1. Introduction
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines fermented foods as “foods made through controlled microbial growth and enzymatic conversions of food components” [
1]. Fermentation is a biochemical process in which microorganisms such as bacteria, yeasts, or molds metabolize organic compounds from the substrate—usually sugars and starches—typically under anaerobic conditions, producing various derivatives that enhance the nutritional, organoleptic, and technological properties of the food [
2]. Fermented foods are an important component of a healthy human diet [
3]. In recent years, there has been a growing global consensus that the consumption of fermented foods is associated with positive health effects [
4].
The health effects of fermented foods have been extensively studied and are well documented [
5,
6]. Thanks to next-generation sequencing and metagenomic methods, it is now possible to also precisely track the effects of fermented foods on the health of the gut microbiota as well. In cross-sectional studies, the gut microbiota of individuals who regularly consume plant-based fermented foods differs significantly in terms of β-diversity and contains higher proportions of
Bacteroides spp.,
Prevotella spp.,
Dorea spp., and
Lachnospiraceae [
7,
8], whereas among consumers of dairy-based fermented foods (yogurt),
Streptococcus thermophilus and
Bifidobacterium animalis prevail [
9]. Consequently, the gut microbiota is enriched not only with bacteria associated with fermented foods, including various lactic acid bacteria, but also with certain unrelated bacteria such as particular
Prevotella and
Enterococcus species [
8]. A 2024 literature review identified 42 human interventional studies on specific fermented foods, of which 24 examined modulation of the gut microbiota [
10]. Although the outcomes depend on the studied specific aliments, human interventional research generally shows that regular consumption of fermented foods increases α-diversity [
11] and, depending on the specific product, may increase the proportion of bacterial groups associated with short-chain fatty acids (SCFA) production and immunomodulatory effects while reducing the proportion of potentially pathogenic bacteria [
12,
13,
14,
15,
16]. Owing to the above-mentioned effects on the composition of the gut microbiota, the application of fermented foods has the potential to counteract dysbiosis, a disturbance of the gut microbiota. However, due to the heterogeneity of the studies conducted to date, this hypothesis cannot yet be confirmed by scientific evidence [
17].
Whole fermented foods are emerging as key components of sports nutrition due to their multiple health effects but also availability and practicality [
5,
6]. Due to their potential application in targeted gut microbiota personalization, fermented foods are a promising management option for dysbiosis prevention and treatment [
18]. The broad implications of dysbiosis on athletic performance and recovery [
19] underline the importance of whole fermented foods in sports nutrition.
Sauerkraut is probably the most popular fermented whole food preparation in Europe [
6]. Sauerkraut is a fermented vegetable product that is derived from the malolactic fermentation of raw fresh white cabbage (
Brassica oleracea L. var. capitata) in a salt brine with 2–3% (
w/
w) sodium chloride [
20]. Sauerkraut can be regarded as a synbiotic whole food, containing pro-, pre-, and postbiotics, all compounds with beneficial effects on the gut microbiota [
21]. Sauerkraut is enriched with probiotics involved in the fermentation process [
22], such as
Weissella spp.,
Leuconostoc mesenteroides,
Levilactobacillus brevis,
Lactiplantibacillus plantarum, and
Pediococcus pentosaceus [
23], which are resistant to bile salts and low gastric pH (one even to β-hemolysis) and demonstrate antimicrobial activity [
24]. Sauerkraut is rich in fiber by nature, which can act as a prebiotic, and biologically active metabolites, including SCFA and biogenic amines, produced by the controlled bacterial metabolism (activity and growth) [
25]. Due to its synbiotic properties, sauerkraut supplementation could potentially be beneficial for gut microbiota personalization in athletes, thereby improving athletic performance and recovery [
26]. The health benefits of sauerkraut consumption have been studied in a limited body of research, mostly on in vitro models [
27]. Human clinical trials are still scarce. Based on earlier scientific findings, one can conclude that the use of sauerkraut can potentially exert beneficial effects on digestive function disorders, such as various functional disorders (e.g., IBS) [
28]; enhance immune functions, including the body’s defense against infections and immune response to tumor processes (via glucosinolate); and positively influence metabolic disorders such as diabetes [
29,
30].These observed effects underline the potential of sauerkraut supplementation in sports nutrition.
Since a knowledge gap was found regarding the potential role of sauerkraut in sports nutrition for gut microbiota personalization, a proof-of-concept study was conducted in 2023 [
31].
The original study was limited due to the small sample size, which was reflected in high false-positivity rates in the results regarding the gut microbiota. But even when adjusted for false positives, the proof-of-concept study yielded a comparably large number of statistically significant results. Consequently, a decision was made to replicate the study and conduct a pilot follow-up study to attempt to reproduce the positive outcomes of the intervention in a new sample of participants with similar characteristics but of greater quantity. An additional objective of the confirmatory study was to also assess the long-term effects of the intervention by including an additional sampling time point for stool and laboratory parameters measurements 30 days postintervention, thereby introducing a washout period (T40). With the newly obtained data, we further close the literature gap on the potential of the sauerkraut supplementation in sports nutrition for gut microbiota personalization.
2. Materials and Methods
2.1. Study Design
The study aimed to investigate the short- and long-term effects of a short course of sauerkraut supplementation on the gut microbiota of active athletes. The primary goal was to potentially replicate the effects observed in the original study within a similar cohort of active athletes [
31]. The intervention itself was identical to that in the original study, lasting 10 days. In this study, its comparably short duration was based on earlier studies, where potent synbiotics altered the gut microbiota already after a short administration of 10 days [
32,
33], and chosen to facilitate study execution. However, it is important to note that the fermented food used in this study (sauerkraut) was produced a year later, in 2023. Outcome measures included changes in gut microbiota composition and laboratory parameters, as in the first study. Gut microbiota analyses followed the same protocol and were conducted in the same laboratory to minimize potential deviations due to technical aspects of amplicon sequencing or laboratory testing. Confounding factors such as diet, training, and sleep were monitored using identical research diaries [
34]. In addition to the athlete diet index (ADI) [
35], additional questionnaires were introduced to evaluate adherence to the Mediterranean diet, providing further objectivity regarding dietary intake during the intervention. As in the first study, stool characteristics were monitored using the Bristol Stool Scale (BSS), and potential side effects of sauerkraut intake were recorded.
Recruitment was conducted through collaboration with sports clubs and nutritionists specializing in athlete nutrition as well as via the researchers’ local networks. Unlike the first study, recreational athletes were included to facilitate and accelerate recruitment. The number of participants remained the same as in the first study.
As in the first study, an initial consultation was conducted at the research facility (The Gut Microbiome Center, CCM), where potential participants were provided with detailed verbal and written instructions about the study by the research team. Written informed consent forms were signed by both the participants and the researchers after all study procedures, risks, and benefits were thoroughly explained. Participants were provided with stool sampling kits and research materials, including sauerkraut. Body composition was assessed using bioelectrical impedance (Tanita MC-780, Tanita Corporation, Tokyo, Japan) at the initial consultation.
Participants were instructed to minimize deviations from their usual lifestyle during the intervention, particularly regarding diet, to allow precise monitoring of the intervention’s effects on gut microbiota. Therefore, they were asked to record food intake, supplementation, sleep duration, and physical activity for three to four days prior to and during the intervention. To simplify participation based on the experience from the first study, the pre-intervention monitoring period was shortened. Three questionnaires were used to objectively assess dietary intake: the athlete diet index (ADI), the Mediterranean Diet Adherence Screener (MEDAS) [
36], and the Short Questionnaire on Mediterranean Diet Adherence and Diet Sustainability (SQM) [
37].
To evaluate short- and long-term effects, stool sampling was performed by participants at home at three time points: the day before the intervention, the day after the intervention, and one month after the intervention, following the washout period.
The intervention consisted of daily supplementation with 250 g of sauerkraut. As this was a confirmatory study, the daily amount of sauerkraut remained unchanged from the first study. For logistical reasons, the amount was not adjusted for participants’ body weight. However, participants were allowed to vary the timing and combination of sauerkraut intake throughout the day, as long as they consumed 500 g every two days (equivalent to two daily doses). Participants recorded the amount and timing of sauerkraut intake in research diaries. Sauerkraut could be consumed alone or in combination with other foods (e.g., in salads or as a side dish). Any side effects, particularly gastrointestinal symptoms (e.g., constipation, diarrhea, bloating, flatulence, or abdominal pain), were recorded in the research diary throughout the study.
All procedures associated with this study adhered to the Declaration of Helsinki and were approved by the Ethics Committee of the University of Zagreb School of Medicine (reference number: 380-59-10106-23-111/36) on 27 March 2023. The study was registered at
ClinicalTrials.gov under registration number NCT06087146.
2.2. Participants
The objective of this study was to recruit active athletes, as in the proof-of-concept study, to replicate its findings as closely as possible. However, due to recruitment challenges in the initial study, the inclusion criteria were broadened from professional athletes or those professionally engaged in sports to individuals engaged in regular physical activity, even at a recreational level. Athletes from various disciplines were included again to minimize the influence of any specific sport on the gut microbiota. While male participants were again included, female participants were explicitly excluded in this study. To further replicate the initial study, the median age of participants from the first study was used as an inclusion criterion. The minimal sample size was established based on the original study power analysis results, which utilized a paired t-test and found that a sample size of 10 participants is sufficient to detect a large effect with 80% power and a significance level of 0.05. Therefore, the aim was to enroll around 20 subjects for adequate follow-up and, in the case of recruitment difficulties, at least 10 active athletes.
The inclusion criteria were as follows:
Age approximately 30 years;
Male sex;
Regular weekly engagement in physical activity (minimum four trainings weekly);
Good general physical health (assessed through annual occupational health check-ups, medical commissions of the Olympic Committee, sports medicine, or primary healthcare).
The exclusion criteria were identical to those of the initial study:
Use of antibiotics within six months prior to or during the intervention;
Probiotic supplementation within six months prior to or during the intervention;
History of gastrointestinal surgery;
Chronic medication use;
Known allergy to fermented or raw cabbage.
Since professional athletes were no longer the focus of this research, the study’s timeline was not aligned with training or competition schedules. The “Participant Classification Framework” [
38] was used to classify participants based on their physical activity levels and athletic achievements into different levels.
2.3. Supplementation Protocol
For the confirmatory study, sauerkraut from the same producer as in the proof-of-concept study, Eko Imanje Zrno d.o.o. (Vrbovec, Croatia), was used. This was pasteurized sauerkraut grown locally under biodynamic agricultural conditions. The sauerkraut was produced using the same process, recipe, personnel, and location, fermented in a 2% saline solution without preservatives. The only technological difference was that the cabbage was harvested and processed a year later, in 2023 [
39].
Each participant received five 500 g jars of sauerkraut. The nutritional and microbiological properties were not re-tested; instead, results from the analysis of the sauerkraut produced the previous year (2022) were assumed.
2.4. Standardization of Physical Activity, Sleep, and Diet
To eliminate key confounding factors, participants recorded their lifestyle habits, specifically physical activity, diet, and sleep, in a virtual Excel research diary (Microsoft, Palo Alto, CA, USA) designed for the proof-of-concept study. Participants completed the research diary in two phases: seven days before the intervention and during the ten-day intervention. For physical activity, participants recorded the duration of training sessions, including start and end times as well as the type of training. Regarding diet, participants logged the time, quantity, and specific foods consumed in each meal. If exact quantities could not be measured using scales, the Capnutra Food Atlas for this specific region of Europe [
40] was used. For sleep, participants recorded their bedtime and approximate wake-up time.
The collected data were analyzed by researchers, while dietary intake was analyzed by a nutritionist involved in the study. Dietary intake was quantified using the USDA database (U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center) to calculate energy (kilocalories) and macronutrient intake for the two phases: before and during the intervention. For physical activity and sleep, the average number of weekly training sessions, daily training duration, and average hours of sleep were calculated for both phases.
To further standardize dietary intake before and during the intervention, the athlete diet index (ADI) and two questionnaires on adherence to the Mediterranean diet were used: the Mediterranean Diet Adherence Screener (MEDAS) and the Short Questionnaire on Mediterranean Diet Adherence and Diet Sustainability (SQM).
The athlete diet index is a validated tool for assessing diet quality in professional and recreational athletes, providing an overall diet score and specific component scores (e.g., intake of key nutrients) [
35].
To further evaluate dietary adherence objectively, additional questionnaires assessing adherence to the Mediterranean diet were included. The Mediterranean diet, along with plant-based diets, is considered optimal for gut microbiota composition and function, increasing diversity and short-chain fatty acid (SCFA) production [
41]. The MEDAS questionnaire consists of 14 questions on the intake of key food groups in the Mediterranean diet, such as olive oil, legumes, and red wine [
42]. Although validated in Croatia and globally [
36,
43,
44], MEDAS assigns points for alcohol consumption, which has adverse effects on gut microbiota [
45]. Therefore, the SQM questionnaire, which excludes alcohol, was also used. SQM is a reliable tool consisting of eight questions on key Mediterranean food groups and diet sustainability [
46].
2.5. 16S rRNA NGS Analysis of Gut Microbiota
Participants collected stool samples at three time points: the day before the intervention (day 0, T0), the day after the intervention (day 11, T10), and one month after the intervention (day 30 after washout, T40). Samples were collected using cotton swabs and toilet paper at home, following provided instructions.
Procedures related to sample storage and the amplicon sequencing process were identical to those in the proof-of-concept study, where the process is described in detail [
31].
2.6. Laboratory Analysis
Samples for laboratory parameters and gut microbiota were collected at three time points: the day before, the day after, and one month after the intervention. Laboratory parameters were selected based on statistically significant findings from the proof-of-concept study and included the following:
Blood count parameters: leukocytes, neutrophils, and lymphocytes;
Vitamins: vitamin B12 and folic acid.
Laboratory analysis was performed in the same tertiary healthcare facility as in the first study, using EDTA or citrate blood depending on the measurement, as described in the original study [
31].
2.7. Statistical Analysis
Statistical analysis followed the same principles as the proof-of-concept study. Online forms were coded, and data were imported into SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Macintosh, Version 27.0. IBM Corp., Armonk, NY, USA) for descriptive and inferential statistical analyses.
Due to differences in study design, minor changes were made to the statistical analysis, particularly for outcome measures. Confounding factors, such as dietary intake and other lifestyle habits, were analyzed during two phases (pre- and postintervention). Mean differences in confounding factors were treated as quantitative variables and analyzed using paired t-tests after testing for distribution type.
Outcome measures, including gut microbiota and laboratory parameters, were assessed at three time points. Laboratory results were tested for distribution using the Shapiro–Wilk test. Depending on normality, differences across three time points were analyzed using repeated-measures ANOVA or the Friedman test. Two laboratory parameters, leukocyte concentration and vitamin B12 concentration, were assed at two time-points with the paired t-test.
Gut microbiota analysis used α-diversity metrics calculated based on ASVs subsampled to 10,000 reads per sample. Shannon’s index was calculated using the Qiime2 diversity function [
47]. Hypotheses were tested using repeated-measures correlation (rmcorr 0.7.0 function in R) [
48]. We performed Levene’s test for homogeneity of variances to assess the variance of α-diversity at the different time-points.
p-values were adjusted for multiple hypothesis testing using the Benjamini–Hochberg correction, with significance set at
q < 0.05 (non-FDR) [
49].
Taxonomic composition and metabolic pathway differences were assessed using repeated-measures analysis, filtered to a minimum threshold of 0.1% average relative abundance. Centered log-ratio (clr) transformation with pseudocounts was applied, and visualizations were generated using ggplot2 3.5.1 in R [
50].
Stool data were processed similarly to the first study. The probability of Bristol Stool Scale types 3 or 4 was analyzed using binomial tests in R with chi-squared tests, and statistically significant results were defined as p-values < 0.05.
4. Discussion
In this pilot follow-up study, we investigated whether the supplementation of 250 g of sauerkraut over a course of ten days would elicit the same or similar changes in the gut microbiota of active athletes as in an earlier proof-of-concept study [
31]. However, the confirmatory study did not yield the same results as the proof-of-concept study.
The original study explored the effects of a 10-day sauerkraut supplementation on gut microbiota composition and functionality in active, professional athletes. The study demonstrated significant and favorable changes in gut microbiota composition, including increased relative abundances of health-promoting anaerobic bacteria, particularly those within the Lachnospiraceae family, which are known short-chain fatty acid (SCFA) producers. These changes were accompanied by alterations in metabolic pathways related to nucleotide metabolism and cell wall synthesis, suggesting improved functionality of the gut microbiota. Interestingly, sauerkraut supplementation did not result in increased α-diversity, contrary to previous studies on fermented foods. Instead, a reduction in the variance of α-diversity values was observed, indicating a stabilizing effect on the gut microbiota. This effect was consistent across participants regardless of baseline microbiota composition, supporting the broad applicability of sauerkraut in gut health interventions. Physiologically, the intervention led to an increase in lymphocyte proportions and a decrease in vitamin B12 levels. Adverse gastrointestinal effects, such as bloating and abdominal pain, were reported during the initial week of supplementation but resolved in all participants by the eighth day. The original study implied that sauerkraut is indeed a synbiotic food, which, when administered in adequate amounts even for a short course of time, can induce a multitude of favorable alterations in the gut microbiota, independent of its baseline composition.
As in the original study, the same confounding factors (diet, sleep, and physical activity) and outcome measures (bowel movement, indigestion, laboratory parameters, and gut microbiota composition and functionality) were assessed. The sole differences in the supplementation protocol and study design between the original and follow-up study were the production year of the sauerkraut, the utilization of novel self-report instruments on dietary intake, and the addition of a washout phase to the study protocol by conducting repeated stool sampling 30 days after sauerkraut supplementation cessation.
Due to the recruitment difficulties experienced during the original study, recreational athletes were included in this follow-up study. The study was conducted on a sample of 11 male active athletes, on average 30 ± 1.56 years old, who were on average athletes with organized training (average athlete classification 2.64 ± 0.98). Although no significant impact of potential confounding factors and similar outcomes were found regarding bowel movement, indigestion, and laboratory parameters, the pilot follow-up study could not confirm the hypotheses stated by proof-of-concept study. Despite all our efforts, we found that despite the identical intervention, sauerkraut could not induce identical favorable changes.
Regarding the gut microbiota composition and functionality, sauerkraut could not be confirmed as a synbiotic fermented food since no uniform significant changes were seen consistently among all of the participants gut microbiota postintervention. Contrary to the original study, no significant changes in β-diversity metrics and no significant increase in the abundances of genera from family Lachnospiraceae and decrease of genera from the family Oscillospiraceae were observed as well as no significant changes in the abundances of metabolic pathways. The identical intervention did not result in even remotely similar results. Furthermore, opposing results were found. In the original study, the median Shannon index decreased postintervention, and in the present study, it increased, albeit in both non-significantly. Genus
Oscillibacter abundance significantly decreased in the original study and increased in the follow-up study; the same occurred for the relative abundance of genus
Lachnospiraceae UCG-001. A possible explanation for the discordance of the results could be attributed to the greater heterogeneity of gut microbiota composition before the intervention in the follow-up study when compared to the original study. In the original study, we recruited exclusively professional athletes (average classification 3.9 ± 1.22), which, due to their specific lifestyle, have a distinct gut microbiota profile with less interindividual variability [
53,
54,
55], specifically higher α-diversity and greater abundance of metabolically relevant taxa (family Lachnospiraceae) [
56,
57,
58,
59]. Therefore, we observed a greater number of alterations in the same taxonomic and functional groups of the same direction in the original study. This could explain why in the original study, the change in β-diversity was significant, and there was a greater number of significantly altered bacterial genera (eight versus three,
Bifidobacterium,
Oscillibacter, and
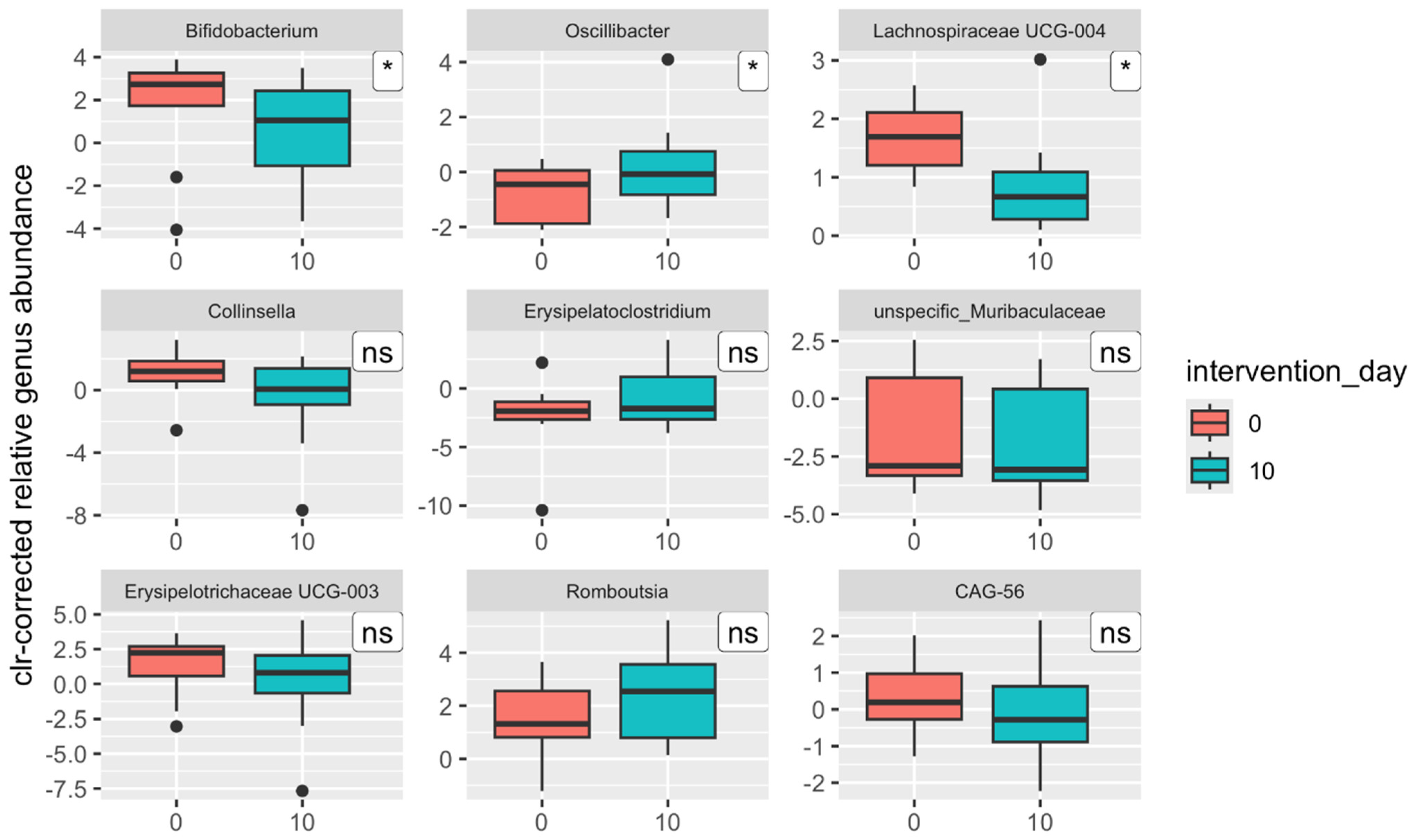
Lachnospiraceae UCG-004), metabolic pathways (35 (18.4% of all) versus 10 (5.3% of all)), and functional modules (five versus none).
These results refute one of the key findings of the original study: sauerkraut does not invoke a specific shift in the gut microbiota but rather, like probiotics and other fermented foods, impacts the gut microbiota depending on its baseline composition [
60,
61].
Curiously, neither the original nor follow-up study found any significant increase in the abundance of lactic acid bacteria (LAB), which is a common phenomenon associated with the intake of fermented food [
62]. However, given the microbial population of the sauerkraut sequenced in the first study and the number of LAB demonstrated through cultural methods, this result is not unexpected. Due to the production process, primarily pasteurization, the initial number of LAB is likely lower than in probiotic formulations or fermented products studied in other research, which might have a greater potential to colonize the digestive tract [
25,
63].
On the other hand, we found in the present study that the relative abundance of the most prominent genera of the sequenced sauerkraut’s microbial population [
31] counterintuitively declined postintervention in the host’s gut microbiota, e.g.,
Ruminococcus,
Blautia, unspecified
Lachnospiraceae, and
Subdoligranulum.
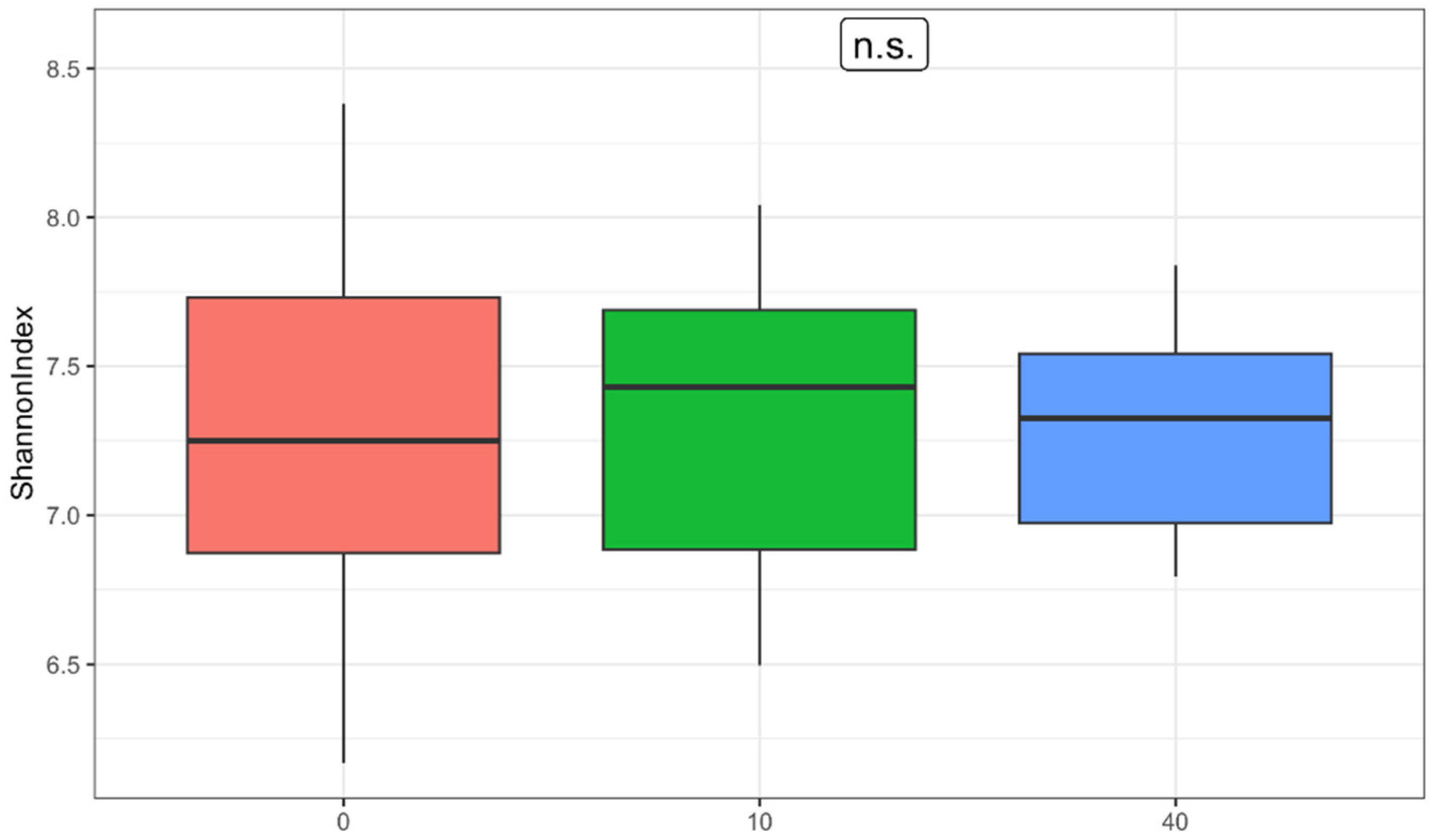
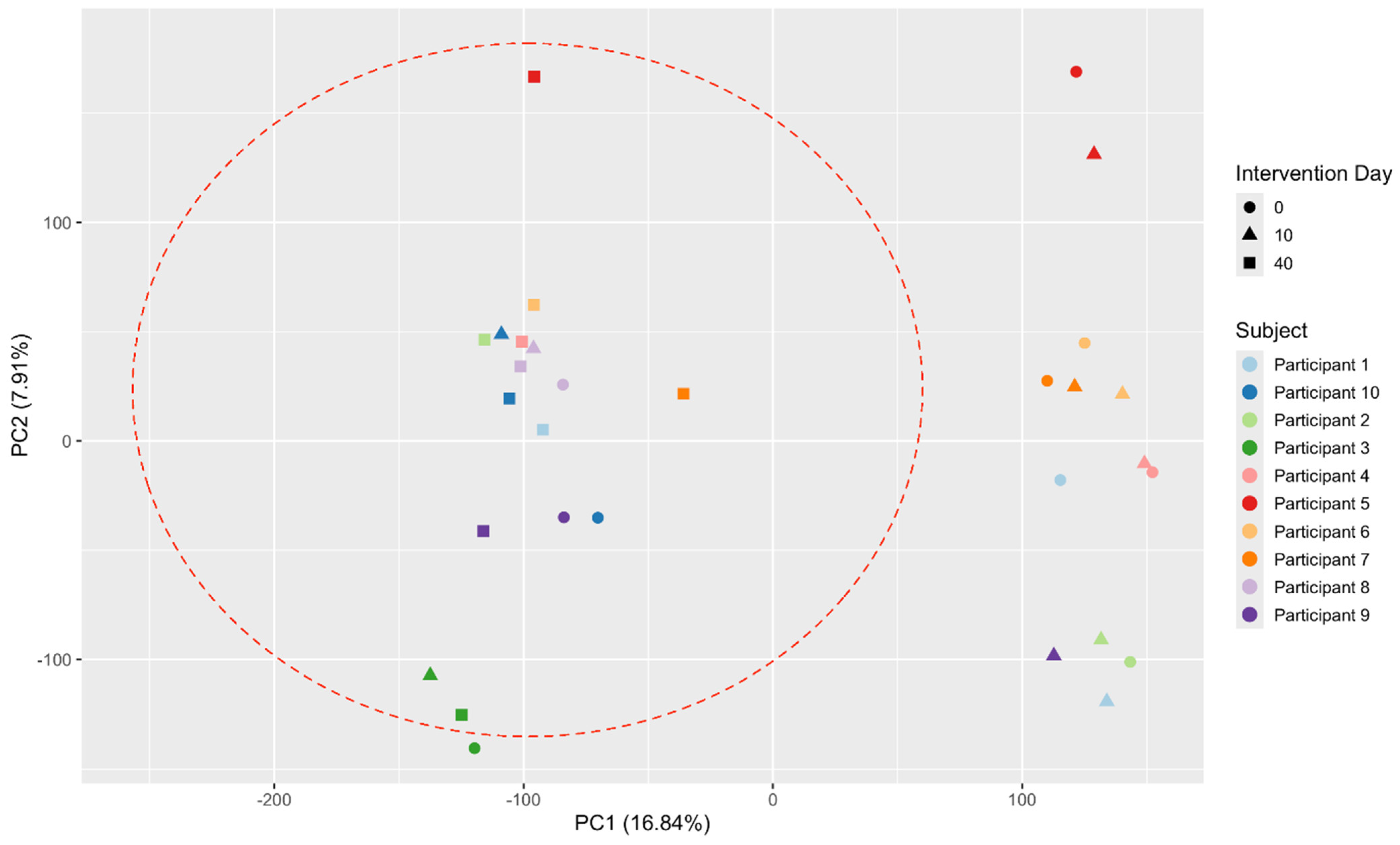
However, by including results from a third time point, after a month-long washout period after the intervention, significant changes in gut microbiota composition and functionality were observed, which were greater in magnitude than those seen immediately after the intervention. Although the increase in α-diversity observed immediately postintervention was short-lived, a significant reduction in the range of Shannon index values between samples was noted (p = 0.011), from 6.17–8.38 to 6.79–7.84. A similar trend was observed regarding β-diversity: one month postintervention, the gut microbiota composition of participants became more similar than in the pre-intervention measurement. When comparing the relative abundance of taxonomic groups across three time points rather than just pre- and postintervention, the number of significant changes increased substantially. Four phyla—Firmicutes, Actinobacteria, Lentisphaerae, and Bacteroidetes (q ≤ 0.045)—showed significant differences in contrast to only one phylum (Actinobacteria) postintervention (q = 0.243). In addition, ten bacterial genera (q ≤ 0.047) demonstrated significant changes compared with just three (q = 0.958) postintervention. The same applied to functional groups: significant differences were detected in 14 metabolic pathways (7.5% of all pathways), most of which were associated with nucleotide metabolism, without indications of false positivity (q ≤ 0.481). These findings underscore the intervention’s substantial impact following the washout period.
These results indicate that supplementation with sauerkraut in this sample of recreational athletes had certain significant long-term effects on the composition and functionality of their gut microbiota, regardless of its initial state. In the pilot follow-up study, we could not replicate the short-term synbiotic effects of sauerkraut seen in the original study; however, its long-term effects on both composition as well as functionality were notable. We can state that the supplementation of 250 g of sauerkraut over a course of 10 days can induce significant alterations in the gut microbiota that are detectable by sequencing methods even one month after cessation of sauerkraut intake. Our research suggests that the synbiotic effect of sauerkraut is not transient, lasting at least for a whole month postintervention.
Contrary to its short-term effects on the gut microbiota, the pilot follow-up study confirmed the short-term effects of sauerkraut on digestion and indigestion, as seen in the original study. The probability of normal stool (Bristol stool types 3 and 4) increased after one week, although this finding was not statistically significant, as in the original study. The highest incidence of reported adverse effects in the follow-up study occurred on days 5 and 7 of the intervention, while in the original study, it occurred on days 5 and 6. When calculated relative to all participant-days for the respective study (proof-of-concept = 100 days; pilot follow-up = 110 days), our findings indicate that sauerkraut supplementation at the specified dose of 250 g carries the highest probability of causing bloating (approximately 16%), with much lower probabilities of diarrhea (3.3%) or abdominal pain (2.3%). Our results suggest that while adverse effects such as bloating are relatively common during the early stages of sauerkraut supplementation, they diminish substantially over time, with minimal adverse effects observed on days 8 to 10.
The fact that participants in the present study also reported constipation and nausea as adverse effects, unlike in the original study, could also be attributed to the greater heterogeneity of their gut microbiota when compared to participants of the original study.
It seems that with the regular intake of sauerkraut, the digestion tract and the gut microbiota apparently adapt to its compounds, and then, sauerkraut becomes safe to use, similarly to probiotics and other fermented food. Hence, we can confirm the recommendation for a seven-day long adaptation period for sauerkraut supplementation from the original study, which is the also case for probiotics [
64,
65,
66].
Regarding laboratory parameters, a nearly significant increase in leukocyte concentration and a significant decrease in serum vitamin B12 levels concentration was observed postintervention in the present study. One month after the intervention, serum vitamin B12 levels normalized (p = 0.097), while leukocyte concentration showed an almost significant further increase (p = 0.052). The significant decrease in vitamin B12 levels is, as seen in the original study (p = 0.012), probably is a short-term effect of sauerkraut supplementation, which warrants further research.
There are several substantial limitations of this study. The main limitation of the pilot follow-up study was that it was repeated with a very small subject number. This is represented by high false-positive rates regarding the many findings of the study. Studies on greater samples with different population characteristics (age, gender, and health status) are required to yield a clearer picture of the synbiotic effect of sauerkraut.
Due to knowledge, population, and practical gaps, we cannot conclude whether the observed long-term effects of sauerkraut supplementation on the abundance of taxonomic and functional groups are favorable.
Although the complete amplicon sequencing process utilized the same sampling, sequencing, and bioinformatical methods, there is the possibility of technological bias due to non-foreseeable factors around this very delicate diagnostic method. Since the original and follow-up studies were performed at different times, there is the slight chance that minor technical issues during sampling, the handling of the samples, or sequencing caused the discrepancy between the results. One other important limitation of the pilot follow-up study is the fact that the investigated sauerkraut was pasteurized. This could also explain why no increase in the relative abundances of lactic acid bacteria was observed and why the abundance of certain genera, which are abundant in the sauerkraut microbial population, did not increase in the host’s gut microbiota after the intervention.
Additionally, one should be cautious when calling the used sauerkraut a synbiotic since it rather contained pre- and postbiotics and not probiotics.