Exploring Antioxidant Properties of Standardized Extracts from Medicinal Plants Approved by the Thai FDA for Dietary Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Standardized Extracts and Chemicals
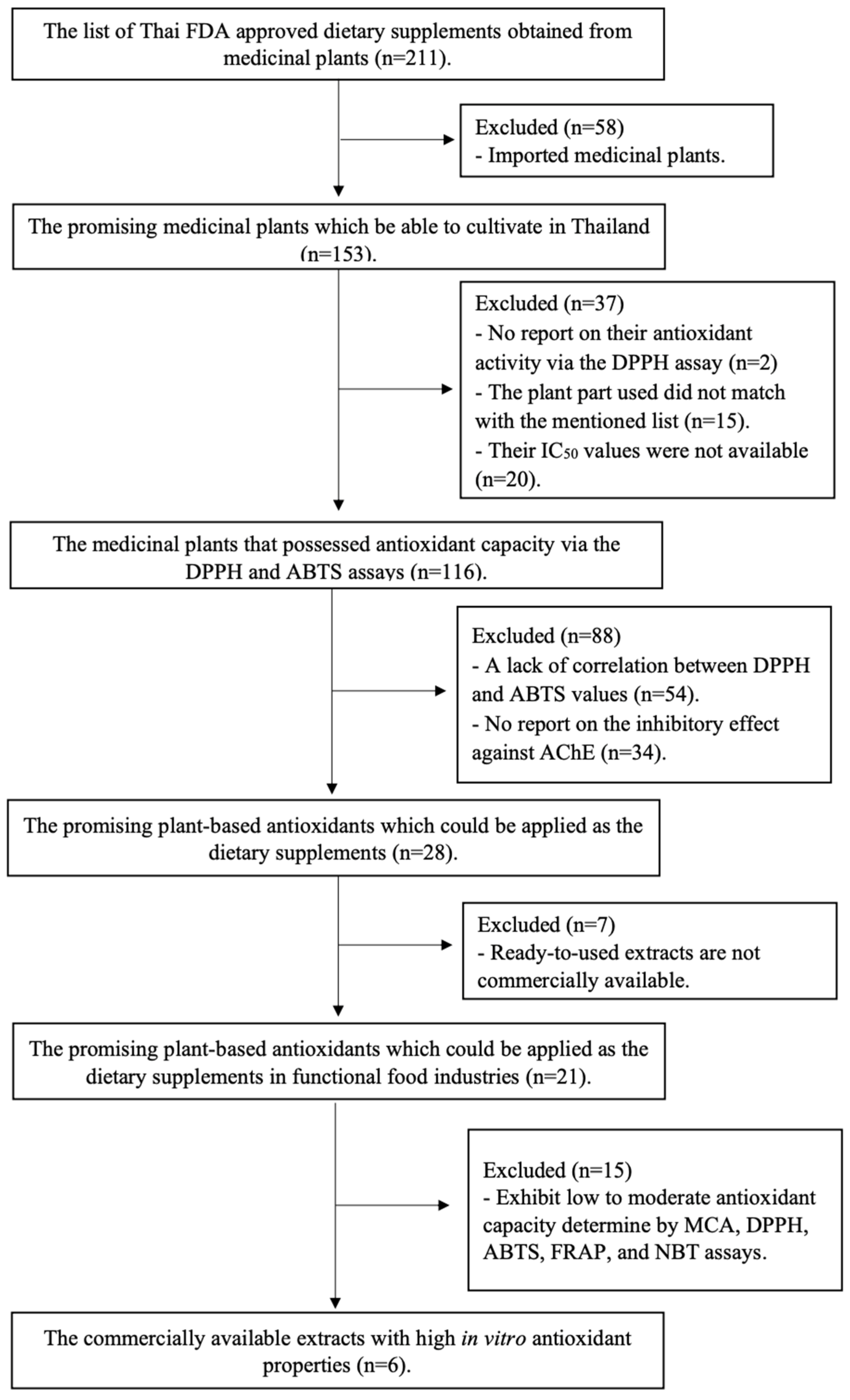
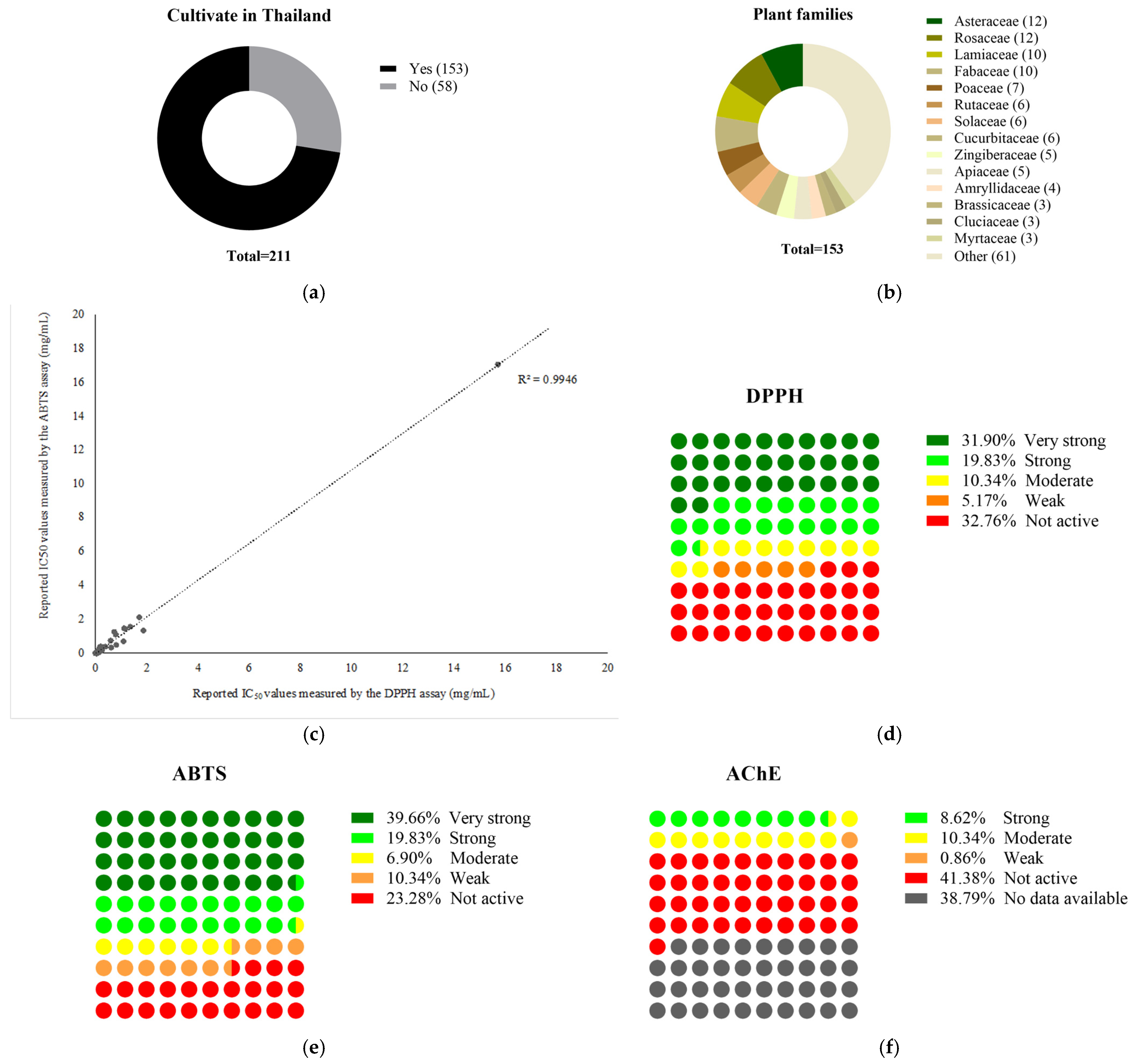
2.2. A Systematic Review-Based Approach for Identifying Potent, Reliable, and Highly Feasible Medicinal Plant Extracts, as Described in the Thai FDA Approval List for Dietary Supplementation
2.3. Measurement of Total Phenolics and Flavonoids Content of Promising Standardized Extracts
2.4. Metal-Chelating Activity
2.5. DPPH and ABTS Free Radical Scavenging Activity
2.6. Ferric-Reducing Antioxidant Power
2.7. Superoxide Anion Scavenging Method
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, A.S.; Ferreira-Pêgo, C.; Costa, J.G. Functional Foods for Health: The antioxidant and anti-inflammatory role of fruits, vegetables and culinary herbs. Foods 2023, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Palthur, M.P.; Palthur, S.S.; Chitta, S.K. Nutraceuticals: Concept and regulatory scenario. Int. J. Pharm. Pharm. Sci. 2010, 2, 14–20. [Google Scholar]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse effects of nutraceuticals and dietary supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, C.; Zang, E.; Shi, R.; Liu, Q.; Zhang, M.; Zhang, K.; Li, M. Review on herbal tea as a functional food: Classification, active compounds, biological activity, and industrial status. J. Future Foods 2023, 3, 206–219. [Google Scholar] [CrossRef]
- Gardiner, P.; Whelan, J.; White, L.F.; Filippelli, A.C.; Bharmal, N.; Kaptchuk, T.J. A systematic review of the prevalence of herb usage among racial/ethnic minorities in the United States. J. Immigr. Minor. Health 2013, 15, 817–828. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of In Vitro and In Vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Pedret, A.; Valls, R.M.; Motilva, M.J.; Rubió, L.; Solà, R. Exploring the effects of phenolic compounds to reduce intestinal damage and improve the intestinal barrier integrity: A systematic review of in vivo animal studies. Clin. Nutr. 2021, 40, 1719–1732. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Harty, P.S.; Erickson, J.L.; Tinsley, G.M.; Garner, D.; Galpin, A.J. Prevalence of adulteration in dietary supplements and recommendations for safe supplement practices in sport. Front. Sports Act. Living 2023, 5, 1239121. [Google Scholar] [CrossRef] [PubMed]
- Ćwieląg-Drabek, M.; Piekut, A.; Szymala, I.; Oleksiuk, K.; Razzaghi, M.; Osmala, W.; Jabłońska, K.; Dziubanek, G. Health risks from consumption of medicinal plant dietary supplements. Food Sci. Nutr. 2020, 8, 3535–3544. [Google Scholar] [CrossRef]
- Starr, R.R. Too little, too late: Ineffective regulation of dietary supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef]
- Food and Drug Administration, Thailand. List Of Plants That Can Be Used in Dietary Supplements. Available online: https://fdakoratcom/wp-content/uploads/2019/06/food_supplementpdf (accessed on 30 August 2021).
- Kalin, P. Determinants of purchase intention of functional food in Thailand: A study on young adults. NIDA Develop. J. 2023, 63, 61–82. [Google Scholar]
- Sumngern, C.; Azeredo, Z.; Subgranon, R.; Matos, E.; Kijjoa, A. The perception of the benefits of herbal medicine consumption among the Thai elderly. J. Nutr. Health Aging 2011, 15, 59–63. [Google Scholar] [CrossRef]
- Chanthasri, W.; Puangkeaw, N.; Kunworarath, N.; Jaisamut, P.; Limsuwan, S.; Maneenoon, K.; Choochana, P.; Chusri, S. Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complement. Altern. Med. 2018, 18, 73. [Google Scholar] [CrossRef]
- Ruangchuay, S.; Wang, Q.Q.; Wang, L.Y.; Lin, J.; Wang, Y.C.; Zhong, G.H.; Maneenoon, K.; Huang, Z.B.; Chusri, S. Antioxidant and antiaging effect of traditional Thai rejuvenation medicines in Caenorhabditis elegans. J. Integr. Med. 2021, 19, 362–373. [Google Scholar] [CrossRef]
- Wetchakul, P.; Goon, J.A.; Adekoya, A.E.; Olatunji, O.J.; Ruangchuay, S.; Jaisamut, P.; Issuriya, A.; Kunworarath, N.; Limsuwan, S.; Chusri, S. Traditional tonifying polyherbal infusion, Jatu-Phala-Tiga, exerts antioxidant activities and extends lifespan of Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 209. [Google Scholar] [CrossRef]
- Wangsawat, N.; Nahar, L.; Sarker, S.D.; Phosri, C.; Evans, A.R.; Whalley, A.J.S.; Choowongkomon, K.; Suwannasai, N. Antioxidant activity and cytotoxicity against cancer cell lines of the extracts from novel xylaria species associated with termite nests and lc-ms analysis. Antioxidants 2021, 10, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, H.; Abas, F.; Permana, D.; Lajis, N.; Ali, A.; Sukari, M.; Hin, T.; Kikuzaki, H.; Nakatani, N. DPPH free radical scavenger components from the fruits of Alpinia rafflesiana Wall. ex. Bak. (Zingiberaceae). Z. Naturforschung C 2004, 59, 811–815. [Google Scholar] [CrossRef]
- Pase, M.P.; Kean, J.; Sarris, J.; Neale, C.; Scholey, A.B.; Stough, C. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J. Altern. Complement. Med. 2012, 18, 647–652. [Google Scholar] [CrossRef]
- Kongkeaw, C.; Dilokthornsakul, P.; Thanarangsarit, P.; Limpeanchob, N.; Norman Scholfield, C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J. Ethnopharmacol. 2014, 151, 528–535. [Google Scholar] [CrossRef]
- Abdul Manap, A.S.; Vijayabalan, S.; Madhavan, P.; Chia, Y.Y.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Bacopa monnieri, a neuroprotective lead in Alzheimer disease: A review on its properties, mechanisms of action, and preclinical and clinical studies. Drug Target Insights 2019, 13, 1177392819866412. [Google Scholar] [CrossRef]
- Goyal, A.; Gopika, S.; Kumar, A.; Garabadu, D. A comprehensive review on preclinical evidence-based neuroprotective potential of Bacopa monnieri against Parkinson’s Disease. Curr. Drug Targets 2022, 23, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, M.; Busceti, C.L.; Lazzeri, G.; Biagioni, F.; Puglisi-Allegra, S.; Frati, A.; Lenzi, P.; Fornai, F. Bacopa protects against neurotoxicity induced by MPP(+) and methamphetamine. Molecules 2022, 27, 5204. [Google Scholar] [CrossRef]
- Saraf, M.K.; Prabhakar, S.; Khanduja, K.L.; Anand, A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid. Based Complement. Altern. Med. 2011, 2011, 236186. [Google Scholar] [CrossRef]
- Vohora, D.; Pal, S.N.; Pillai, K.K. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J. Ethnopharmacol. 2000, 71, 383–390. [Google Scholar] [CrossRef]
- Basheer, A.; Agarwal, A.; Mishra, B.; Gupta, A.; Padma Srivastava, M.V.; Kirubakaran, R.; Vishnu, V. Use of Bacopa monnieri in the treatment of dementia due to Alzheimer disease: Systematic review of randomized controlled trials. Interact. J Med. Res. 2022, 11, e38542. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- da Costa, I.M.; de Moura Freire, M.A.; de Paiva Cavalcanti, J.R.; de Araújo, D.P.; Norrara, B.; Moreira Rosa, I.M.M.; de Azevedo, E.P.; do Rego, A.C.M.; Guzen, F.P. Supplementation with Curcuma longa reverses neurotoxic and behavioral damage in models of Alzheimer’s disease: A systematic review. Curr. Neuropharmacol. 2019, 17, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective effects of curcumin in neurodegenerative diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective activities of curcumin in Parkinson’s disease: A review of the literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Rogelj, B.; Župunski, V. Therapeutic potential of polyphenols in amyotrophic lateral sclerosis and frontotemporal dementia. Antioxidants 2021, 10, 1328. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The mechanisms of action of curcumin in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The clinical use of curcumin on neurological disorders: An updated systematic review of clinical trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef]
| No. | Botanical Name | Bioactive Markers/Analysis Items (%; w/w) | ||
|---|---|---|---|---|
| Compounds | Required Amount | Reported Amount | ||
| 1. | Allium sativum | Allicin | >1.00 | 1.20 |
| 2. | Aloe vera | Aloin content | NA | ≤0.1 ppm |
| 3. | Bacopa monnieri | Bacosides | ≥20.00 | 21.24 |
| 4. | Camellia sinensis | Polyphenols | >10.00 | 10.40 |
| 5. | Capsicum annuum | Capsaicin | ≥10.00 | 12.40 |
| 6. | Carthamus tinctorius | Flavonoids | >300.00 | 375.10 |
| 7. | Centella asiatica | Asiaticoside | >2.50 | 2.82 |
| Triterpene | >1.00 | 1.74 | ||
| 8. | Citrus aurantium | Hesperidin | >99.00 | 99.91 |
| 9. | Coffea arabica | Chlorogenic acid | ≥50.00 | 50.25 |
| 10. | Curcuma longa | Curcumin | >5.00 | 7.40 |
| 11. | Daucus carota | Beta-carotene | ≥10.00 | 13.67 |
| 12. | Ganoderma lucidum | Polysaccharides | >50.00 | 72.80 |
| Triterpenoids | >1.00 | 1.50 | ||
| 13. | Garcinia mangostana | Xanthone | >1.00 | 1.30 |
| 14. | Gynostemma pentaphyllum Saponins | ≥1000.00 | 1392.00 | |
| 15. | Kaempferia parviflora | Flavonoids | >1.50 | 1.51 |
| 16. | Matricaria chamomilla | Apigenin | >1.00 | 1.26 |
| 17. | Moringa oleifera | Flavonoids | >500.00 | 567.10 |
| 18. | Piper nigrum | Piperine | ≥10.00 | 10.20 |
| 19. | Tagetes erecta | Lutein | ≥10.00 | 10.26 |
| 20. | Terminalia chebula | Gallic acid | ≥500.00 | 603.90 |
| 21. | Zingiber officinale | Gingerols | ≥5.00 | 5.21 |
| Medicinal Plants | Part Used | Total Contents of (mg Equivalence/g of Extract) | |
|---|---|---|---|
| Phenolic | Flavonoid | ||
| Allium sativum (GCL) | Rhizome | 242.52 ± 17.14 de | 70.22 ± 11.79 d |
| Aloe vera (AVR) | Gel | 211.52 ± 15.78 defgh | 35.41 ± 2.85 gh |
| Bacopa monnieri (BCP) | Whole tree | 258.02 ± 12.80 d | 80.35 ± 3.95 c |
| Camellia sinensis (GNT) | Fruit | 387.43 ± 31.26 c | 15.79 ± 1.10 j |
| Capsicum annuum (CSC) | Leaf | 180.14 ± 10.55 fgh | 36.36 ± 2.51 fg |
| Carthamus tinctorius (SAF) | Flower | 222.37 ± 11.09 defg | 28.45 ± 6.10 ghi |
| Centella asiatica (CTL) | Leaves | 210.75 ± 21.78 defgh | 1.65 ± 0.16 k |
| Citrus aurantium (CIT) | Fruit | 165.02 ± 8.25 h | 15.16 ± 1.45 j |
| Coffea arabica (GCB) | Seeds | 1378.19 ± 85.34 a | 521.47 ± 10.16 a |
| Curcuma longa (TMR) | Rhizome | 514.13 ± 50.55 b | 185.22 ± 8.87 b |
| Daucus carota (CRR) | Root | 215.78 ± 17.45 defgh | 34.15 ± 3.95 gh |
| Ganoderma lucidum (RSH) | Fruit | 205.32 ± 16.94 defgh | 33.51 ± 0.95 gh |
| Garcinia mangostana (MGT) | Peel | 241.74 ± 16.82 de | 11.95 ± 1.11 j |
| Gynostemma pentaphyllum (JGL) | Leaves | 203.38 ± 21.19 defgh | 28.45 ± 2.19 ghi |
| Kaempferia parviflora (BGL) | Rhizome | 200.28 ± 10.65 efgh | 27.82 ± 1.64 hi |
| Matricaria chamomilla (CMM) | Flower | 169.67 ± 13.87 gh | 26.55 ± 1.45 ghij |
| Moringa oleifera (MOR) | Leaf | 204.16 ± 14.53 defgh | 32.56 ± 1.64 gh |
| Piper nigrum (BPP) | Seed | 230.51 ± 5.07 def | 20.22 ± 1.90 ij |
| Tagetes erecta (MRG) | Flower | 173.94 ± 8.16 gh | 13.26 ± 0.55 j |
| Terminalia chebula (TML) | Fruit | 215.01 ± 5.85 defgh | 43.96 ± 0.95 f |
| Zingiber officinale (GIN) | Rhizome | 218.49 ± 17.14 defgh | 51.87 ± 3.84 e |
| Medicinal Plants | Metal-Chelating Activity | Ferric-Reducing Antioxidant Power |
|---|---|---|
| (%Inhibition ± SD at conc. 1 mg/mL) | (µM Fe2SO4/mg) | |
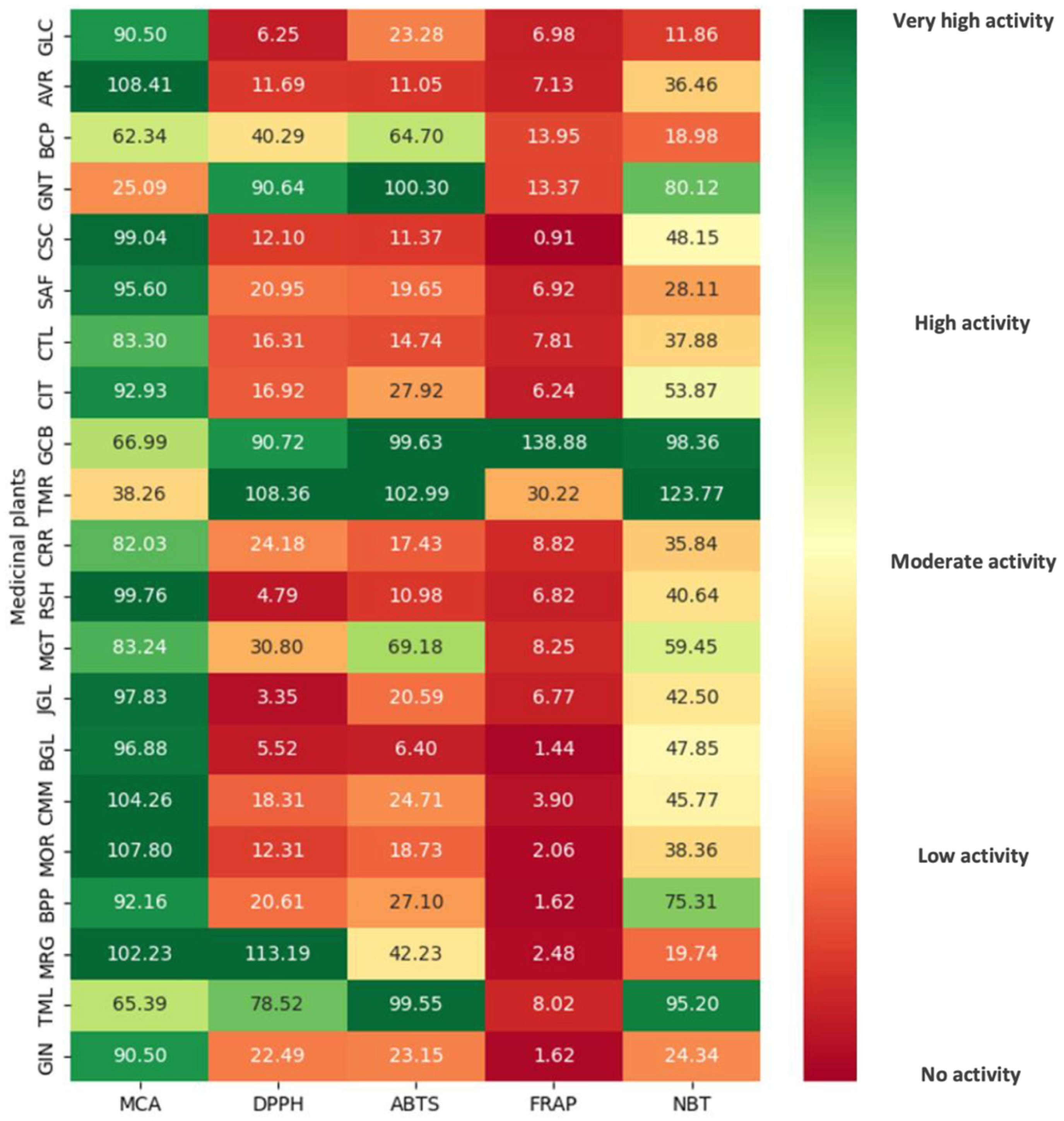
| Allium sativum (GCL) | 90.50 ± 6.98 efg | 6.98 ± 0.19 d |
| Aloe vera (AVR) | 108.41 ± 4.41 a | 7.13 ± 0.70 d |
| Bacopa monnieri (BCP) | 62.34 ± 4.90 h | 13.95 ± 0.35 c |
| Camellia sinensis (GNT) | 25.09 ± 1.28 j | 13.37 ± 0.22 c |
| Capsicum annuum (CSC) | 99.04 ± 0.96 bcde | 0.91 ± 0.02 f |
| Carthamus tinctorius (SAF) | 95.60 ± 4.60 cde | 6.92 ± 0.15 de |
| Centella asiatica (CTL) | 83.30 ± 5.71 fg | 7.81 ± 0.15 d |
| Citrus aurantium (CIT) | 92.93 ± 4.83 de | 6.24 ± 0.65 de |
| Coffea arabica (GCB) | 66.99 ± 6.23 h | 138.88 ± 7.13 a |
| Curcuma longa (TMR) | 38.26 ± 2.49 i | 30.22 ± 2.27 b |
| Daucus carota (CRR) | 82.03 ± 1.72 g | 8.82 ± 0.17 d |
| Ganoderma lucidum (RSH) | 99.76 ± 1.04 abcde | 6.82 ± 0.06 de |
| Garcinia mangostana (MGT) | 83.24 ± 7.66 fg | 8.25 ± 0.58 d |
| Gynostemma pentaphyllum (JGL) | 97.83 ± 1.63 cde | 6.77 ± 0.02 de |
| Kaempferia parviflora (BGL) | 96.88 ± 0.22 cde | 1.44 ± 0.11 f |
| Matricaria chamomilla (CMM) | 104.26 ± 9.12 abc | 3.90 ± 0.19 ef |
| Moringa oleifera (MOR) | 107.80 ± 9.80 ab | 2.06 ± 0.08 f |
| Piper nigrum (BPP) | 92.16 ± 3.01 ef | 1.62 ± 0.05 f |
| Tagetes erecta (MRG) | 102.23 ± 4.98 abcd | 2.48 ± 0.11 f |
| Terminalia chebula (TML) | 65.39 ± 4.34 h | 8.02 ± 0.17 d |
| Zingiber officinale (GIN) | 90.50 ± 2.98 efg | 1.62 ± 0.10 f |
| Medicinal Plants | Free Radical Scavenging Activities Against (%Inhibition ± SD at conc. 1 mg/mL) | ||
|---|---|---|---|
| DPPH Radicals | ABTS Radicals | Superoxide Radicals | |
| Allium sativum | 6.25 ± 0.58 l | 23.28 ± 0.67 h | 11.86 ± 0.68 n |
| Aloe vera | 11.69 ± 0.44 k | 11.05 ± 0.52 l | 32.46 ± 1.40 jk |
| Bacopa monnieri | 40.29 ± 2.05 e | 64.70 ± 0.93 d | 18.98 ± 1.34 m |
| Camellia sinensis | 90.64 ± 0.04 c | 100.30 ± 0.07 b | 80.12 ± 4.79 c |
| Capsicum annuum | 12.10 ± 0.71 k | 11.37 ± 1.06 l | 48.15 ± 2.69 g |
| Carthamus tinctorius | 19.65 ± 0.48 ij | 6.92 ± 0.15 de | 28.11 ± 1.54 l |
| Centella asiatica | 16.31 ± 0.41 j | 14.74 ± 0.62 k | 37.88 ± 2.37 jk |
| Citrus aurantium | 16.92 ± 1.14 j | 27.92 ± 0.99 f | 53.87 ± 3.74 f |
| Coffea arabica | 90.72 ± 0.34 c | 99.63 ± 0.41 b | 98.36 ± 0.96 b |
| Curcuma longa | 108.36 ± 2.35 b | 102.99 ± 0.23 a | 123.77 ± 0.72 a |
| Daucus carota | 24.18 ± 0.38 g | 17.43 ± 1.19 j | 35.84 ± 2.75 k |
| Ganoderma lucidum | 4.79 ± 0.21 l | 10.98 ± 1.06 l | 40.64 ± 1.65 ij |
| Garcinia mangostana | 30.80 ± 1.45 f | 69.18 ± 3.91 c | 59.45 ± 5.39 e |
| Gynostemma pentaphyllum | 3.35 ± 0.24 l | 20.59 ± 1.26 i | 42.50 ± 2.16 hi |
| Kaempferia parviflora | 5.52 ± 0.28 l | 6.40 ± 0.49 m | 47.85 ± 1.93 g |
| Matricaria chamomilla | 18.31 ± 1.09 ij | 24.71 ± 2.13 gh | 45.77 ± 1.66 gh |
| Moringa oleifera | 12.31 ± 0.38 k | 18.73 ± 0.91 ij | 38.36 ± 1.94 jk |
| Piper nigrum | 20.61 ± 1.09 hi | 27.10 ± 1.07 fg | 75.31 ± 0.74 d |
| Tagetes erecta | 113.19 ± 6.95 a | 42.23 ± 3.50 e | 19.74 ± 0.38 m |
| Terminalia chebula | 78.52 ± 2.15 d | 99.55 ± 0.08 b | 95.20 ± 1.05 b |
| Zingiber officinale | 22.49 ± 0.96 gh | 23.15 ± 1.73 h | 24.34 ± 1.69 l |
| Medicinal Plants | IC50; mg/mL | |||
|---|---|---|---|---|
| Metal Chelating 1 | DPPH 2 | ABTS 3 | NBT 4 | |
| Bacopa monnieri | 0.21 ± 0.01 | 0.38 ± 0.10 a | 2.82 ± 0.29 b | 0.21 ± 0.10 bc |
| Camellia sinensis | 1.23 ± 0.31 | 0.55 ± 0.03 a | 2.97 ± 0.24 b | 0.06 ± 0.02 a |
| Coffea arabica | 1.19 ± 0.19 | 0.17 ± 0.00 a | 0.12 ± 0.01 a | 0.29 ± 0.13 cd |
| Curcuma longa | 0.06 ± 0.00 | 0.42 ± 0.02 a | 0.18 ± 0.01 a | 038 ± 0.07 d |
| Tagetes erecta | 0.91 ± 0.07 | 12.40 ± 3.03 b | 8.73 ± 1.00 d | 0.06 ± 0.01 a |
| Terminalia chebula | 0.41 ± 0.01 | 0.98 ± 0.03 a | 6.43 ± 0.13 c | 0.13 ± 0.06 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limsuwan, S.; Awaeloh, N.; Na-Phatthalung, P.; Kaewmanee, T.; Chusri, S. Exploring Antioxidant Properties of Standardized Extracts from Medicinal Plants Approved by the Thai FDA for Dietary Supplementation. Nutrients 2025, 17, 898. https://doi.org/10.3390/nu17050898
Limsuwan S, Awaeloh N, Na-Phatthalung P, Kaewmanee T, Chusri S. Exploring Antioxidant Properties of Standardized Extracts from Medicinal Plants Approved by the Thai FDA for Dietary Supplementation. Nutrients. 2025; 17(5):898. https://doi.org/10.3390/nu17050898
Chicago/Turabian StyleLimsuwan, Surasak, Nurulhusna Awaeloh, Pinanong Na-Phatthalung, Thammarat Kaewmanee, and Sasitorn Chusri. 2025. "Exploring Antioxidant Properties of Standardized Extracts from Medicinal Plants Approved by the Thai FDA for Dietary Supplementation" Nutrients 17, no. 5: 898. https://doi.org/10.3390/nu17050898
APA StyleLimsuwan, S., Awaeloh, N., Na-Phatthalung, P., Kaewmanee, T., & Chusri, S. (2025). Exploring Antioxidant Properties of Standardized Extracts from Medicinal Plants Approved by the Thai FDA for Dietary Supplementation. Nutrients, 17(5), 898. https://doi.org/10.3390/nu17050898







