Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Synthesis of DSPE-PEG-SC and DSPE-PEG-MAN
2.3. Preparation of NaA-Loaded Liposomes
2.4. In Vitro Cytotoxicity Evaluation
2.5. Endocytosis Pathway Activity Using Uptake Inhibitors
2.6. In Vivo Distribution of Fluorescent Liposomes Following Oral Intaking
2.7. Tissue Distribution of 13C-NaA-Loaded Liposomes Following Oral Intaking
2.8. In Vitro Alleviation Effect of Liposomes in NAFLD Cell Models
2.9. In Vivo Alleviation Effect of Liposomes in NAFLD Model Mice
2.10. qRT-PCR
2.11. Western Blot
2.12. Statistical Analyses
3. Results
3.1. Synthesis of DSPE-PEG2000-SC and DSPE-PEG2000-MAN
3.2. Design and Preparation of NaA Liposomes
3.3. Characterization of NaA Liposomes
3.4. Cell Cytotoxicity Test of NaA Liposomes
3.5. Endocytosis Pathway Activities Using Uptake Inhibitors
3.6. Biodistribution and In Vivo Imaging of Fluorescent Liposomes
3.7. Tissue Distribution of 13C-NaA Liposomes
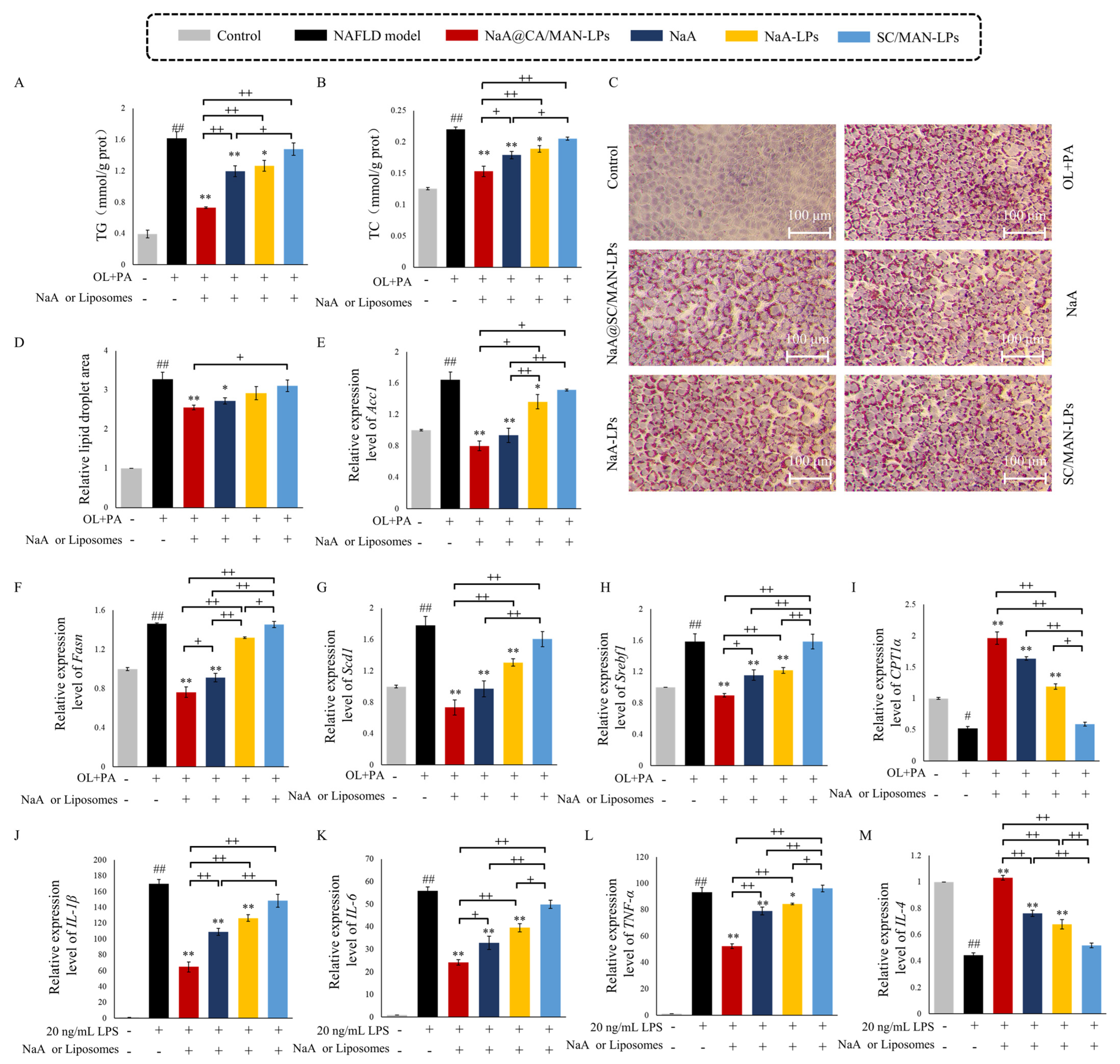
3.8. In Vitro Inhibition Efficacy on Lipid Accumulation in NAFLD Cells
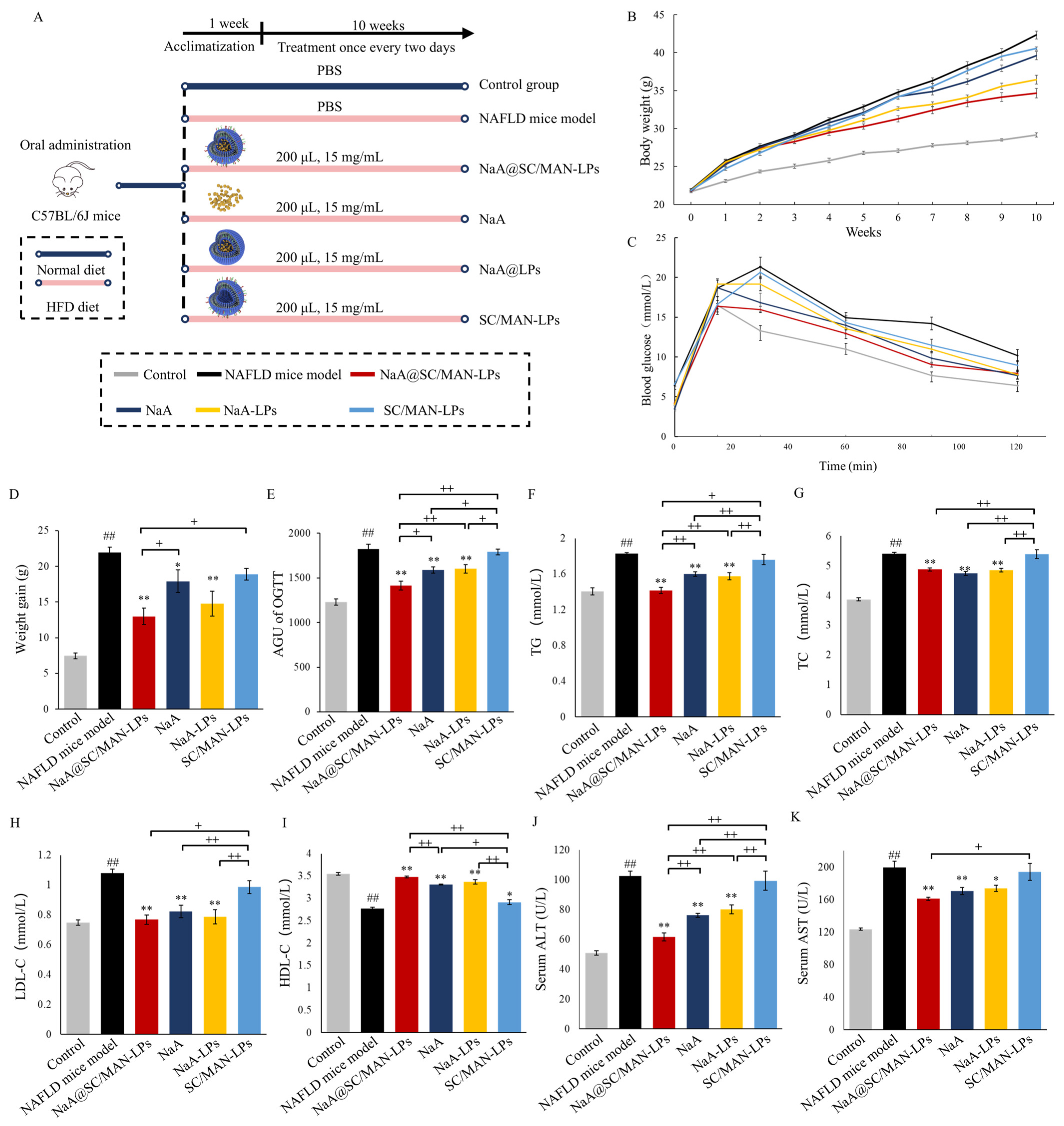
3.9. In Vivo Alleviation Efficacy on Metabolic Abnormalities in NAFLD Mice
3.10. In Vivo Inhibition on Lipid Accumulation in NAFLD Mice
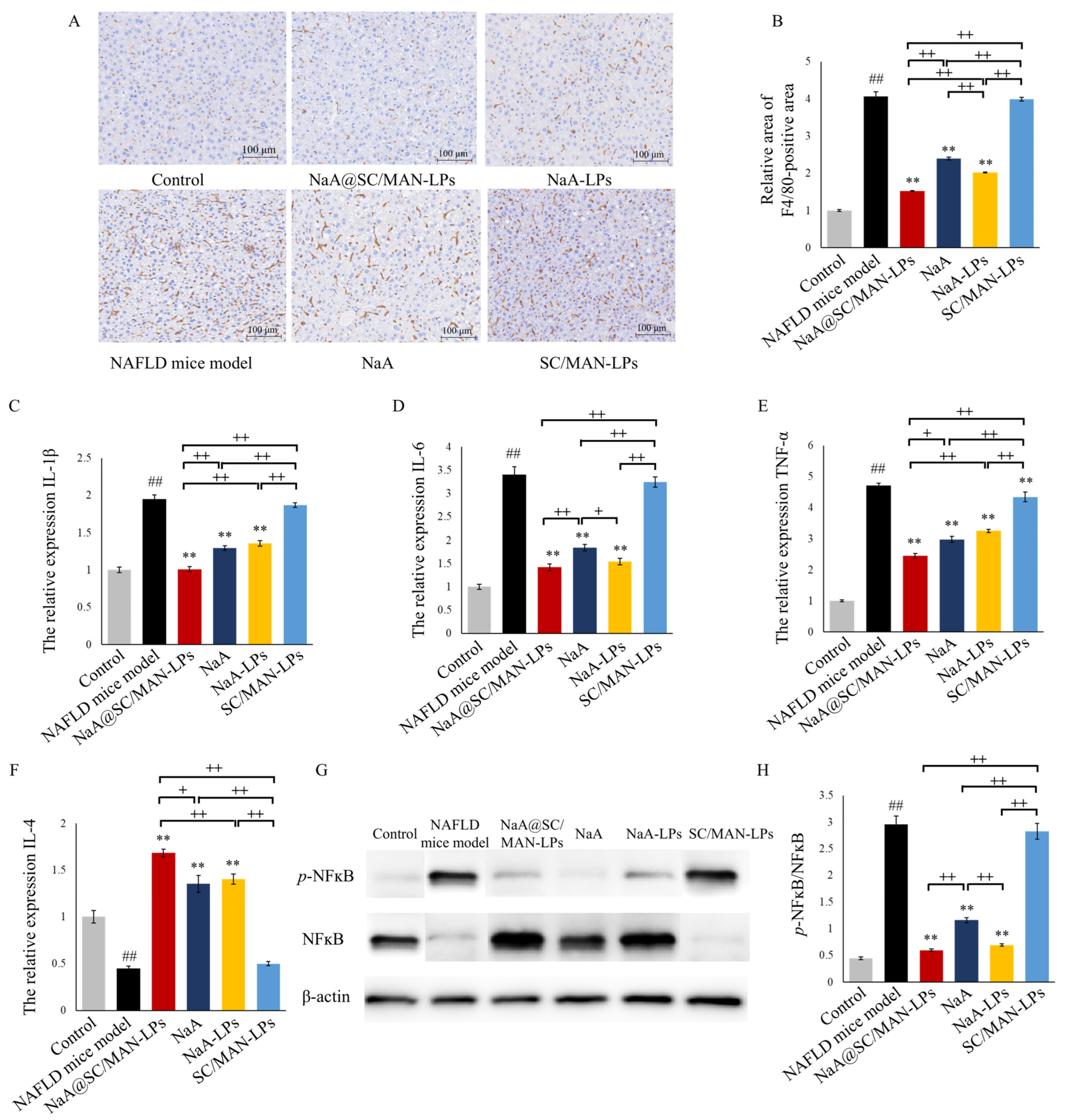
3.11. In Vivo Exhibition on Hepatic Oxidative and Inflammation in NAFLD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaA | sodium acetate |
| NAFLD | non-alcoholic fatty liver disease |
| SC | sodium cholate |
| MAN | mannose |
| TG | triglycerides |
| TC | total cholesterol |
| NASH | non-alcoholic steatohepatitis |
| HCC | hepatocellular carcinoma |
| SCFAs | short-chain fatty acids |
| AMPKα | AMP-activated protein kinase alpha |
| FFAR2 | free fatty acid receptor 2 |
| LPS | lipopolysaccharide |
| HFD | high-fat diet |
| GLP-1 | glucagon-like peptide 1 |
| PYY | peptide YY |
| LMW | low-molecular-weight |
| NTCP | Na+-taurocholate co-transporting polypeptide |
| ASBT | bile acid-na+-dependent bile acid transporter |
| DSPE-PEG2000-NH2 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene gly-col)-2000] |
| DSPC | 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine |
| DC-cholesterol | 3β-[N-(Dimethylaminoethane) carbamoyl] cholesterol |
| EDC | N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-Hydroxysuccinimide |
| CY7-SE triethylamine | sulfo-cyanine7 succinimidyl ester triethylamine |
| DMSO | dimethyl sulfoxide |
| HPLC | high-performance liquid chromatography |
| AM | amiloride hydrochloride |
| CH | chlorpromazine |
| M-β-CD | Me-Thyl-β-Cyclodextrin |
| GE | genistrin |
| NY | nystatin |
| GC-MS | triple quadrupole gas chromatography–mass spectrometry |
| DSC | differential scanning calorimeter |
| FTIR | Fourier transform infrared spectrometer |
| PDI | poly-dispersity indexes |
| EE | encapsulation efficiency |
| DL | drug loading |
| TEM | transmission electron microscopy |
| OL | sodium oleate |
| PA | sodium palmitate |
| Acc1 | acetyl-CoA carboxylase 1 |
| Fasn | atty acid synthase |
| Scd1 | stearoyl-CoA desaturase 1 |
| Srebf1 | sterol regulatory element-binding transcription factor 1 |
| CPT1α | carnitine palmitoyltransferase 1α |
| OGTT | oral glucose tolerance tests |
| LDL-C | low-density lipoprotein cholesterol |
| HDL-C | high-density lipoprotein cholesterol |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ROS | reactive oxygen species |
References
- Fatima, H.; Rangwala, H.S.; Mustafa, M.S.; Shafique, M.A.; Abbas, S.R.; Rangwala, B.S. Analyzing and evaluating the prevalence and metabolic profile of lean NAFLD compared to obese NAFLD: A systemic review and meta-analysis. Ther. Adv. Endocrinol. Metab. 2024, 15, 1–16. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Deepa, H.; Kim, S.; Tami, P. Evaluating bioactive-substance-based interventions for adults with MASLD: Results from a systematic scoping Review. Nutrients 2025, 17, 453. [Google Scholar] [CrossRef]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Zhao, J.W.; Xie, F.; He, H.X.; Johnston, L.J.; Dai, X.F.; Wu, C.D.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.F.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Dangana, E.O.; Omolekulo, T.E.; Areola, E.D.; Olaniyi, K.S.; Soladoye, A.O.; Olatunji, L.A. Sodium acetate protects against nicotine-induced excess hepatic lipid in male rats by suppressing xanthine oxidase activity. Chem. Biol. Interact. 2020, 316, 108929. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Li, L.; He, M.L.; Xiao, H.; Liu, X.Q.; Wang, K.; Zhang, Y.S. Acetic acid influences BRL-3A cell lipid metabolism via the AMPK signalling pathway. Cell Physiol. Biochem. 2018, 45, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yanada, T.; Kamikado, K.; Kim, Y.G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Deng, M.J.; Gong, J.H.; Hou, Y.C.; Zhao, L. Bidirectional regulation of sodium acetate on macrophage activity and its role in lipid metabolism of hepatocyte. Int. J. Mol. Sci. 2023, 24, 5536. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Cherta-Murillo, A.; Pugh, J.E.; Alaraj-Alshehhi, S.; Hajjar, D.; Chambers, E.S.; Frost, G.S. The effects of SCFAs on glycemic control in human, a systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 335–361. [Google Scholar] [CrossRef]
- Suokas, A.; Kupapi, M.; Heikkila, J.; Lindros, K.; Ylikahri, R. Acute cardiovascular and metabolic effects of acetate in men. Alcohol. Clin. Exp. Res. 1998, 12, 52–58. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.W.M.O.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Elliott, G.A.; Purmalis, A.; Vandermeed, D.A.; Denlinger, R.H. The propionic acids-gastrointestinal toxicity in various species. Toxicol. Pathol. 1988, 16, 245–250. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to supercritical fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Liu, W.L.; Hou, Y.Y.; Jin, Y.Y.; Wang, Y.P.; Xu, X.K.; Han, J.Z. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazu, S.; Kiselev, M.A. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef]
- Fan, W.W.; Xia, D.N.; Zhu, Q.L.; Li, X.Y.; He, S.F.; Zhu, C.L.; Guo, S.Y.; Hovgaard, L.; Yang, M.S.; Gan, Y. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials 2018, 151, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.Y. Enhanced hepatic-targeted delivery via oral administration using nanoliposomes functionalized with a novel DSPE-PEG-cholic acid conjugate. RSC Adv. 2016, 6, 28110–28120. [Google Scholar] [CrossRef]
- Lei, K.L.; Yuan, M.H.; Zhou, T.; Ye, Q.; Zeng, B.; Zhou, Q.; Wei, A.L.; Guo, L. Research progress in the application of bile acid-drug conjugates: A “trojan horse” strategy. Sterodies 2021, 173, 108879. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.L.; Li, H.H.; Zhang, H.Y.; Zhu, Y.; Omari-Siaw, E.; Sun, C.Y.; Wei, Q.Y.; Deng, W.W.; Yu, J.N.; et al. Galangin-loaded, liver targeting liposomes: Optimization and hepatoprotective efficacy. J. Drug Deliv. Sci. Technol. 2018, 46, 339–347. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.D.; Wang, Y.; Li, Z.; Zhum, C.Y. Co-delivery doxorubicin and silybin for anti-hepatoma via enhanced oral hepatic-targeted efficiency. Int. J. Nanomedicine 2018, 14, 301–315. [Google Scholar] [CrossRef]
- Hu, S.W.; Niu, M.M.; Hu, F.Q.; Lu, Y.; Qi, J.P.; Yin, Z.N.; Wu, W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- Xiong, M.H.; Lei, Q.; You, X.Y.; Gao, T.T.; Song, X.J.; Xia, Y.; Ye, T.H.; Zhang, L.D.; Wang, N.Y.; Yu, L.T. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J. Microencapsul. 2017, 34, 513–521. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhang, M.L.; Chen, R.N.; Niu, R.W.; Deng, Y.H. Mannose derivative and lipid a dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 2014, 88, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Nacaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ’browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhao, R.; Kang, Z.Y.; Cao, Z.Q.; Liu, N.H.; Shen, J.J.; Wang, C.; Pan, F.; Zhou, X.; Liu, Z.; et al. Delivery of short chain fatty acid butyrate to overcome Fusobacterium nucleatum-induced chemoresistance. J. Control. Release 2023, 363, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mody, N.; Kushwah, V.; Jain, S.; Vyas, S.P. Development, characterization and ex vivo assessment of lipid-polymer based nanocomposite(s) as a potential carrier for site-specific delivery of immunogenic molecules. J. Drug Deliv. Sci. Technol. 2019, 51, 310–319. [Google Scholar] [CrossRef]
- Garg, M.; Dutta, T.; Jain, N.K. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. Eur. J. Pharm. Biopharm. 2007, 67, 76–85. [Google Scholar] [CrossRef]
- Shubitowski, T.B.; Pluznick, J.L. Short chain fatty acid delivery: Assessing exogenous administration of the microbiome metabolite acetate in mice. Physiol. Rep. 2019, 7, e14005. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Fushimi, T.; Kishi, M.; Irie, S.; Tsuji, S.; Hosokawa, N.; Kaga, T. Bioavailability of acetate from two vinegar supplements: Capsule and drink. J. Nutr. Sci. Vitaminol. 2010, 56, 266–269. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Jiang, Z.H.; Xu, J.Z.; Zhang, J.Y.; Sun, R.B.; Zhou, J.; Lu, Y.; Gong, Z.P.; Huang, J.; Shen, X.C.; et al. Improving the ameliorative effects of berberine and curcumin combination via dextran-coated bilosomes on non-alcohol fatty liver disease in mice. J. Nanobiotechnology 2014, 19, 230. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Varum, K.M.; Marsich, E. Butyrate-loaded chitosan/hyaluronan nanoparticles: A suitable tool for sustained inhibition of ROS release by activated neutrophils. Macromol. Biosci. 2017, 17, 1700214. [Google Scholar] [CrossRef]
- Shashni, B.; Tajika, Y.; Ikeda, Y.; Nishikawa, Y.; Nagasaki, Y. Self-assembling polymer-based short chain fatty acid prodrugs ameliorate non-alcoholic steatohepatitis and liver fibrosis. Biomaterials 2023, 295, 122047. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.Y.; Ho, R.J.Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Tian, F.L.; Nie, D.; Liu, Y.; Qian, K.; Yu, M.R.; Wang, A.H.; Zhang, Y.Q.; Shi, X.H.; Gan, Y. Rapid transport of germ-mimetic nanoparticles with dual conformational polyethylene glycol chains in biological tissues. Sci. Adv. 2020, 6, eaay9937. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Omran, S.; Hazzah, H.A.; Abdallah, O.Y. Anionic versus cationic bilosomes as oral nanocarriers for enhanced delivery of the hydrophilic drug risedronate. Int. J. Pharm. 2019, 564, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- He, H.S.; Lu, Y.; Qi, J.P.; Zhu, Q.G.; Chen, Z.J.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Lee, M.K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.L.; Zhang, J.C. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Baipaywad, P.; Kim, Y.; Wi, J.S.; Paik, T.; Park, H. Size-controlled synthesis, characterization, and cytotoxicity study of monodisperse poly (dimethylsiloxane) nanoparticles. J. Ind. Eng. Chem. 2017, 53, 177–182. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Anstee, Q.M.; Loomba, R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024, 36, 912–926. [Google Scholar] [CrossRef]
- Lanthier, N. Targeting Kupffer cells in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why and how? World J. Hepatol. 2015, 7, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.H.; Gao, X.L.; Ge, S.Y.; Li, H.L.; Wang, R.; Zhao, L. The role of cGAS-STING signalling in metabolic diseases: From signalling networks to targeted intervention. Int. J. Biol. Sci. 2024, 20, 152–174. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.Z.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]



| Group | Partial Size (nm) | PDI | Zeta Potential (mV) | EE% | DL% |
|---|---|---|---|---|---|
| NaA@SC/MAN-LPs | 94.15 ± 0.23 | 0.23 ± 0.01 | +33.00 ± 3.71 | 76.6 ± 0.47 | 33.16 ± 0.16 |
| NaA@SC-LPs | 100.46 ± 1.56 | 0.24 ± 0.01 | +33.10 ± 3.56 | 81.57 ± 0.94 | 37.94 ± 0.36 |
| NaA@MAN-LPs | 98.35 ± 1.55 | 0.24 ± 0.02 | +32.40 ± 6.19 | 78.90 ± 0.07 | 33.29 ± 0.03 |
| NaA@LPs | 95.29 ± 1.07 | 0.22 ± 0.01 | +27.50 ± 5.06 | 79.03 ± 0.30 | 35.28 ± 0.26 |
| SC/MAN-LPs | 96.53 ± 0.68 | 0.23 ± 0.00 | +33.00 ± 3.71 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Gao, X.; Gong, J.; Dong, X.; Hao, Y.; Zhai, Z.; Zhang, H.; Zhang, M.; Liu, R.; Wang, R.; et al. Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation. Nutrients 2025, 17, 930. https://doi.org/10.3390/nu17050930
Hou Y, Gao X, Gong J, Dong X, Hao Y, Zhai Z, Zhang H, Zhang M, Liu R, Wang R, et al. Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation. Nutrients. 2025; 17(5):930. https://doi.org/10.3390/nu17050930
Chicago/Turabian StyleHou, Yichao, Xilong Gao, Jiahui Gong, Xinrui Dong, Yanling Hao, Zhengyuan Zhai, Hao Zhang, Ming Zhang, Rong Liu, Ran Wang, and et al. 2025. "Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation" Nutrients 17, no. 5: 930. https://doi.org/10.3390/nu17050930
APA StyleHou, Y., Gao, X., Gong, J., Dong, X., Hao, Y., Zhai, Z., Zhang, H., Zhang, M., Liu, R., Wang, R., & Zhao, L. (2025). Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation. Nutrients, 17(5), 930. https://doi.org/10.3390/nu17050930








