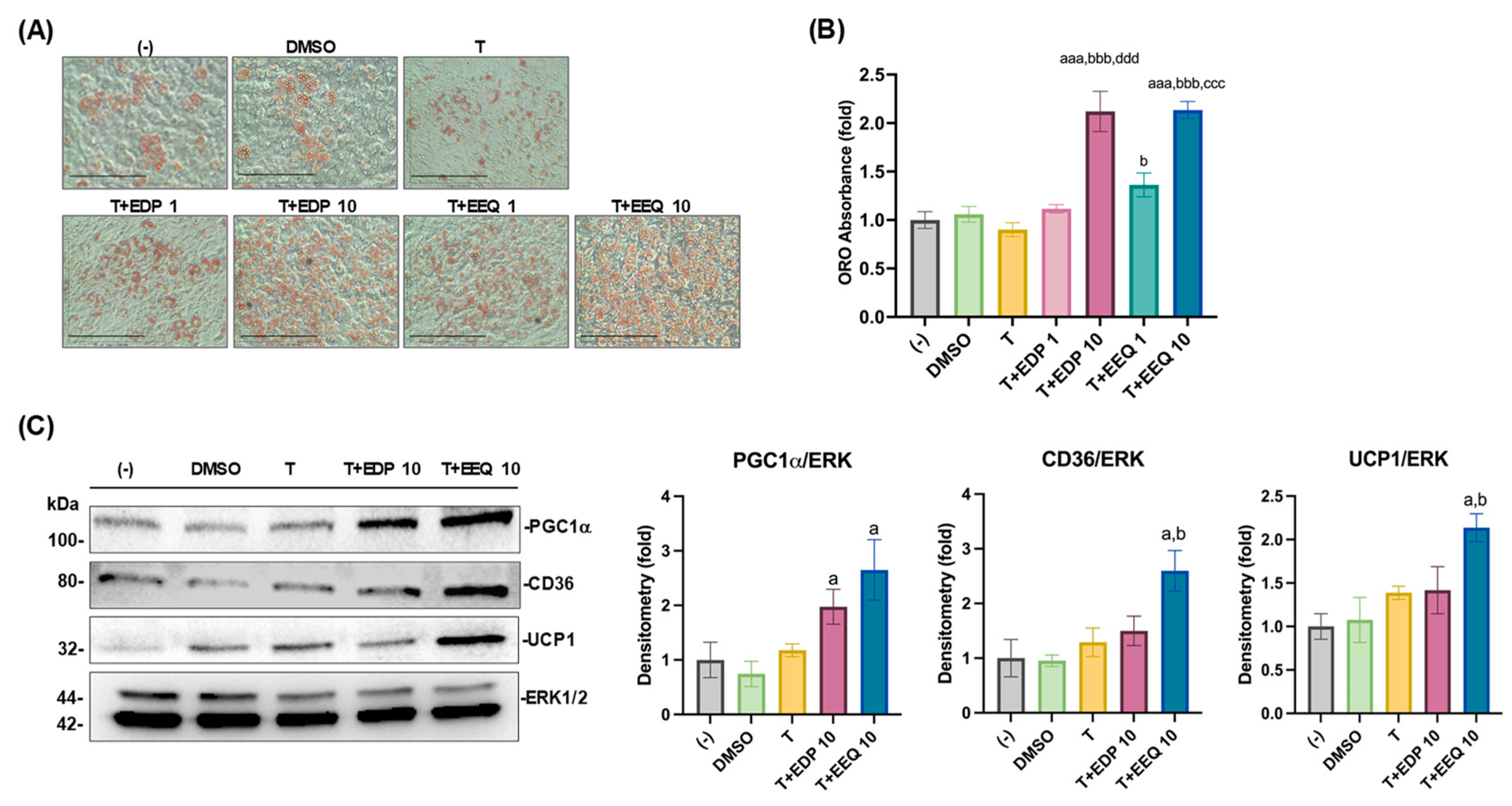
3.1. 17,18-EEQ and 19,20-EDP Combined with t-TUCB Differentially Promote Murine Brown Adipogenesis and Upregulate Protein Expression of Thermogenic and Lipid Metabolic Genes
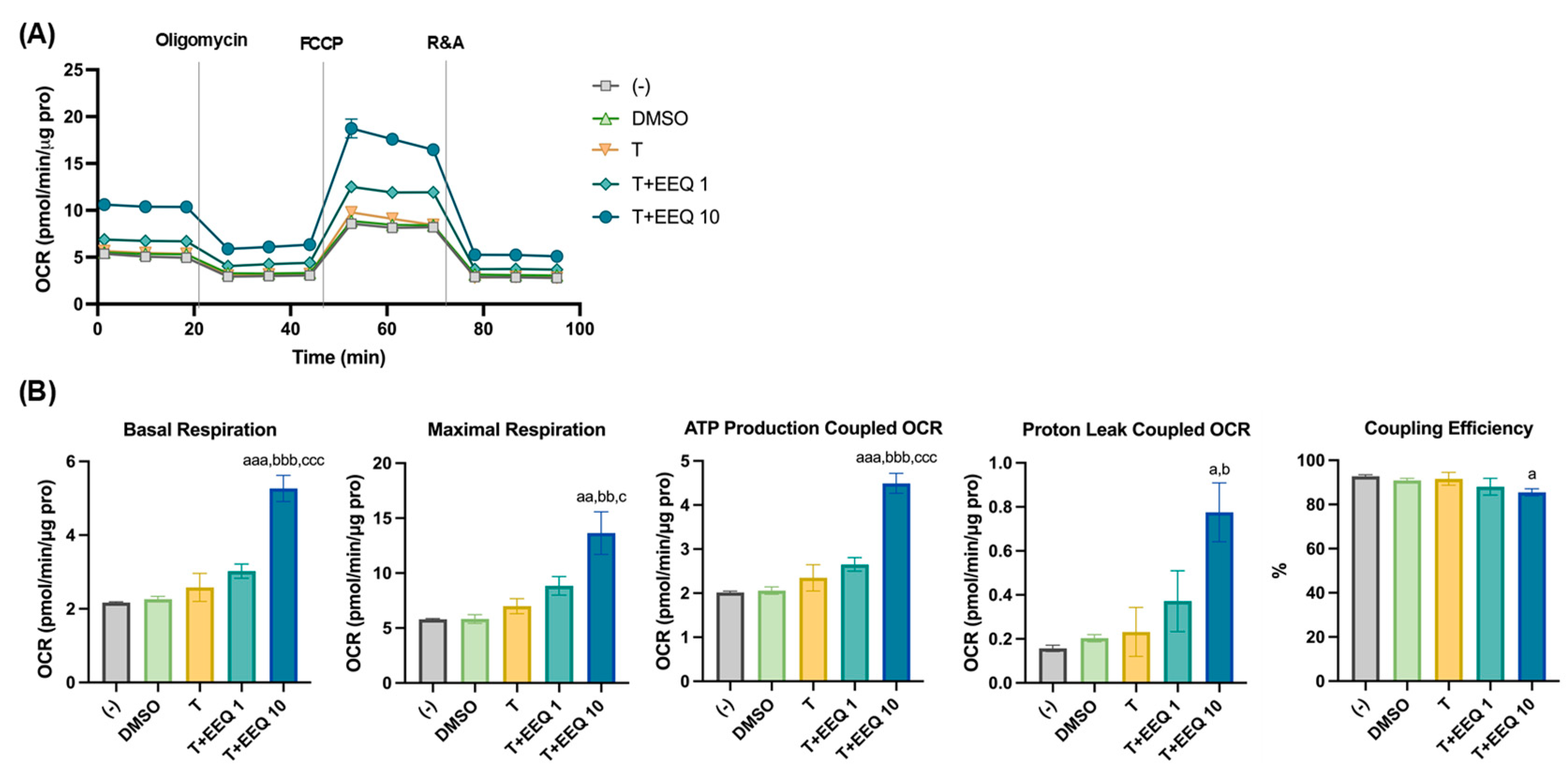
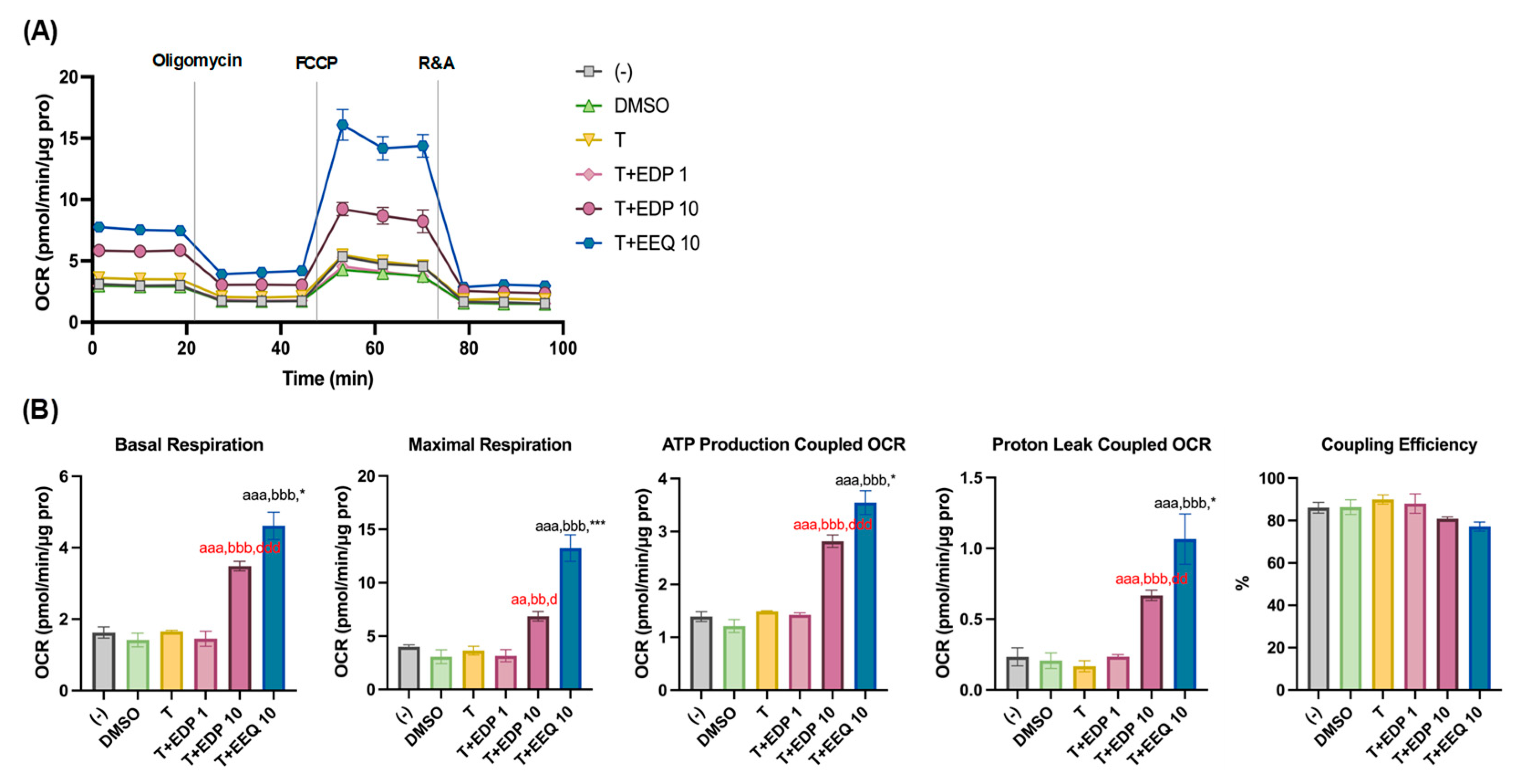
3.2. 17,18-EEQ and 19,20-EDP Combined with t-TUCB Increase Mitochondrial Respiration and Proton Leak in Differentiating Murine Brown Adipocytes
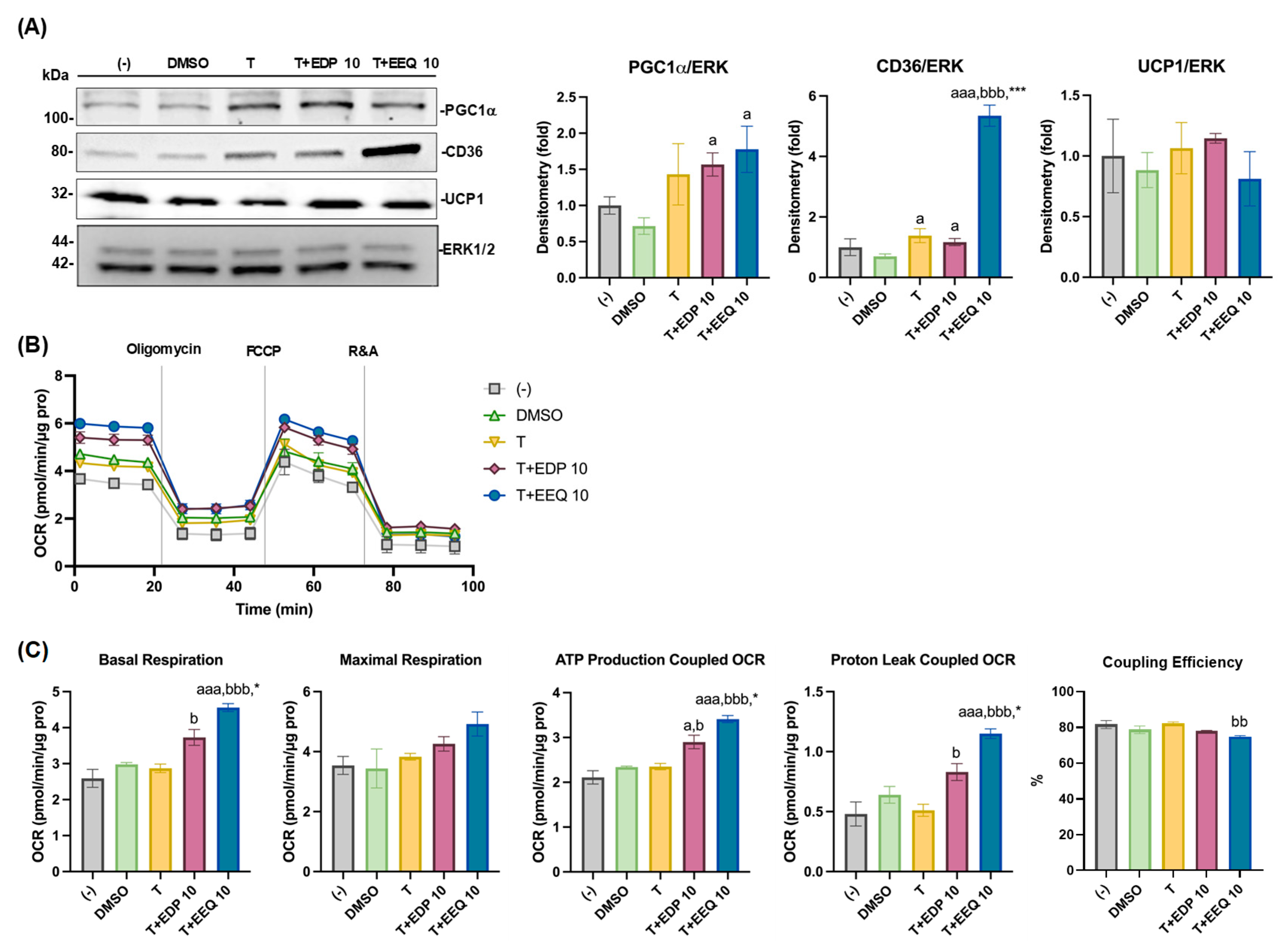
3.3. 17,18-EEQ with t-TUCB Upregulates Thermogenic and Lipid Metabolic Genes and Promotes Mitochondrial Respiration and Uncoupling in Differentiated Murine Brown Adipocytes
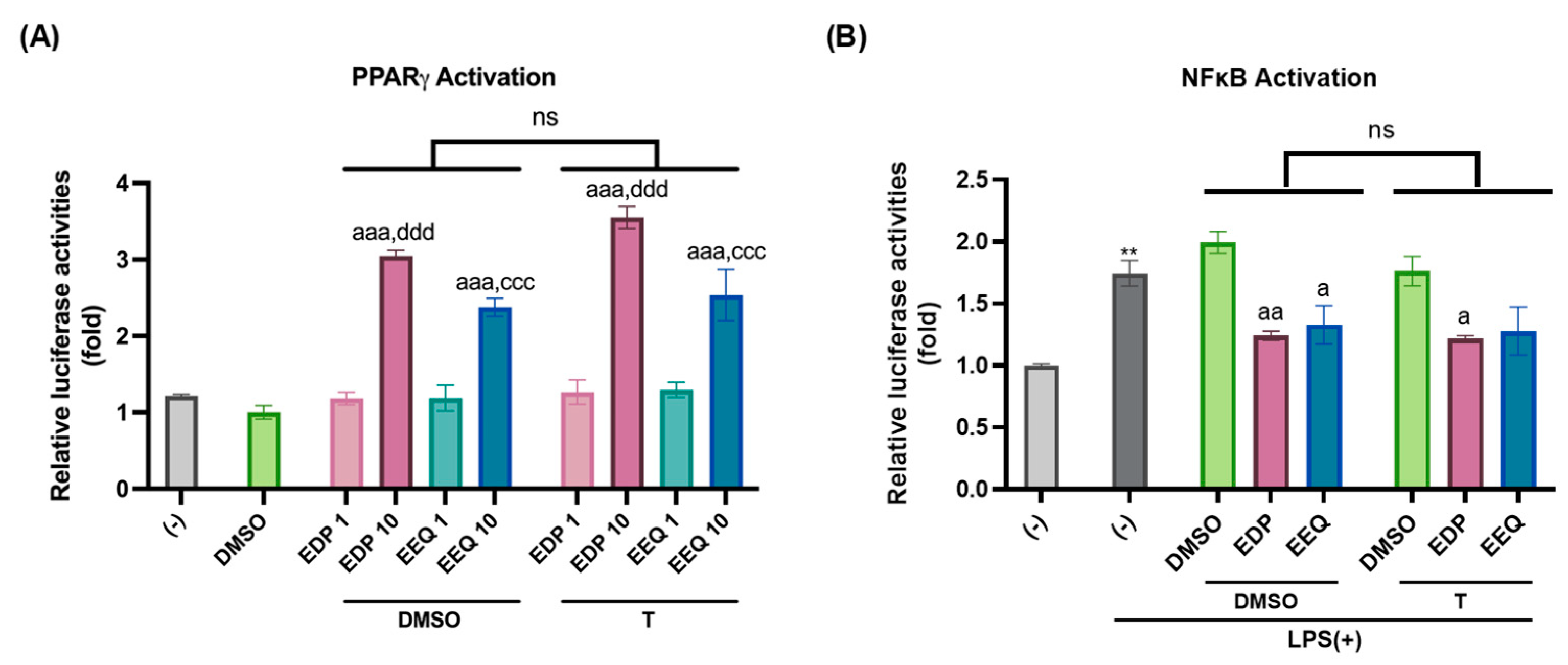
3.4. 17,18-EEQ and 19,20-EDP Activate PPARγ and Inhibit LPS-Induced NFκB Activation in Murine Brown Preadipocytes
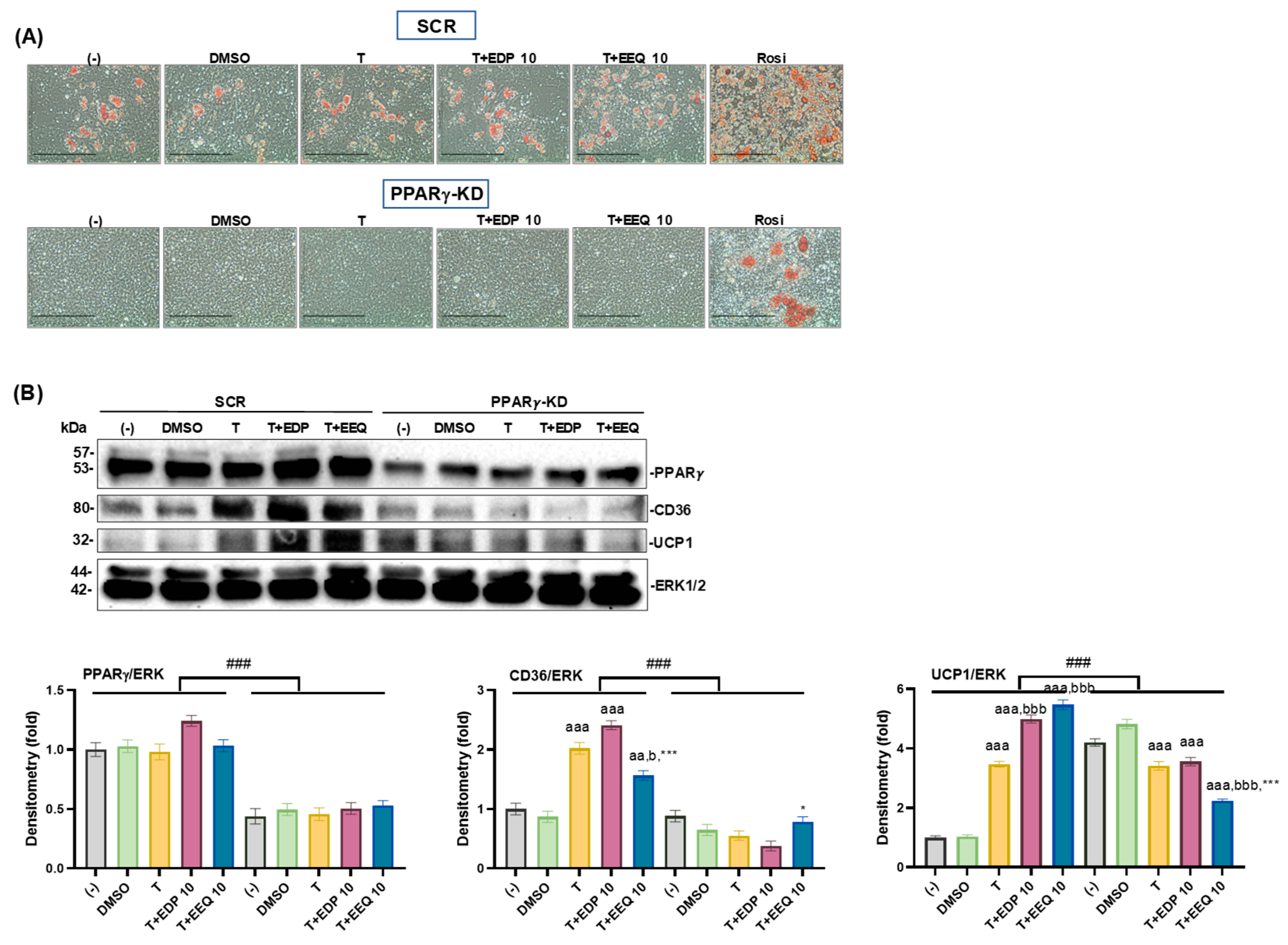
3.5. The Effects of PPARγ Knockdown on the Brown Adipocyte Differentiation Treated by 17,18-EEQ and 19,20-EDP Combined with t-TUCB
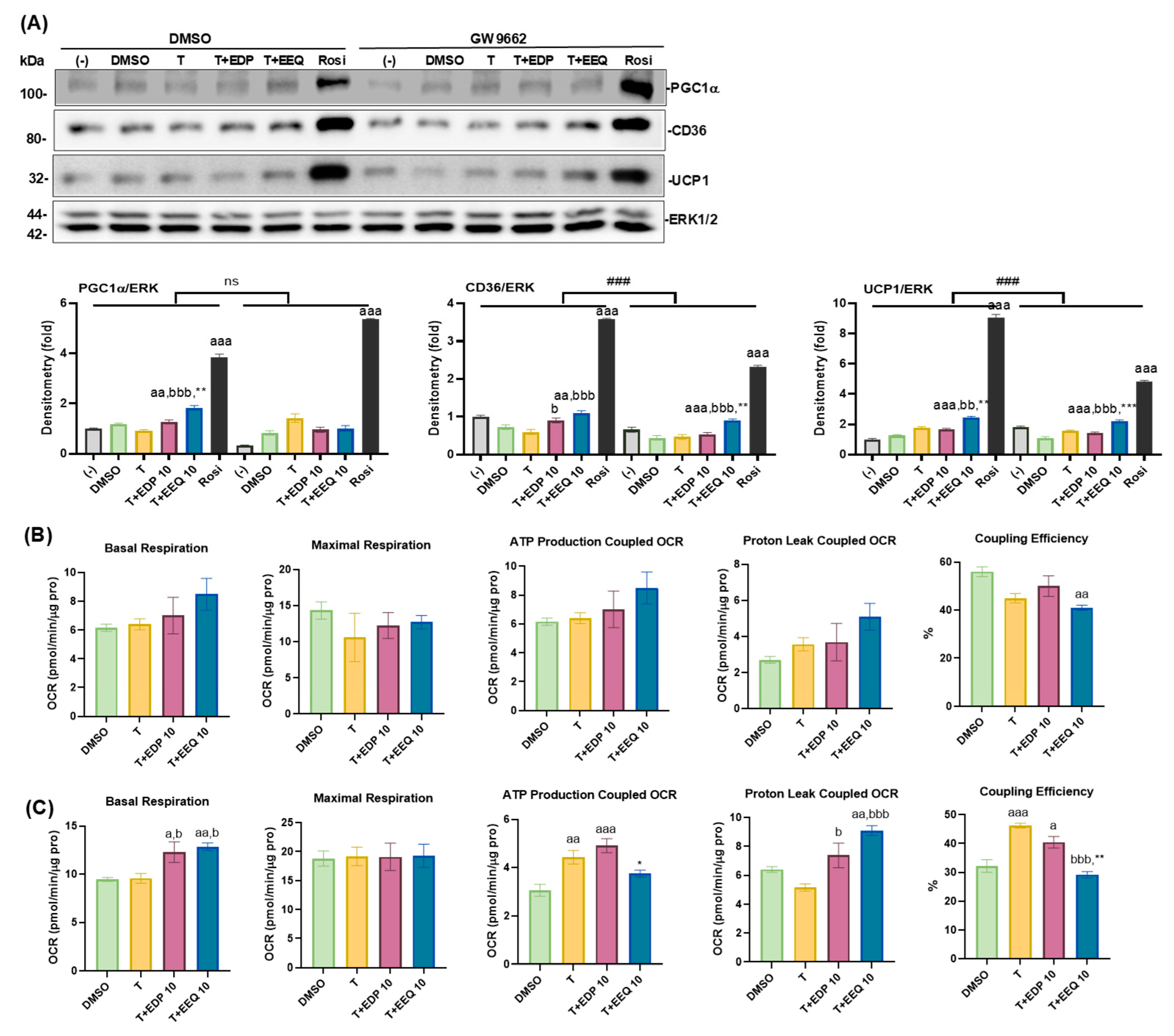
3.6. The Effects of PPARγ Antagonism on the Differentiated Brown Adipocytes Treated with 17,18-EEQ and 19,20-EDP Combined with t-TUCB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The World Health Organization Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 7 December 2024).
- CDC. The US Center for Disease Control and Prevention, Adult Obesity Prevalence Maps. Available online: https://www.cdc.gov/obesity/data-and-statistics/adult-obesity-prevalence-maps.html (accessed on 7 December 2024).
- CDC. The US Center for Disease Control and Prevention, Consequences of Obesity. Available online: https://www.cdc.gov/obesity/basics/consequences.html (accessed on 7 December 2024).
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Novikoff, A.; Liu, X.; Muller, T.D. Recent achievements and future directions of anti-obesity medications. Lancet Reg. Health Eur. 2024, 47, 101100. [Google Scholar] [CrossRef] [PubMed]
- Kupnicka, P.; Krol, M.; Zychowska, J.; Lagowski, R.; Prajwos, E.; Surowka, A.; Chlubek, D. GLP-1 Receptor Agonists: A Promising Therapy for Modern Lifestyle Diseases with Unforeseen Challenges. Pharmaceuticals 2024, 17, 1470. [Google Scholar] [CrossRef]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.H. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef]
- Morisseau, C.; Inceoglu, B.; Schmelzer, K.; Tsai, H.J.; Jinks, S.L.; Hegedus, C.M.; Hammock, B.D. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 2010, 51, 3481–3490. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, X.; Wu, H.; Yang, J.; Chen, J.; Morisseau, C.; Hammock, B.D.; Bettaieb, A.; Zhao, L. Differential Effects of 17,18-EEQ and 19,20-EDP Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2021, 22, 8267. [Google Scholar] [CrossRef]
- Inceoglu, B.; Bettaieb, A.; Haj, F.G.; Gomes, A.V.; Hammock, B.D. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediat. 2017, 133, 68–78. [Google Scholar] [CrossRef]
- McReynolds, C.; Morisseau, C.; Wagner, K.; Hammock, B. Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation. Adv. Exp. Med. Biol. 2020, 1274, 71–99. [Google Scholar] [CrossRef]
- Fan, R.; Koehler, K.; Chung, S. Adaptive thermogenesis by dietary n-3 polyunsaturated fatty acids: Emerging evidence and mechanisms. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 59–70. [Google Scholar] [CrossRef]
- Fernandez-Galilea, M.; Felix-Soriano, E.; Colon-Mesa, I.; Escote, X.; Moreno-Aliaga, M.J. Omega-3 fatty acids as regulators of brown/beige adipose tissue: From mechanisms to therapeutic potential. J. Physiol. Biochem. 2020, 76, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 2014, 450, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Okla, M.; Erickson, A.; Carr, T.; Natarajan, S.K.; Chung, S. Eicosapentaenoic Acid Potentiates Brown Thermogenesis through FFAR4-dependent Up-regulation of miR-30b and miR-378. J. Biol. Chem. 2016, 291, 20551–20562. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, M.; Razafimanjato, F.; Ramalingam, L.; Kalupahana, N.S.; Moussa, H.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 2017, 39, 101–109. [Google Scholar] [CrossRef]
- Rose, T.E.; Morisseau, C.; Liu, J.Y.; Inceoglu, B.; Jones, P.D.; Sanborn, J.R.; Hammock, B.D. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J. Med. Chem. 2010, 53, 7067–7075. [Google Scholar] [CrossRef]
- Tsai, H.J.; Hwang, S.H.; Morisseau, C.; Yang, J.; Jones, P.D.; Kasagami, T.; Kim, I.H.; Hammock, B.D. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur. J. Pharm. Sci. 2010, 40, 222–238. [Google Scholar] [CrossRef][Green Version]
- Klein, J.; Fasshauer, M.; Klein, H.H.; Benito, M.; Kahn, C.R. Novel adipocyte lines from brown fat: A model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 2002, 24, 382–388. [Google Scholar] [CrossRef]
- Bae, J.; Yang, Y.; Xu, X.; Flaherty, J.; Overby, H.; Hildreth, K.; Chen, J.; Wang, S.; Zhao, L. Naringenin, a citrus flavanone, enhances browning and brown adipogenesis: Role of peroxisome proliferator-activated receptor gamma. Front. Nutr. 2022, 9, 1036655. [Google Scholar] [CrossRef]
- Wu, H.; Adebesin, A.M.; Falck, J.R.; Xu, X.; Chen, J.; Masi, T.J.; Stephenson, S.M.; Zhao, L. Effects of 17,18-EEQ analog (TZ-1) on brown adipogenesis and browning of human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2024, 734, 150660. [Google Scholar] [CrossRef]
- Overby, H.; Yang, Y.; Xu, X.; Graham, K.; Hildreth, K.; Choi, S.; Wan, D.; Morisseau, C.; Zeldin, D.C.; Hammock, B.D.; et al. Soluble Epoxide Hydrolase Inhibition by t-TUCB Promotes Brown Adipogenesis and Reduces Serum Triglycerides in Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 7039. [Google Scholar] [CrossRef]
- Purohit, J.; Hu, P.; Burke, S.J.; Collier, J.J.; Chen, J.; Zhao, L. The effects of NOD activation on adipocyte differentiation. Obesity 2013, 21, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Petrovic, N.; Lindgren, E.M.; Jacobsson, A.; Cannon, B. PPARgamma in the control of brown adipocyte differentiation. Biochim. Biophys. Acta 2005, 1740, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Koppen, A.; Kalkhoven, E. Brown vs white adipocytes: The PPARgamma coregulator story. FEBS Lett. 2010, 584, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Chen, J.; Zhao, L. Chronic activation of pattern recognition receptors suppresses brown adipogenesis of multipotent mesodermal stem cells and brown pre-adipocytes. Biochem. Cell Biol. 2015, 93, 251–261. [Google Scholar] [CrossRef]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef]
- Quesada-Lopez, T.; Cereijo, R.; Turatsinze, J.V.; Planavila, A.; Cairo, M.; Gavalda-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000, 84, 175–184. [Google Scholar] [CrossRef]
- Oliveira, T.E.; Castro, E.; Belchior, T.; Andrade, M.L.; Chaves-Filho, A.B.; Peixoto, A.S.; Moreno, M.F.; Ortiz-Silva, M.; Moreira, R.J.; Inague, A.; et al. Fish Oil Protects Wild Type and Uncoupling Protein 1-Deficient Mice from Obesity and Glucose Intolerance by Increasing Energy Expenditure. Mol. Nutr. Food Res. 2019, 63, e1800813. [Google Scholar] [CrossRef]
- Bargut, T.C.; Silva-e-Silva, A.C.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Dos Santos, L.R.B.; Fleming, I. Role of cytochrome P450-derived, polyunsaturated fatty acid mediators in diabetes and the metabolic syndrome. Prostaglandins Other Lipid Mediat. 2020, 148, 106407. [Google Scholar] [CrossRef]
- Morin, C.; Sirois, M.; Echave, V.; Albadine, R.; Rousseau, E. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: Role of soluble epoxide hydrolase. Am. J. Respir. Cell Mol. Biol. 2010, 43, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.M.; Teruel, T.; Navarro, P.; Benito, M.; Lorenzo, M. Tumor necrosis factor-alpha causes insulin receptor substrate-2-mediated insulin resistance and inhibits insulin-induced adipogenesis in fetal brown adipocytes. Endocrinology 1998, 139, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Ricciardi, C.J.; Esposito, D.; Komarnytsky, S.; Hu, P.; Curry, B.J.; Brown, P.L.; Gao, Z.; Biggerstaff, J.P.; Chen, J.; et al. Activation of pattern recognition receptors in brown adipocytes induces inflammation and suppresses uncoupling protein 1 expression and mitochondrial respiration. Am. J. Physiol. Cell Physiol. 2014, 306, C918–C930. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Putri, M.; Syamsunarno, M.R.; Iso, T.; Yamaguchi, A.; Hanaoka, H.; Sunaga, H.; Koitabashi, N.; Matsui, H.; Yamazaki, C.; Kameo, S.; et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem. Biophys. Res. Commun. 2015, 457, 520–525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, H.; Xu, X.; Morisseau, C.; Lee, K.S.S.; Hammock, B.D.; Chen, J.; Zhao, L. Effects of 17,18-Epoxyeicosatetraenoic Acid and 19,20-Epoxydocosapentaenoic Acid Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Brown Adipogenesis and Mitochondrial Respiration. Nutrients 2025, 17, 936. https://doi.org/10.3390/nu17060936
Yang Y, Wu H, Xu X, Morisseau C, Lee KSS, Hammock BD, Chen J, Zhao L. Effects of 17,18-Epoxyeicosatetraenoic Acid and 19,20-Epoxydocosapentaenoic Acid Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Brown Adipogenesis and Mitochondrial Respiration. Nutrients. 2025; 17(6):936. https://doi.org/10.3390/nu17060936
Chicago/Turabian StyleYang, Yang, Haoying Wu, Xinyun Xu, Christophe Morisseau, Kin Sing Stephen Lee, Bruce D. Hammock, Jiangang Chen, and Ling Zhao. 2025. "Effects of 17,18-Epoxyeicosatetraenoic Acid and 19,20-Epoxydocosapentaenoic Acid Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Brown Adipogenesis and Mitochondrial Respiration" Nutrients 17, no. 6: 936. https://doi.org/10.3390/nu17060936
APA StyleYang, Y., Wu, H., Xu, X., Morisseau, C., Lee, K. S. S., Hammock, B. D., Chen, J., & Zhao, L. (2025). Effects of 17,18-Epoxyeicosatetraenoic Acid and 19,20-Epoxydocosapentaenoic Acid Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Brown Adipogenesis and Mitochondrial Respiration. Nutrients, 17(6), 936. https://doi.org/10.3390/nu17060936








