Anti-Stress Effects of Tremella fuciformis Berk. Enzymatic Extracts: A Preclinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of T. fuciformis Enzymatic Extracts
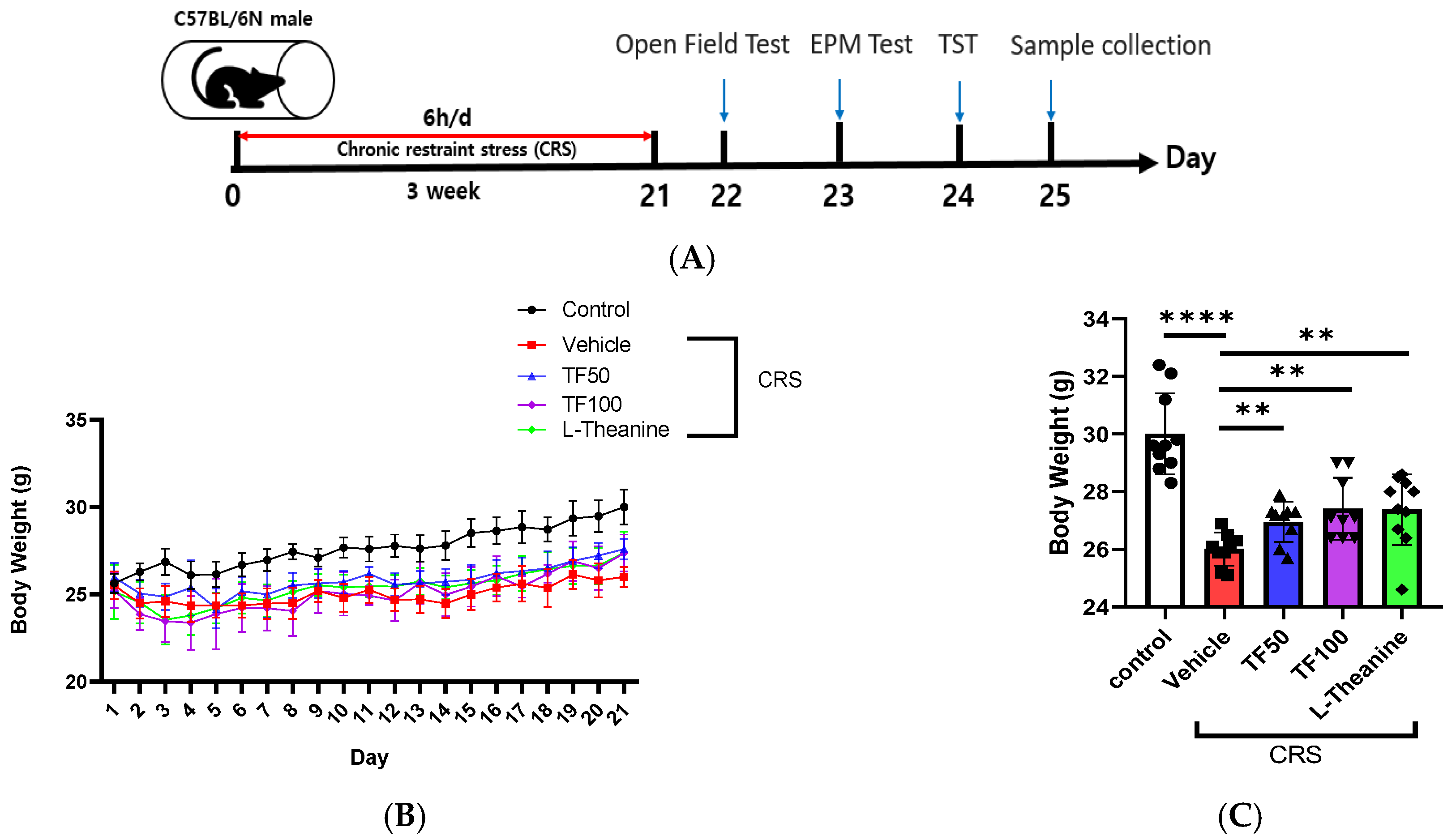
2.2. Animals and Experimental Design
2.3. Serum and Hippocampus Collection
2.4. Chronic Restraint Stress
2.5. Open Field Test
2.6. Elevated Pulse Maze Test
2.7. Tail Suspension Test
2.8. Quantitative Real-Time PCR Analysis
2.9. CORT ELISA
2.10. Hematoxylin and Eosin Staining
2.11. MTT Assay
2.12. MAO Assay
2.13. Statistical Analysis
3. Results
3.1. Effect of TF on Body Weight in Chronically Stressed Mice
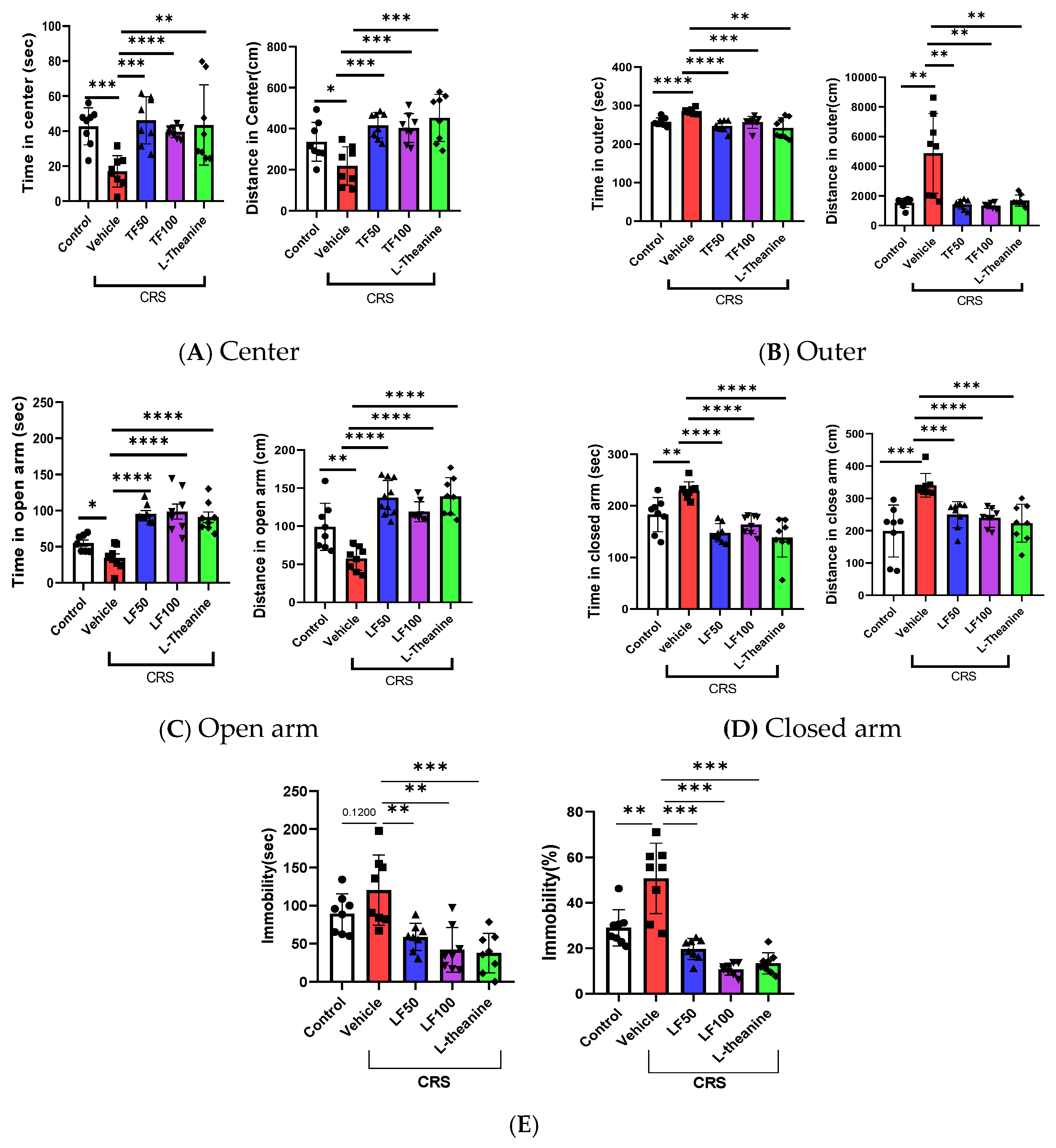
3.2. TF Reduces Anxiety and Depression-like Behaviors in Mice
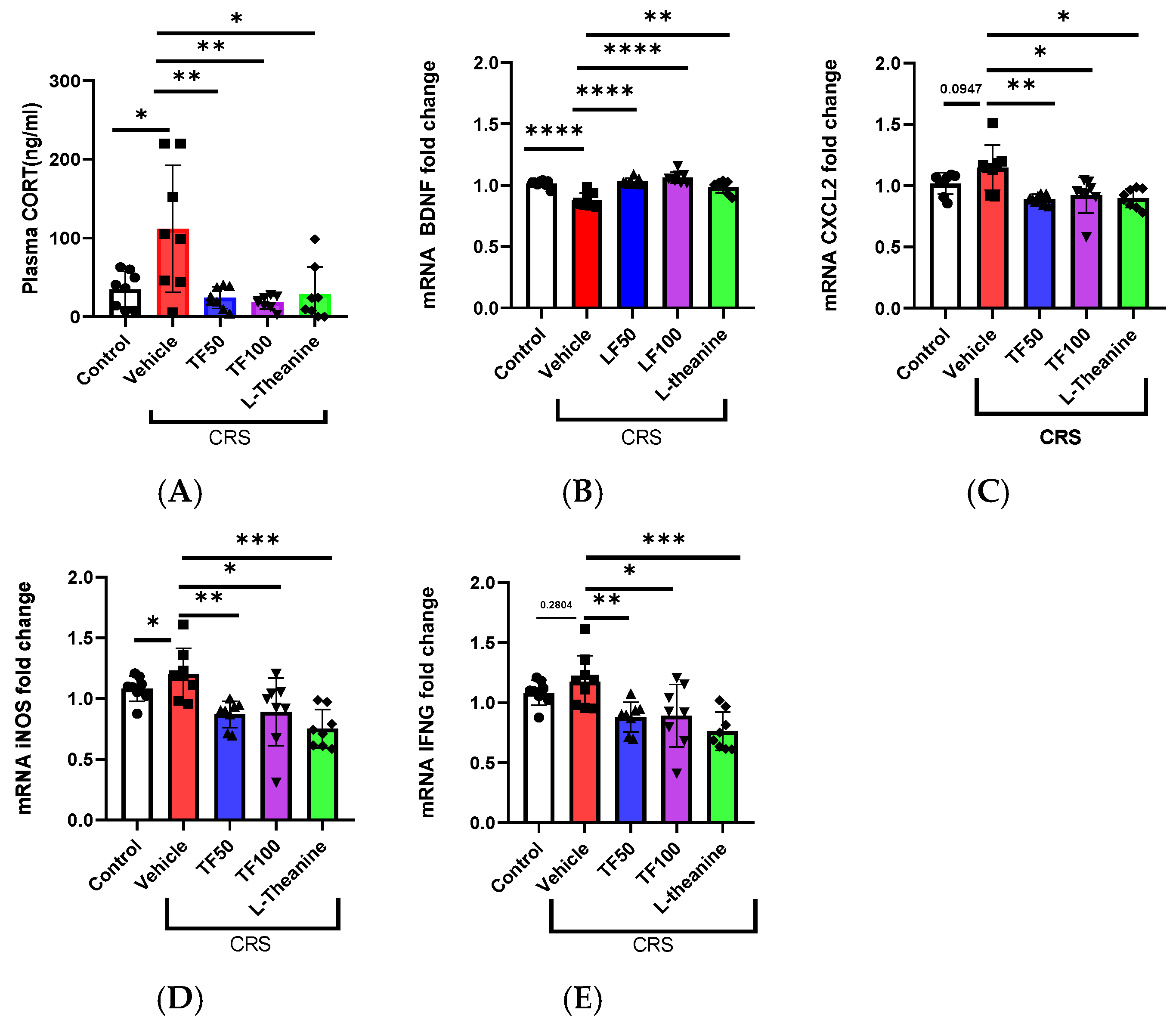
3.3. TF Prevents CRS-Induced Neurochemical and Inflammatory Dysregulation
3.4. Histopathological Analysis of Hippocampal Regions Following TF Treatment
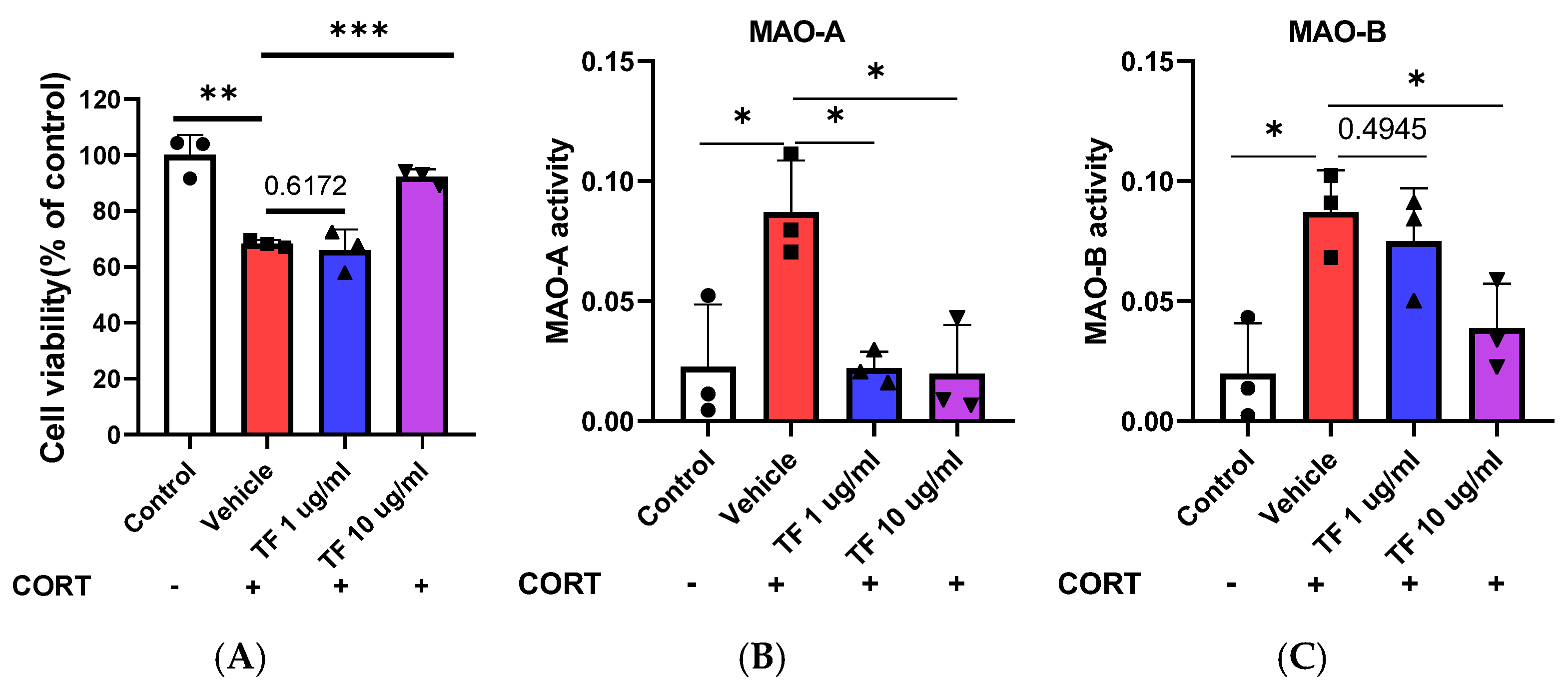
3.5. Neuroprotective Effects of TF on CORT-Induced Stress in Neuronal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- Eiland, L.; McEwen, B.S. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 2012, 22, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxid. Med. Cell Longev. 2019, 2019, 1270397. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- He, G.; Chen, T.; Huang, L.; Zhang, Y.; Feng, Y.; Qu, S.; Yin, X.; Liang, L.; Yan, J.; Liu, W. Tremella fuciformis polysaccharide reduces obesity in high-fat diet-fed mice by modulation of gut microbiota. Front. Microbiol. 2022, 13, 1073350. [Google Scholar] [CrossRef]
- Kong, W.; Liu, X.; Zhu, H.; Zheng, S.; Yin, G.; Yu, P.; Shan, Y.; Ma, S.; Ying, R.; Jin, H. Tremella fuciformis polysaccharides induce ferroptosis in Epstein-Barr virus-associated gastric cancer by inactivating NRF2/HO-1 signaling. Aging 2024, 16, 1767–1780. [Google Scholar] [CrossRef]
- Mineroff, J.; Jagdeo, J. The potential cutaneous benefits of Tremella fuciformis. Arch. Dermatol. Res. 2023, 315, 1883–1886. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.J.; Lee, H.J.; Kim, M.J.; Kim, J.H.; Kim, Y.T.; Song, K.M.; Kim, Y.J.; Kim, H.K.; Jung, S.K. Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-kappaB and MAPK pathways. Food Funct. 2016, 7, 3263–3272. [Google Scholar] [CrossRef]
- Khan, T.J.; Xu, X.; Xie, X.; Dai, X.; Sun, P.; Xie, Q.; Zhou, X. Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Curr. Issues Mol. Biol. 2022, 44, 1224–1234. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Ahn, Y.H.; Kim, K.S.; Park, K.J.; Choi, W.K.; Ha, H.C.; Kang, J.I.; Kim, T.S.; Yeo, I.H.; et al. Tremella fuciformis enhances the neurite outgrowth of PC12 cells and restores trimethyltin-induced impairment of memory in rats via activation of CREB transcription and cholinergic systems. Behav. Brain Res. 2012, 229, 82–90. [Google Scholar] [CrossRef]
- Lazur, J.; Hnatyk, K.; Kala, K.; Sulkowska-Ziaja, K.; Muszynska, B. Discovering the Potential Mechanisms of Medicinal Mushrooms Antidepressant Activity: A Review. Antioxidants 2023, 12, 623. [Google Scholar] [CrossRef]
- Deng, W.; Wu, L.; Xiao, Z.; Li, Y.; Zheng, Z.; Chen, S. Structural Characterization and Anti-Inflammatory Activity of Polysaccharides from Tremella fuciformis on Monosodium Urate-Stimulated RAW264.7 Macrophages. Foods 2023, 12, 4398. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Lee, S.L.; Jeong, H.S.; Lim, S.M.; Park, S.; Hong, Y.S.; Kim, J.E. Efficacy and Safety of Tremella fuciformis in Individuals with Subjective Cognitive Impairment: A Randomized Controlled Trial. J. Med. Food 2018, 21, 400–407. [Google Scholar] [CrossRef]
- Kawasaki, A.; Suzuki, N.; Endo, K.; Ashida, N. Novel Effects of Uridine on Behavioral Changes Due to Social Isolation Stress in Mice. Food Sci. Technol. Res. 2013, 19, 455–461. [Google Scholar] [CrossRef][Green Version]
- Holguin, S.; Martinez, J.; Chow, C.; Wurtman, R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008, 22, 3938–3946. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Ulus, I.H. Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J. Nutr. Health Aging 2009, 13, 189–197. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Guo, Y.; Wu, N.; Cao, B.; Zhan, B.; Zhou, T.; Li, Y.; Zhu, F.; Chen, W.; et al. D-mannose is a rapid inducer of ACSS2 to trigger rapid and long-lasting antidepressant responses through augmenting BDNF and TPH2 levels. Transl. Psychiatry 2023, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Loffler, M.; Carrey, E.A.; Zameitat, E. New perspectives on the roles of pyrimidines in the central nervous system. Nucleosides Nucleotides Nucleic Acids 2018, 37, 290–306. [Google Scholar] [CrossRef]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 2021, 15, 46–59. [Google Scholar]
- Huang, L.; Xiao, D.; Sun, H.; Qu, Y.; Su, X. Behavioral tests for evaluating the characteristics of brain diseases in rodent models: Optimal choices for improved outcomes (Review). Mol. Med. Rep. 2022, 25, 183. [Google Scholar] [CrossRef]
- Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- Horka, P.; Langova, V.; Hubeny, J.; Vales, K.; Chrtkova, I.; Horacek, J. Open field test for the assessment of anxiety-like behavior in Gnathonemus petersii fish. Front. Behav. Neurosci. 2023, 17, 1280608. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019, 1916, 69–74. [Google Scholar] [CrossRef]
- Perals, D.; Griffin, A.S.; Bartomeus, I.; Sol, D. Revisiting the open-field test: What does it really tell us about animal personality? Anim. Behav. 2017, 123, 69–79. [Google Scholar] [CrossRef]
- Luo, D.H.; Liu, X.F.; Guan, J.J.; Jiang, G.L.; Hua, Y.L.; Zhang, X.F.; Xu, X.F. Effects of Polysaccharides with Different Molecular Weight on D-Galactose-Induced Aging of Mice. Pol. J. Food Nutr. Sci. 2023, 73, 163–174. [Google Scholar] [CrossRef]
- Xie, L.; Yang, K.; Liang, Y.; Zhu, Z.; Yuan, Z.; Du, Z. Tremella fuciformis polysaccharides alleviate induced atopic dermatitis in mice by regulating immune response and gut microbiota. Front. Pharmacol. 2022, 13, 944801. [Google Scholar] [CrossRef]
- Khosravi-Nezhad, S.; Hassanpour, S.; Hesaraki, S. L-Theanine Improves Locomotor Function in a Model of Multiple Sclerosis Mice. Arch. Razi Inst. 2023, 78, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, L.; Gou, L.; Ling, X.; Feng, Y.; Wang, L.; Yin, X.; Liu, Y. Protective effect of l-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain Res. 2013, 1503, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bhagya, V.; Christofer, T.; Shankaranarayana Rao, B.S. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian. J. Pharmacol. 2016, 48, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Khames, A.; Mohammad, M.K.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Gad, A.M. Meloxicam Targets COX-2/NOX1/NOX4/Nrf2 Axis to Ameliorate the Depression-like Neuropathology Induced by Chronic Restraint Stress in Rats. Pharmaceuticals 2023, 16, 848. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Sakamoto, T.; Ulus, I. Nutritional modifiers of aging brain function: Use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr. Rev. 2010, 68 (Suppl. S2), S88–S101. [Google Scholar] [CrossRef]
- Wang, J.; Jalali Motlagh, N.; Wang, C.; Wojtkiewicz, G.R.; Schmidt, S.; Chau, C.; Narsimhan, R.; Kullenberg, E.G.; Zhu, C.; Linnoila, J.; et al. d-mannose suppresses oxidative response and blocks phagocytosis in experimental neuroinflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2107663118. [Google Scholar] [CrossRef]
- Zain, M.A.; Pandy, V.; Majeed, A.B.A.; Wong, W.F.; Mohamed, Z. Chronic restraint stress impairs sociability but not social recognition and spatial memoryin C57BL/6J mice. Exp. Anim. 2019, 68, 113–124. [Google Scholar] [CrossRef]
- Hazim, A.I.; Ramanathan, S.; Parthasarathy, S.; Muzaimi, M.; Mansor, S.M. Anxiolytic-like effects of mitragynine in the open-field and elevated plus-maze tests in rats. J. Physiol. Sci. 2014, 64, 161–169. [Google Scholar] [CrossRef]
- Kinlein, S.A.; Phillips, D.J.; Keller, C.R.; Karatsoreos, I.N. Role of CORT in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology 2019, 102, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, J.; Kim, M.; Lim, D.W.; Jung, J.; Kim, M.J.; Song, J.; Cho, S.; Um, M.Y. Sargassum horneri Extract Attenuates Depressive-like Behaviors in Mice Treated with Stress Hormone. Antioxidants 2023, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metab. 2013, 28, 288–296. [Google Scholar] [CrossRef]
- Powell, T.R.; Fernandes, C.; Schalkwyk, L.C. Depression-Related Behavioral Tests. Curr. Protoc. Mouse Biol. 2012, 2, 119–127. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, D.N.; Yanaka, H.T.; Kosaka, H.; Okazawa, H. Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: A functional MRI study. Hippocampus 2014, 24, 214–224. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, S.; Sim, J.; Yang, S.; An, H.; Nam, M.H.; Jang, D.P.; Lee, C.J. Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis. Exp. Mol. Med. 2021, 53, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M. Inhibitors of MAO-B and COMT: Their effects on brain dopamine levels and uses in Parkinson’s disease. J. Neural Transm. 2019, 126, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Labonte, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.E.; Cahill, M.; et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017, 23, 1102–1111. [Google Scholar] [CrossRef]
- Ding, J.; Ji, J.; Rabow, Z.; Shen, T.; Folz, J.; Brydges, C.R.; Fan, S.; Lu, X.; Mehta, S.; Showalter, M.R.; et al. A metabolome atlas of the aging mouse brain. Nat. Commun. 2021, 12, 6021. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, G.; Rustamov, N.; Park, J.; Park, H.; Park, K.; Choi, E.H.; Roh, Y.-S. Anti-Stress Effects of Tremella fuciformis Berk. Enzymatic Extracts: A Preclinical Study. Nutrients 2025, 17, 914. https://doi.org/10.3390/nu17050914
Moon G, Rustamov N, Park J, Park H, Park K, Choi EH, Roh Y-S. Anti-Stress Effects of Tremella fuciformis Berk. Enzymatic Extracts: A Preclinical Study. Nutrients. 2025; 17(5):914. https://doi.org/10.3390/nu17050914
Chicago/Turabian StyleMoon, Gahye, Nodir Rustamov, Junhang Park, Hanseul Park, Kumju Park, Eun Hye Choi, and Yoon-Seok Roh. 2025. "Anti-Stress Effects of Tremella fuciformis Berk. Enzymatic Extracts: A Preclinical Study" Nutrients 17, no. 5: 914. https://doi.org/10.3390/nu17050914
APA StyleMoon, G., Rustamov, N., Park, J., Park, H., Park, K., Choi, E. H., & Roh, Y.-S. (2025). Anti-Stress Effects of Tremella fuciformis Berk. Enzymatic Extracts: A Preclinical Study. Nutrients, 17(5), 914. https://doi.org/10.3390/nu17050914




