Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
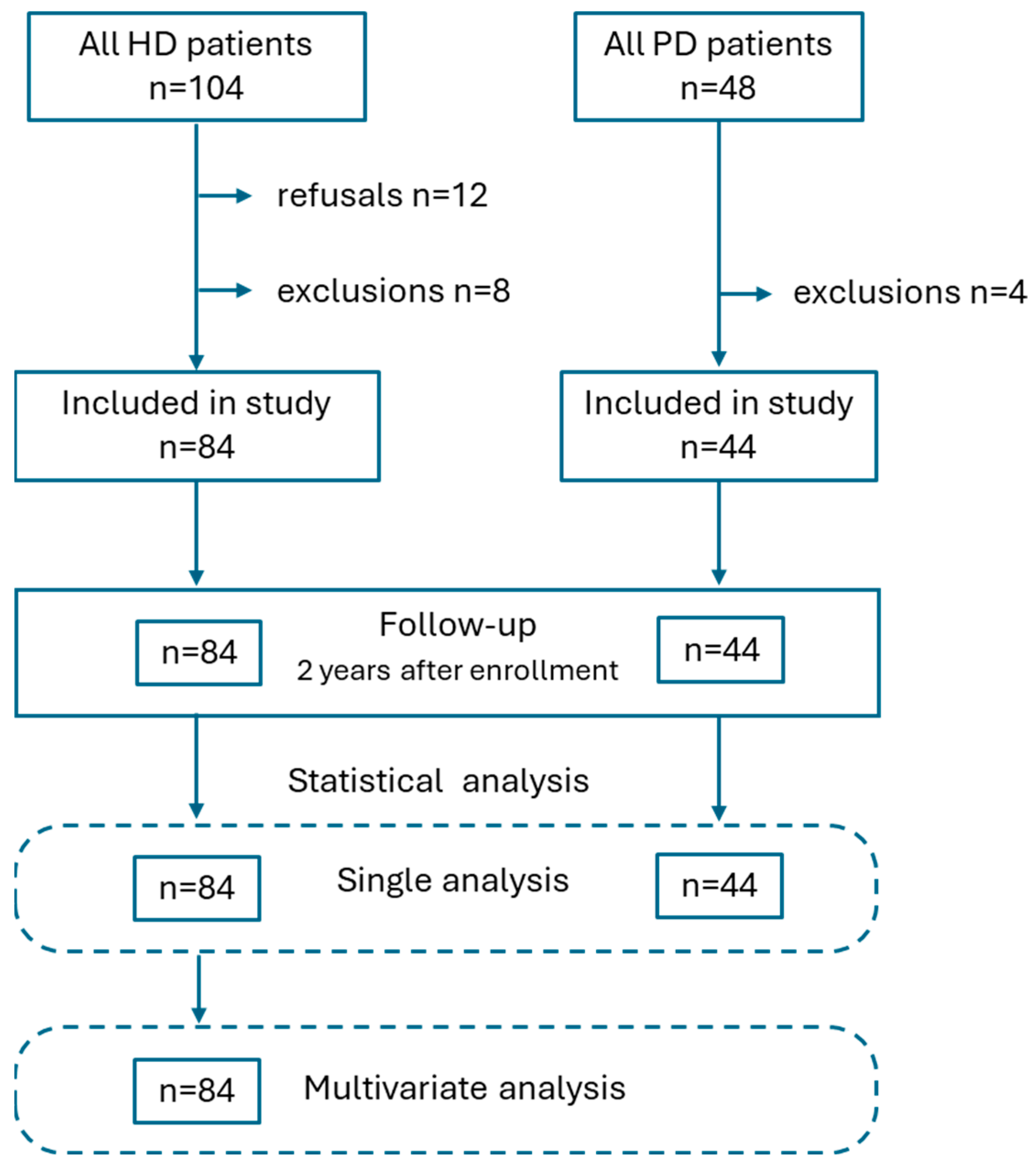
2.2. Study Population
2.3. Assessment of Biochemical Data
2.4. Assessment of Nutritional Status
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
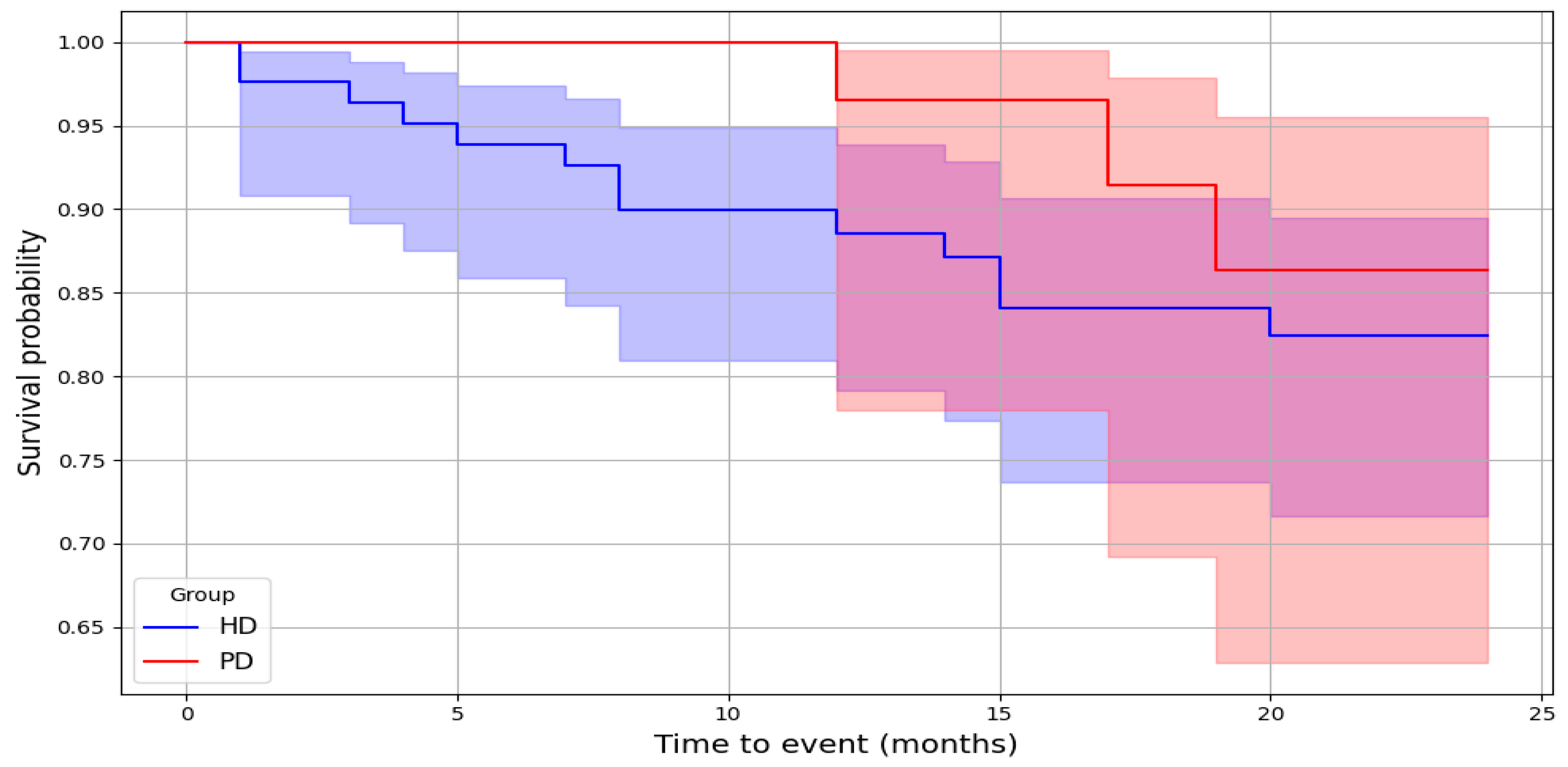
3.2. Cardiovascular Mortality
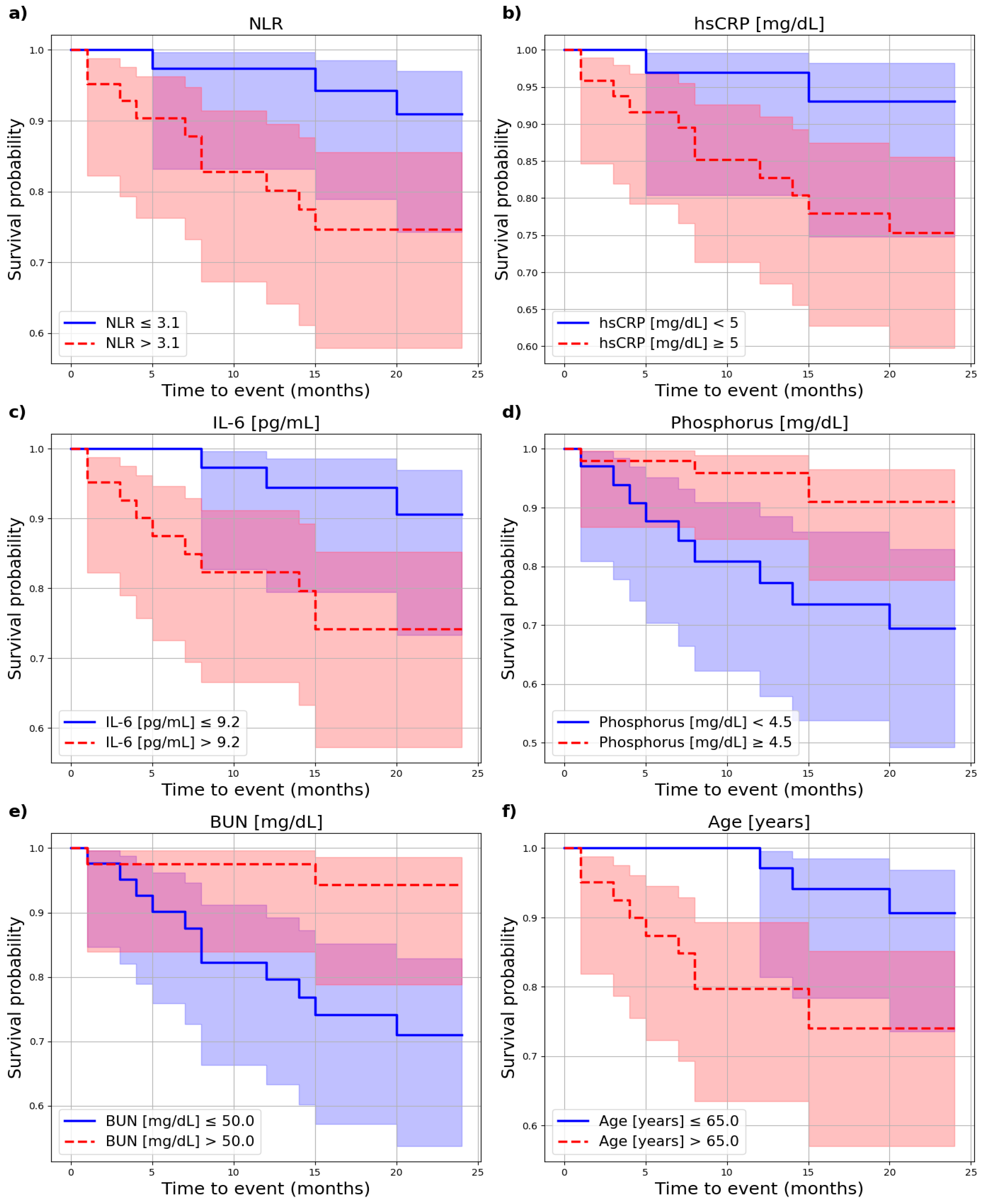
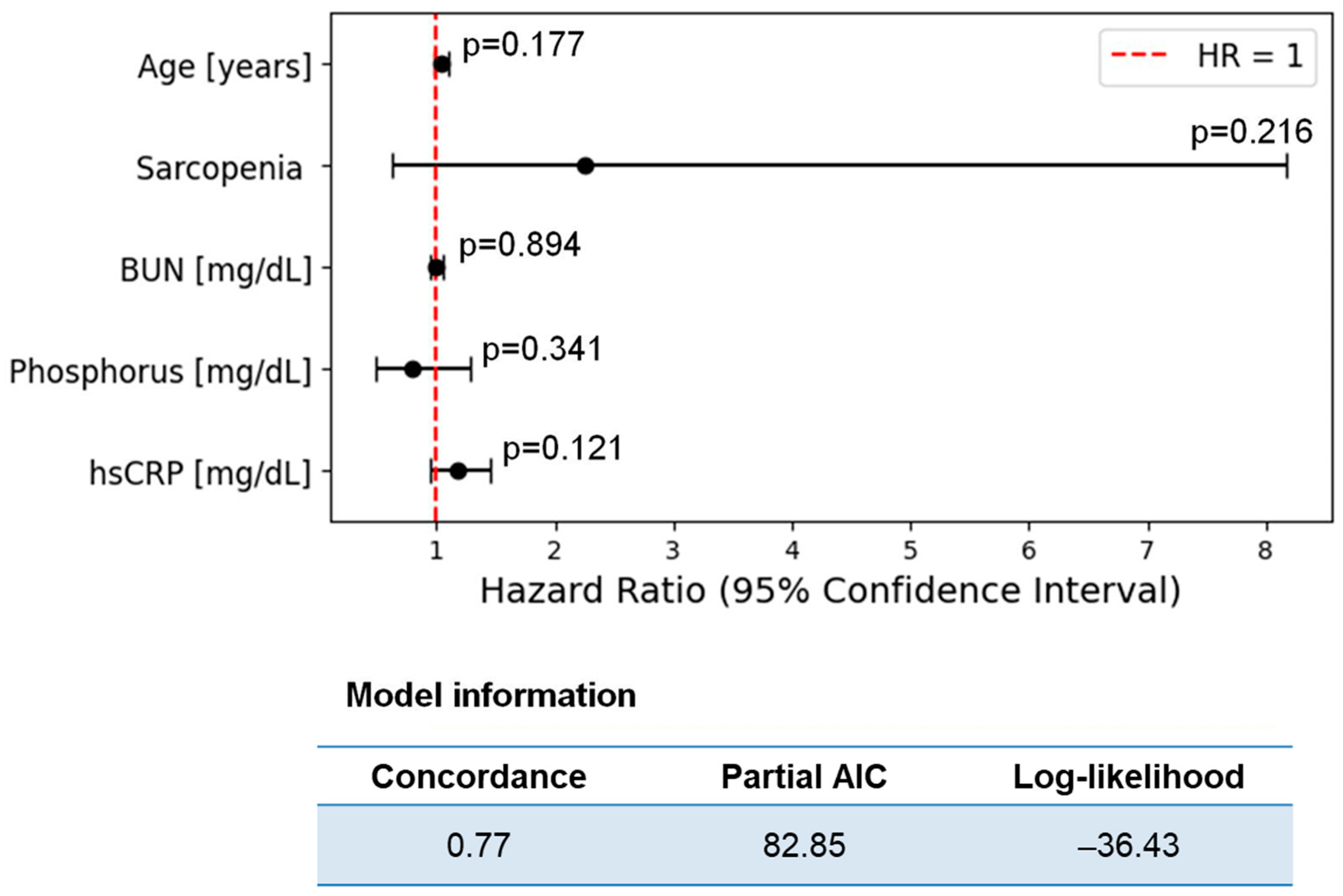
3.3. Cardiovascular Risk
4. Discussion
4.1. Adipokines
4.2. Myokines
4.3. Gut-Microbiota-Derived Uremic Toxins
4.4. Other Biochemical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADMA | asymmetric dimethylarginine |
| APD | automated peritoneal dialysis |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| CAPD | continuous ambulatory peritoneal dialysis |
| CKD | chronic kidney disease |
| CV | cardiovascular |
| CVD | cardiovascular diseases |
| ELISA | enzyme-linked immunosorbent assays |
| Hb | hemoglobin |
| HCT | hematocrit |
| HD | hemodialysis |
| HGS | hand grip strength |
| HR | hazard risk |
| hsCRP | high sensitivity C-reactive protein |
| IL-6 | interleukin 6 |
| IS | indoxyl sulfate |
| KRT | kidney replacement therapy |
| KTRs | kidney transplant recipients |
| LAR | leptin/adiponectin ratio |
| LC─MS/MS | liquid chromatography-tandem mass spectrometry |
| LTI | lean tissue index |
| NLR | neutrophil-to-lymphocyte ratio |
| pCS | p-cresyl sulfate |
| PD | peritoneal dialysis |
| PLR | platelet-to-lymphocyte ratio |
| ROS | reactive oxygen species |
| SGA | 7-Point Subjective Global Assessment |
| TMAO | trimethylamine-N-oxide |
| ZAG | zinc alpha 2-glycoprotein |
References
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef]
- Dębska-Ślizień, A.; Rutkowski, B.; Jagodziński, P.; Rutkowski, P.; Przygoda, J.; Lewandowska, D.; Czerwiński, J.; Kamiński, A.; Gellert, R. Current status of renal replacement therapy in Poland in 2021. Pol. Nephrol. Dial. 2021, 25, 85–103. [Google Scholar]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- The United States Renal Data System. Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. End Stage Renal Disease. 2023; Volume 837. Available online: https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities (accessed on 13 September 2024).
- Srivastava, A.; Kaze, A.D.; McMullan, C.J.; Isakova, T.; Waikar, S. Uric Acid the Risks of Kidney Failure Death in Individuals with CKD. Am. J. Kidney Dis. 2018, 71, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Constantino-Jonapa, L.A.; Espinoza-Palacios, Y.; Escalona-Montaño, A.R.; Hernández-Ruiz, P.; Amezcua-Guerra, L.; Amedei, A.; Aguirre-García, M. Contribution of Trimethylamine N-Oxide (TMAO) to Chronic Inflammatory and Degenerative Diseases. Biomedicines 2023, 11, 431. [Google Scholar] [CrossRef]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Yang, M.; Luo, S.; Yang, J.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. Myokines: Novel therapeutic targets for diabetic nephropathy. Front. Endocrinol. 2022, 13, 1014581. [Google Scholar] [CrossRef]
- Chung, H.S.; Choi, K.M. Adipokines and Myokines: A Pivotal Role in Metabolic and Cardiovascular Disorders. Curr. Med. Chem. 2018, 25, 2401–2415. [Google Scholar] [CrossRef]
- Tanriover, C.; Copur, S.; Mutlu, A.; Peltek, I.; Galassi, A.; Ciceri, P.; Cozzoolino, M.; Kanbay, M. Early aging and premature vascular aging in chronic kidney disease. Clin. Kidney J. 2023, 16, 1751–1765. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Ma, W.; Miao, H.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Chatrenet, A.; Potrykus, M.; Ruszkowski, J.; Torreggiani, M.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Piccoli, G.B.; Małgorzewicz, S. Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients 2024, 16, 2480. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Stolc, S.; Potrykus, M.; Ruszkowski, J.; Styburski, D.; Dębska-Ślizień, A.; Małgorzewicz, S. The associations between nutrition and circulating gut microbiota-derived uremic toxins in patients undergoing kidney replacement therapy: An Observational, Cross-Sectional Study. Clin. Nutr. ESPEN 2024, 65, 105–114. [Google Scholar] [CrossRef]
- Visser, R.; Dekker, F.W.; Boeschoten, E.W.; Stevens, P.; Krediet, R.T. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv. Perit. Dial. 1999, 15, 222–225. [Google Scholar]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, W.; Zou, M.; Zeng, Q.S.; Feng, Y.; Lou, Z.Y.; Gan, H.T. Diagnosis, prevalence, and outcomes of sarcopenia in kidney transplantation recipients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 14, 17–29. [Google Scholar] [CrossRef]
- Roy, N.; Rosas, S.E. IL-6 Is Associated with Progression of Coronary Artery Calcification and Mortality in Incident Dialysis Patients. Am. J. Nephrol. 2021, 52, 745–752. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y. Interleukin-6 levels can be used to estimate cardiovascular and all- cause mortality risk in dialysis patients: A meta-analysis and a systematic review. Immun. Inflamm. Dis. 2023, 11, e818. [Google Scholar] [CrossRef]
- Selim, G.; Stojceva-Taneva, O.; Ivanovski, N.; Zafirovska, K.; Sikole, A.; Trajcevska, L.; Asani, A.; Polenakovic, M. Inflammation and anaemia as predictors of cardiovascular mortality in hemodialysis patients. Hippokratia 2007, 11, 39–43. [Google Scholar] [PubMed]
- Dao Bui Quy, Q.; Pham Ngoc Huy, T.; Nguyen Minh, T.; Nguyen Duc, L.; Nguyen Minh, T.; Nguyen Trung, K.; Tran Viet, T.; Do, Q.; Le Viet, T. High Serum Uric Acid and High-Sensitivity C Reactive Protein Concentrations Predict Three-Year Cardiovascular Mortality in Patients Treated with Continuous Ambulatory Peritoneal Dialysis. Cureus 2021, 13, e17900. [Google Scholar] [CrossRef]
- Menon, V.; Li, L.; Wang, X.; Greene, T.; Balakrishnan, V.; Madero, M.; Pereira, A.A.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; et al. Adiponectin and mortality in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2599–2606. [Google Scholar] [CrossRef]
- Rhee, C.M.; Nguyen, D.V.; Moradi, H.; Brunelli, S.M.; Dukkipati, R.; Jing, J.; Nakata, T.; Kovesdy, C.P.; Brent, G.A.; Kalantar-Zadeh, K. Association of Adiponectin with Body Composition and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2015, 66, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kappelle, P.J.; Dullaart, R.P.; van Beek, A.P.; Hillege, H.L.; Wolffenbuttel, B.H. The plasma leptin/adiponectin ratio predicts first cardiovascular event in men: A prospective nested case-control study. Eur. J. Intern. Med. 2012, 23, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Yoo, T.H.; Kim, J.K.; Oh, H.J.; Kim, S.J.; Yoo, D.E.; Lee, M.J.; Shin, D.H.; Han, S.H.; Han, D.S.; et al. Leptin/adiponectin ratio is an independent predictor of mortality in nondiabetic peritoneal dialysis patients. Perit. Dial. Int. 2013, 33, 67–74. [Google Scholar] [CrossRef]
- Bouchara, A.; Yi, D.; Pastural, M.; Granjon, S.; Selag, J.C.; Laville, M.; Arkouche, W.; Pelletier, S.; Fouque, D.; Soulage, C.O.; et al. Serum levels of the adipokine zinc-alpha2- glycoprotein (ZAG) predict mortality in hemodialysis patients. Kidney Int. 2018, 94, 983–992. [Google Scholar] [CrossRef]
- Lyu, J.X.; Guo, D.D.; Song, Y.C.; Zhang, M.R.; Ge, F.Q.; Zhao, J.; Zhu, H.; Hang, P.Z. Circulating Myokines as Novel Biomarkers for Cardiovascular Diseases. Rev. Cardiovasc. Med. 2024, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, M.; Hamasaki, Y.; Oki, R.; Komaru, Y.; Miyamoto, Y.; Yoshida, T.; Matsuura, R.; Doi, K.; Nangaku, M. Serum Myostatin at Dialysis Initiation May Predict 1-Year Mortality and Hospitalization. Nephron 2024, 148, 544–552. [Google Scholar] [CrossRef]
- Arcidiacono, T.; Magni, G.; Macrina, L.; Sirtori, M.; Belloni, C.; Premaschi, S.; Lanzani, C.; Simonini, M.; Citterio, L.; Locatelli, M.; et al. Serum Irisin May Predict Cardiovascular Events in Elderly Patients with Chronic Kidney Disease Stage 3-5. J. Ren. Nutr. 2022, 32, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, W.; Deng, Y.; Jia, L.; Lin, N.; Li, W.; Zhang, A. Lower serum irisin levels are associated with the increasing mortality of cardiovascular and cerebrovascular diseases in hemodialysis patients. Ann. Palliat. Med. 2021, 10, 6052–6061. [Google Scholar] [CrossRef]
- Behrens, F.; Bartolomaeus, H.; Wilck, N.; Holle, J. Gut-immune axis and cardiovascular risk in chronic kidney disease. Clin. Kidney J. 2023, 17, sfad303. [Google Scholar] [CrossRef]
- Shafi, T.; Powe, N.R.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Hostetter, T.H. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J. Am. Soc. Nephrol. 2017, 28, 321–331. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, J.Z.; Chen, J.; Tan, X.; Xiang, F.F.; Shen, B.; Hu, J.C.; Wang, J.L.; Wang, Y.Q.; Yu, J.B.; et al. Association of trimethylamine N-Oxide with cardiovascular and all-cause mortality in hemodialysis patients. Ren. Fail. 2020, 42, 1004–1014. [Google Scholar] [CrossRef]
- Stubbs, J.R.; Stedman, M.R.; Liu, S.; Long, J.; Franchetti, Y.; West, R.E., 3rd; Prokopienko, A.J.; Mahnken, J.D.; Chertow, G.M.; Nolin, T.D. Trimethylamine N-Oxide and Cardiovascular Outcomes in Patients with ESKD Receiving Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 261–267. [Google Scholar]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J. Am. Heart Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Dong, Z. Trimethylamine N-Oxide and mortality in hemodialysis patients: Like a mystery. Ren. Fail. 2021, 43, 463–464. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Wu, Q.J.; Xiao, J.; Wang, Z.H.; Mu, X.W.; Zhang, Y.; Wang, X.N.; You, L.L.; Wang, S.N.; et al. Serum total indoxyl sulfate levels and all-cause and cardiovascular mortality in maintenance hemodialysis patients: A prospective cohort study. BMC Nephrol. 2022, 23, 231. [Google Scholar] [CrossRef]
- Kendrick, J.; Kestenbaum, B.; Chonchol, M. Phosphate and cardiovascular disease. Adv. Chronic Kidney Dis. 2011, 18, 113–119. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Yu, W.; Zhuo, Y.; Yuan, Q.; Wu, X. Association of Dose and Frequency on the Survival of Patients on Maintenance Hemodialysis in China: A Kaplan-Meier and Cox- Proportional Hazard Model Analysis. Med. Sci. Monit. 2018, 24, 5329–5337. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.H.; Oh, T.R.; Suh, S.H.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Kim, S.W.; Bae, E.H. Prognostic role of the neutrophil-to-lymphocyte ratio in patients with chronic kidney disease. Korean J. Intern. Med. 2023, 38, 725–733. [Google Scholar] [CrossRef]
- Neuen, B.L.; Leather, N.; Greenwood, A.M.; Gunnarsson, R.; Cho, Y.; Mantha, M.L. Neutrophil-lymphocyte ratio predicts cardiovascular and all-cause mortality in hemodialysis patients. Ren. Fail. 2016, 38, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Torlén, K.; Kalantar-Zadeh, K.; Molnar, M.Z.; Vashistha, T.; Mehrotra, R. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin. J. Am. Soc. Nephrol. 2012, 7, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Kadoshi, H.; Shapiro, G.; Feldman, L.; Sandbank, J.; Averbukh, Z. Low serum concentration of obestatin as a predictor of mortality in maintenance hemodialysis patients. BioMed Res. Int. 2013, 2013, 796586. [Google Scholar] [CrossRef] [PubMed]

| HD Patients (N = 84) | PD Patients (N = 44) | |||||
|---|---|---|---|---|---|---|
| CV Deaths During Follow-Up | Survivors | p-Values | CV Deaths During Follow-Up | Survivors | p-Values | |
| N | 13 | 71 | p-values | 3 | 41 | p-values |
| Males, n (%) | 9 (69.2) | 39 (54.9) | 0.33 | 3 (100) | 20 (48.8) | 0.04 |
| Age (years) | 71.0 (12.4) | 60.0 (16.6) | 0.025 | 70.0 [70.0–79.0] | 49.0 [36.0–66.0] | 0.029 |
| Dialysis vintage (months) | 27.0 [8.0–95.0] | 30.0 [9.5–71.0] | 0.643 | 12.0 [9.5–38.0] | 14.5 [8.5–25.2] | 0.886 |
| BMI (kg/m2) | 23.1 [22.1–28.6] | 24.8 [22.5–27.6] | 0.961 | 32.4 (3.1) | 26.2 (4.9) | 0.035 |
| LTI (kg/m2) | 10.8 (2.4) | 11.7 (2.7) | 0.309 | 15.5 [14.5–16.5] | 13.1 [11.1–14.7] | 0.183 |
| BUN (mg/L) | 45.5 (15.0) | 53.5 (13.9) | 0.06 | 63.0 (18.7) | 48.4 (13.6) | 0.087 |
| HCT (%) | 33.1 (4.3) | 31.3 (3.8) | 0.126 | 33.2 (2.7) | 33.6 (4.8) | 0.879 |
| Hb (g/dL) | 10.7 (1.4) | 10.3 (1.2) | 0.288 | 11.1 (1.0) | 11.4 (1.7) | 0.767 |
| NLR | 3.5 [3.2–3.9] | 3.0 [2.1–4.4] | 0.19 | 3.3 (1.4) | 3.8 (1.6) | 0.585 |
| PLR | 157.4 [127.7–192.4] | 138.7 [101.5–190.3] | 0.466 | 193.4 (64.5) | 192.6 (69.5) | 0.983 |
| Potassium (mmol/L) | 5.3 (0.5) | 5.3 (0.8) | 0.858 | 5.3 [5.2–5.3] | 4.4 [4.1–5.0] | 0.084 |
| Calcium (mg/dL) | 8.7 (0.6) | 8.7 (0.9) | 0.922 | 8.6 (0.1) | 8.8 (0.7) | 0.649 |
| Phosphorus (mg/dL) | 4.0 [3.3–4.6] | 5.4 [4.0–6.5] | 0.046 | 6.3 (0.6) | 5.8 (1.8) | 0.686 |
| Albumin (g/dL) | 3.25 [3.1–3.42] | 3.4 [3.2–3.60] | 0.191 | 32.0 [31.5–34.0] | 34.0 [29.0–37.0] | 0.797 |
| TMAO (μM/L) | 109.4 [95.6–168.4] | 124.9 [76.3–203.4] | 0.855 | 119 [115.7–149.2] | 117.9 [78.4–196.1] | 0.759 |
| pCS (μM/L) | 170.4 (95.8) | 193.2 (85.1) | 0.426 | 169.8 (83.1) | 146.3 (94.8) | 0.679 |
| (IS μM/L) | 33.9 [28.4–47.1] | 43.5 [30.3–64.7] | 0.225 | 38.6 [17.1–53.6] | 34.7 [15.9–33.2] | 0.852 |
| leptin (ng/mL) | 5.9 [1.8–14.6] | 7.6 [4.1–19.9] | 0.335 | 13.5 [11.8–23.1] | 14.1 [5.3–36.1] | 0.861 |
| adiponectin (μg/mL) | 4.8 [2.8–9.3] | 5.3 [2.7–9.3] | 0.678 | 4.8 (4.6) | 8.5 (4.9) | 0.21 |
| ZAG (μg/mL) | 11.7 [10.7–15.9] | 11.0 [8.4–14.3] | 0.367 | 12.8 [9.9–39.1] | 10.7 [8.5–15.0] | 0.627 |
| IL-6 (pg/mL) | 12.6 [11.0–14.7] | 8.2 [5.0–18.2] | 0.107 | 7.9 [7.4–11.4] | 5.7 [2.8–13.7] | 0.328 |
| irisin (μg/mL) | 7.6 [6.8–8.1] | 7.2 [6.1–8.4] | 0.496 | 7.8 [6.8–8.1] | 9.3 [8.1–10.2] | 0.061 |
| myostatin (pg/mL) | 2816.8 [1698.4–4000.0] | 3482.4 [2356.0–4710.0] | 0.182 | 5214.9 (2503.6) | 6735.1 (2753.8) | 0.359 |
| LAR | 0.8 [0.3–5.9] | 1.7 [0.5–7.4] | 0.282 | 10.7 [5.8–10.7] | 2.7 [0.6–6.1] | 0.394 |
| irisin/IL-6 | 0.6 [0.5–0.8] | 0.7 [0.4–1.5] | 0.266 | 0.9 [0.7–1.0] | 1.8 [0.7–3.5] | 0.258 |
| myostatin/IL-6 | 215.9 [115.5–392.0] | 352.2 [194.1–702.3] | 0.031 | 765.4 [473.7–872.3] | 831.4 [508.2–2804.2] | 0.421 |
| hsCRP (mg/dL) | 9.8 [7.5–10.0] | 6.7 [3.0–10.0] | 0.176 | 9.2 [8.8–9.5] | 3.3 [1.9–9.8] | 0.258 |
| obestatin (pg/mL) | 158.0 [157.0–575.0] | 160.0 [154.0–425.0] | 0.872 | 154.0 [119.0–154.5] | 155.0 [87.0–488.0] | 0.402 |
| ADMA (μM/L) | 1.0 [0.7–1.3] | 0.8 [0.5–1.3] | 0.328 | 0.9 [0.6–1.2] | 0.6 [0.4–1.0] | 0.816 |
| HD Patients | PD Patients | |||||
|---|---|---|---|---|---|---|
| Cut-Off Points | Log-Rank | p-Values | Cut-Off Points | Log-Rank | p-Values | |
| Gender [0-W; 1-M] | binary | 1.0 | 0.321 | binary | 3.3 | 0.070 |
| Age (years) | 65.0 | 4.7 | 0.031 | 50.5 | 3.8 | 0.049 |
| Dialysis vintage (months) | 28.5 | 0.4 | 0.540 | 14.0 | 0.4 | 0.527 |
| BMI (kg/m2) * | 25.0 | 0.1 | 0.822 | 25.0 | 2.5 | 0.114 |
| malnutrition [SGA ≤ 5] | binary | 0.1 | 0.748 | 0.0 | 1.9 | 0.165 |
| sarcopenia | binary | 3.0 | 0.081 | 0.0 | 1.3 | 0.250 |
| LTI (kg/m2) * | 14.0 | 0.3 | 0.565 | 14.0 | 0.8 | 0.375 |
| BUN (mg/L) | 50.0 | 6.8 | 0.009 | 49.5 | 2.5 | 0.114 |
| HCT (%) | 31.4 | 2.2 | 0.140 | 33.2 | 0.1 | 0.715 |
| Hb (g/dL) | 10.3 | 0.3 | 0.613 | 11.1 | 0.1 | 0.715 |
| NLR | 3.1 | 4.2 | 0.040 | 3.8 | 0.6 | 0.449 |
| PLR | 142.2 | 0.6 | 0.453 | 190.6 | 0.5 | 0.488 |
| Potassium (mmol/L) * | 5.1 | 0.7 | 0.388 | 5.1 | 7.9 | 0.005 |
| Calcium (mg/dL) | 8.8 | 0.9 | 0.354 | 8.8 | 3.1 | 0.076 |
| Phosphorus (mg/dL) * | 4.5 | 6.2 | 0.013 | 4.5 | 1.3 | 0.259 |
| Albumin (g/dL) * | 35.0 | 1.0 | 0.321 | 35.0 | 0.2 | 0.686 |
| TMAO (μM/L) | 124.5 | 0.1 | 0.727 | 118.2 | 0.7 | 0.408 |
| pCS (μM/L) | 172.6 | 0.1 | 0.821 | 143.3 | 0.5 | 0.488 |
| (IS μM/L) | 41.8 | 1.8 | 0.177 | 36.7 | 1.1 | 0.290 |
| leptin (ng/mL) | 7.2 | 1.2 | 0.279 | 13.8 | 0.3 | 0.614 |
| adiponectin (μg/mL) | 5.3 | 0.2 | 0.694 | 7.7 | 0.2 | 0.686 |
| ZAG (μg/mL) | 11.5 | 1.0 | 0.315 | 10.9 | 0.3 | 0.598 |
| IL-6 (pg/mL) | 9.2 | 4.3 | 0.038 | 5.8 | 3.3 | 0.070 |
| irisin (μg/mL) | 7.2 | 0.0 | 0.826 | 9.1 | 2.2 | 0.135 |
| myostatin (pg/mL) | 3333.8 | 0.7 | 0.395 | 6418.0 | 0.2 | 0.625 |
| leptin/adiponectin | 1.57 | 1.14 | 0.28 | 2.65 | 0.04 | 0.84 |
| irisin/IL-6 | 0.69 | 1.09 | 0.30 | 1.4 | 3.83 | 0.05 |
| myostatin/IL-6 | 320.1 | 0.89 | 0.34 | 771.1 | 0.43 | 0.51 |
| hsCRP (mg/dL) * | 5.0 | 4.0 | 0.046 | 5.0 | 3.4 | 0.064 |
| obestatin (pg/mL) | 159.5 | 0.9 | 0.343 | 155.0 | 2.1 | 0.144 |
| ADMA (μM/L) | 0.9 | 1.3 | 0.263 | 0.7 | 0.2 | 0.625 |
| HD Patients | PD Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | z | p-Values | Concordance | Hazard Ratio (HR) | z | p-Values | Concordance | |
| Gender [0-W; 1-M] | 1.80 | 0.98 | 0.327 | 0.57 | complete separation—excluded from analysis | |||
| Age (years) | 1.04 | 2.05 | 0.040 | 0.69 | 1.14 | 2.05 | 0.041 | 0.90 |
| Dialysis vintage (months) | - | - | - | - | 1.00 | 0.00 | 0.998 | 0.41 |
| BMI (kg/m2) | 1.01 | 0.20 | 0.842 | 0.51 | 1.26 | 1.83 | 0.068 | 0.81 |
| malnutrition [SGA ≤ 5] | 1.20 | 0.32 | 0.747 | 0.53 | 0.00 | −0.01 | 0.996 | 0.71 |
| sarcopenia | 2.93 | 1.66 | 0.096 | 0.65 | 4.43 | 1.05 | 0.293 | 0.61 |
| LTI (kg/m2) | 0.91 | −0.73 | 0.468 | 0.56 | 1.60 | 1.50 | 0.134 | 0.88 |
| BUN (mg/L) | 0.96 | −1.91 | 0.057 | 0.66 | 1.10 | 2.18 | 0.030 | 0.82 |
| HCT (%) | 1.11 | 1.45 | 0.146 | 0.60 | 0.95 | −0.41 | 0.684 | 0.53 |
| Hb (g/dL) | - | - | - | - | 0.84 | −0.46 | 0.646 | 0.56 |
| NLR | 1.03 | 0.66 | 0.510 | 0.61 | 0.80 | −0.54 | 0.589 | 0.65 |
| PLR | 1.00 | 0.45 | 0.652 | 0.56 | 1.00 | 0.19 | 0.846 | 0.60 |
| Potassium (mmol/L) | 1.09 | 0.22 | 0.823 | 0.55 | 3.68 | 1.63 | 0.104 | 0.80 |
| Calcium (mg/dL) | 0.99 | −0.03 | 0.979 | 0.52 | 0.48 | −0.75 | 0.451 | 0.61 |
| Phosphorus (mg/dL) | 0.70 | −1.93 | 0.053 | 0.68 | 1.21 | 0.67 | 0.504 | 0.60 |
| Albumin (g/dL) | 0.93 | −1.17 | 0.243 | 0.64 | 1.02 | 0.18 | 0.856 | 0.51 |
| TMAO (μM/L) | 1.00 | −0.57 | 0.566 | 0.51 | 1.00 | 0.05 | 0.963 | 0.63 |
| pCS (μM/L) | 1.00 | 0.76 | 0.449 | 0.54 | 1.00 | 0.69 | 0.487 | 0.57 |
| (IS μM/L) | 0.99 | −1.01 | 0.312 | 0.59 | 1.00 | −0.02 | 0.984 | 0.49 |
| leptin (ng/mL) | 0.98 | −1.10 | 0.272 | 0.60 | 0.99 | −0.38 | 0.703 | 0.51 |
| adiponectin (μg/mL) | 1.00 | −0.04 | 0.966 | 0.49 | 0.83 | −1.13 | 0.260 | 0.67 |
| ZAG (μg/mL) | 0.99 | −0.28 | 0.782 | 0.39 | 1.05 | 1.94 | 0.053 | 0.52 |
| IL−6 (pg/mL) | 1.06 | 1.22 | 0.222 | 0.65 | 1.06 | 0.70 | 0.484 | 0.70 |
| irisin (μg/mL) | 1.08 | 0.52 | 0.603 | 0.54 | 0.54 | −1.45 | 0.147 | 0.76 |
| myostatin (pg/mL) | 1.00 | −1.40 | 0.161 | 0.63 | 1.00 | −1.03 | 0.301 | 0.63 |
| LAR | 0.96 | −0.8 | 0.42 | 0.59 | 1.1 | 0.94 | 0.35 | 0.6 |
| irisin/IL-6 | 0.41 | −1.52 | 0.13 | 0.61 | 0.35 | −1.1 | 0.27 | 0.7 |
| myostatin/IL-6 | - | - | - | - | 1 | −0.96 | 0.34 | 0.63 |
| hsCRP (mg/dL) | 1.18 | 1.68 | 0.094 | 0.60 | 1.43 | 1.35 | 0.177 | 0.73 |
| obestatin (pg/mL) | 1.00 | 0.20 | 0.844 | 0.52 | 1.00 | −0.73 | 0.468 | 0.64 |
| ADMA (μM/L) | 1.03 | 0.08 | 0.939 | 0.60 | 2.04 | 0.45 | 0.654 | 0.51 |
| Variables that do not meet the assumptions of proportionality of distributions; Log-Normal model | ||||||||
| exp (coef) | z | p-values | Concordance index | - | - | - | - | |
| Dialysis vintage (months) | 0.99 | −0.80 | 0.422 | 0.51 | - | - | - | - |
| Hb (g/dL) | 0.82 | −0.67 | 0.504 | 0.56 | - | - | - | - |
| myostatin/IL-6 | 1.0039 | 2.01 | 0.044 | 0.7 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja-Stolc, S.; Potrykus, M.; Ruszkowski, J.; Dębska-Ślizień, A.; Małgorzewicz, S. Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study. Nutrients 2025, 17, 1043. https://doi.org/10.3390/nu17061043
Czaja-Stolc S, Potrykus M, Ruszkowski J, Dębska-Ślizień A, Małgorzewicz S. Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study. Nutrients. 2025; 17(6):1043. https://doi.org/10.3390/nu17061043
Chicago/Turabian StyleCzaja-Stolc, Sylwia, Marta Potrykus, Jakub Ruszkowski, Alicja Dębska-Ślizień, and Sylwia Małgorzewicz. 2025. "Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study" Nutrients 17, no. 6: 1043. https://doi.org/10.3390/nu17061043
APA StyleCzaja-Stolc, S., Potrykus, M., Ruszkowski, J., Dębska-Ślizień, A., & Małgorzewicz, S. (2025). Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study. Nutrients, 17(6), 1043. https://doi.org/10.3390/nu17061043






