Phytochemical-Mediated Ah Receptor Activity Is Dependent on Dietary Context
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Mice
2.3. Diets and Feeding Studies
2.4. Cell Culture and Luciferase AHR Reporter Assays
2.5. RNA Isolation and Quantitative PCR
2.6. Isolation of Microsomes
2.7. CYP1A1 Activity Assay
2.8. Apigenin Competitive Metabolism Assay
2.9. Apigenin Quantification Using LC/MS/MS
2.10. Computational Docking Analysis
2.11. Statistical Analysis
3. Results
3.1. Plant Foods as a Source of AHR Ligands
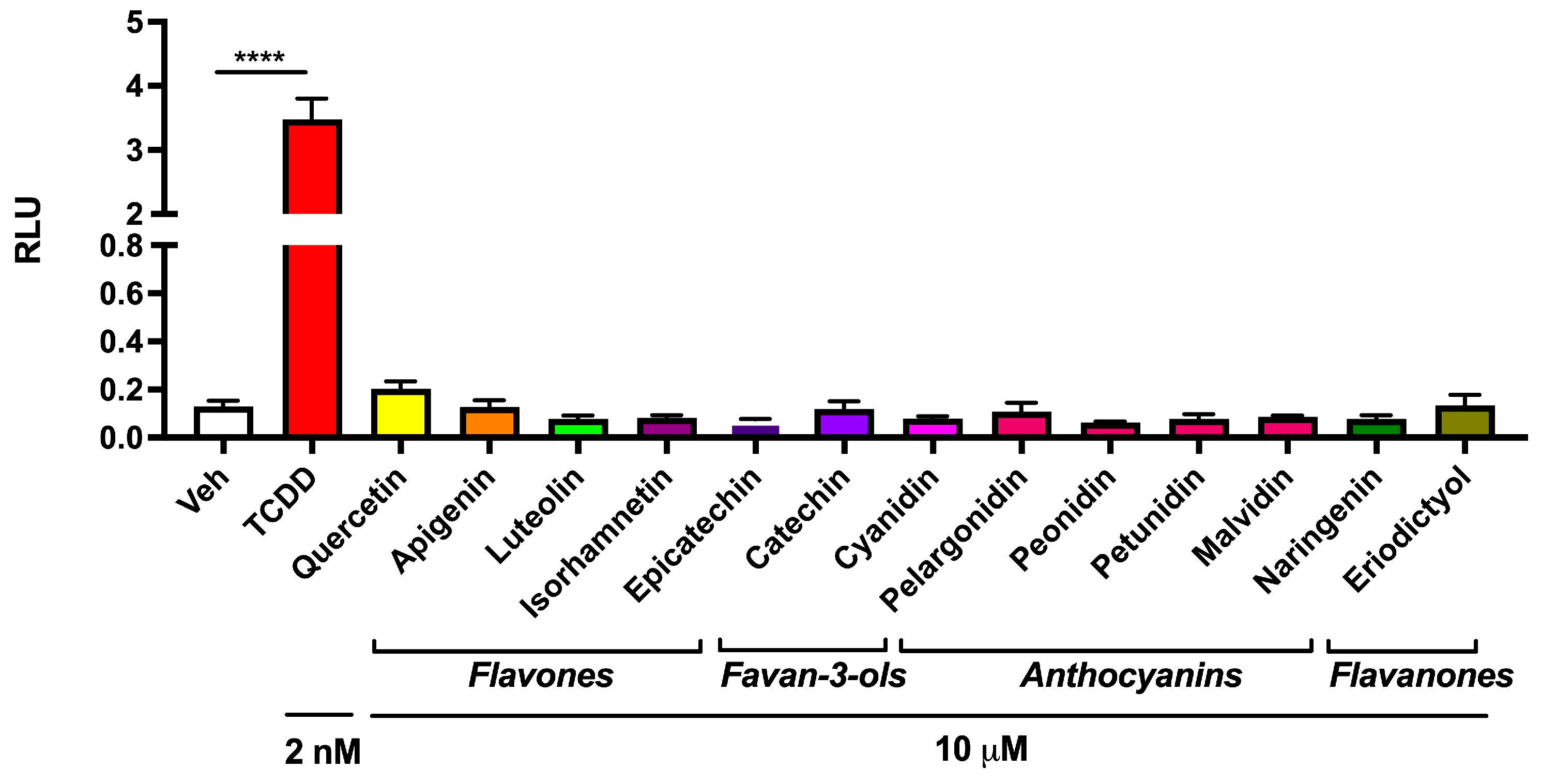
3.2. Abundant Flavonoids Do Not Exbibit AHR Agonist Activity at Relevant Concentrations
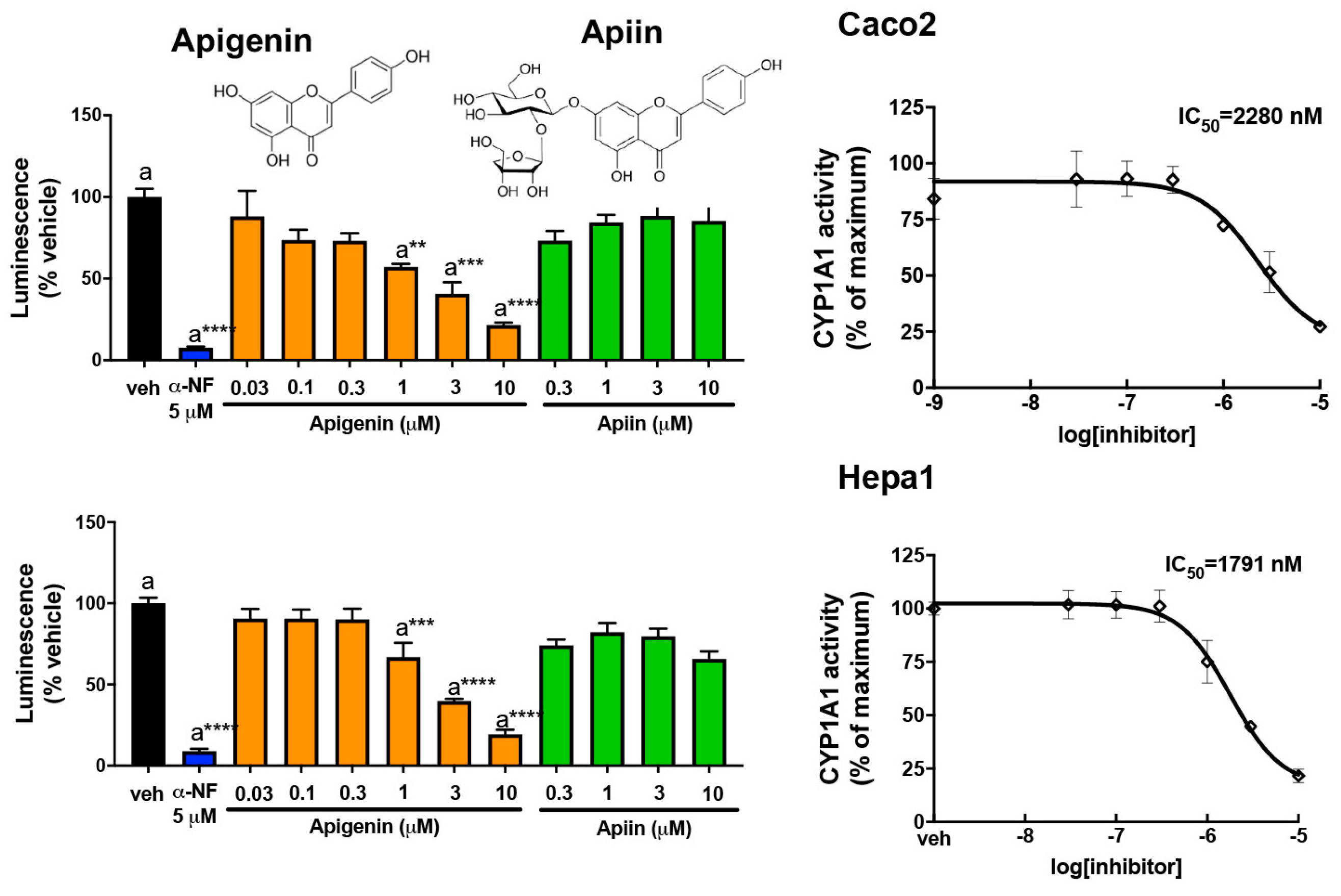
3.3. Abundant Flavonoids Are Inhibitors of or Substrates for CYP1A1
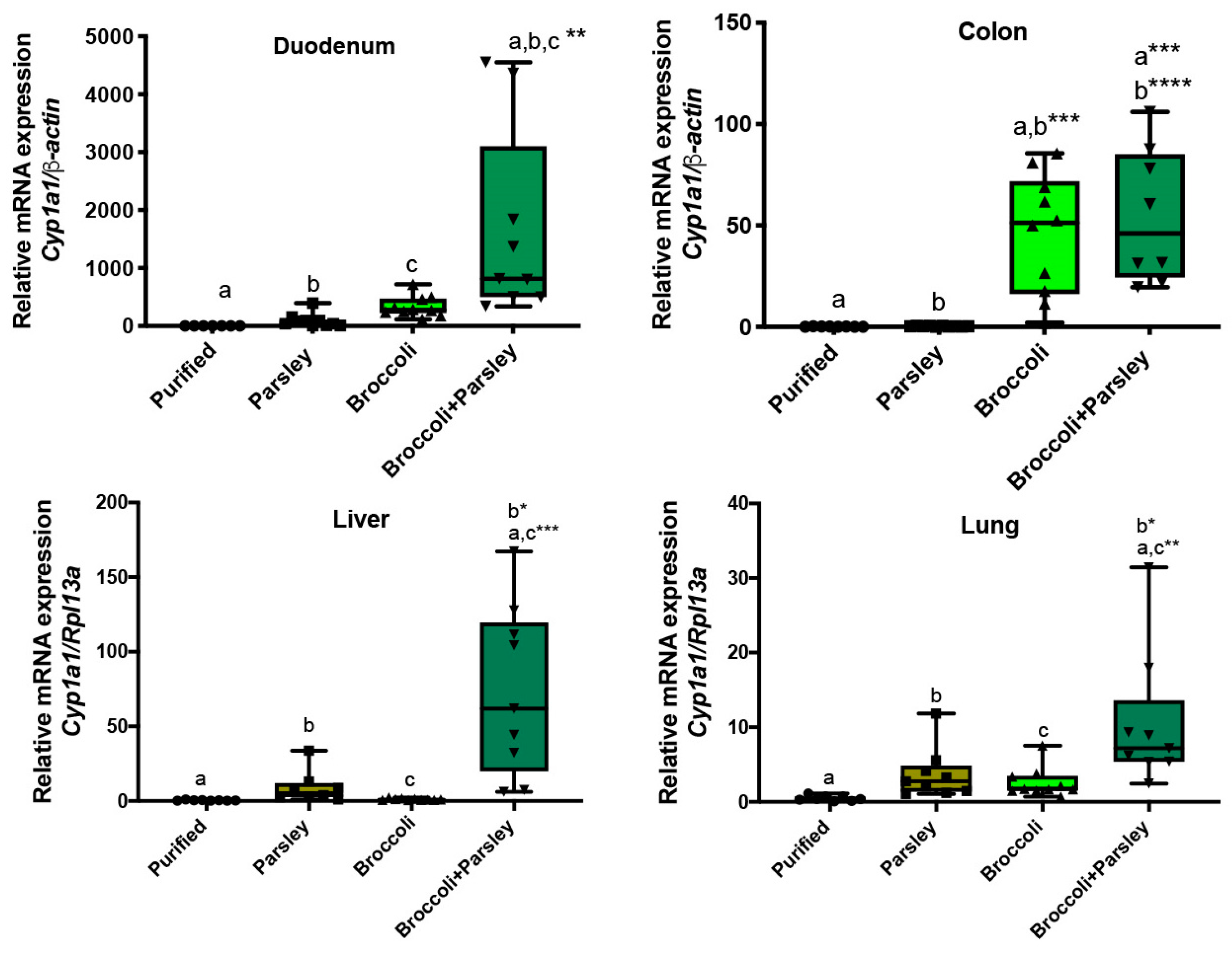
3.4. Parsley/Broccoli Diet Induces Cyp1a1 Expression
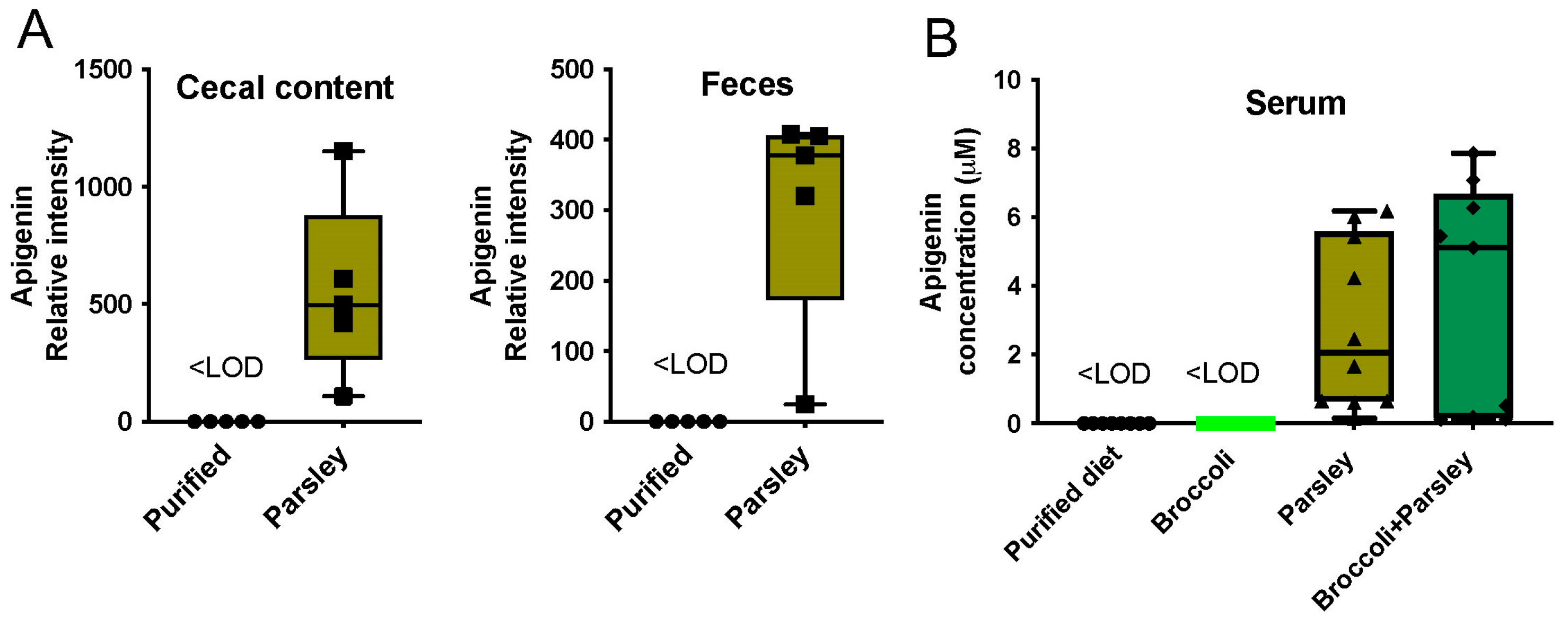
3.5. Parsley and the Generation of Apigenin in the Intestinal Tract
3.6. Apigenin Is a Substrate for or Competitive Inhibitor of CYP1A1/1B1
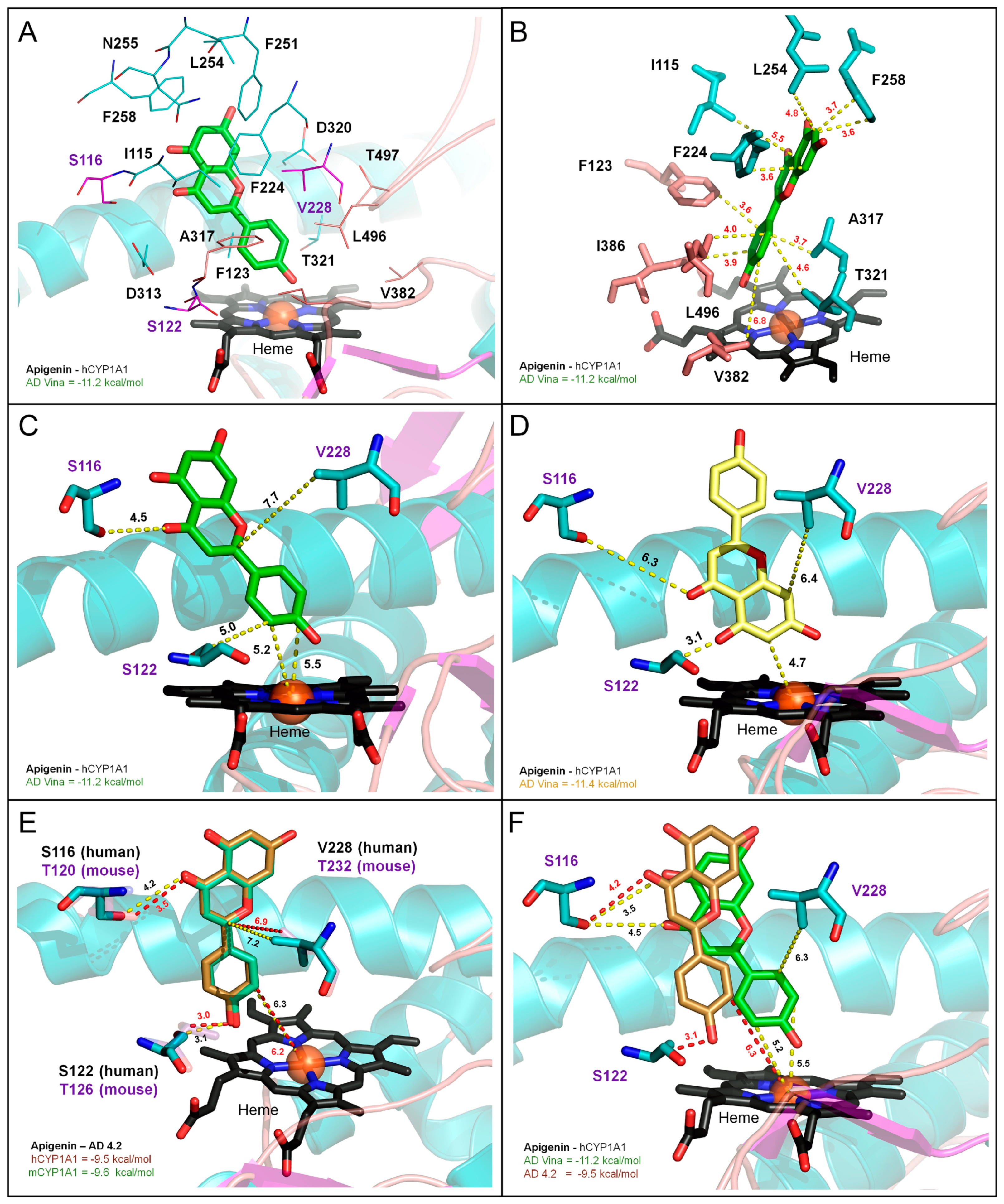
3.7. Molecular Modeling of CYP1A1/1B1 Metabolism of Apigenin
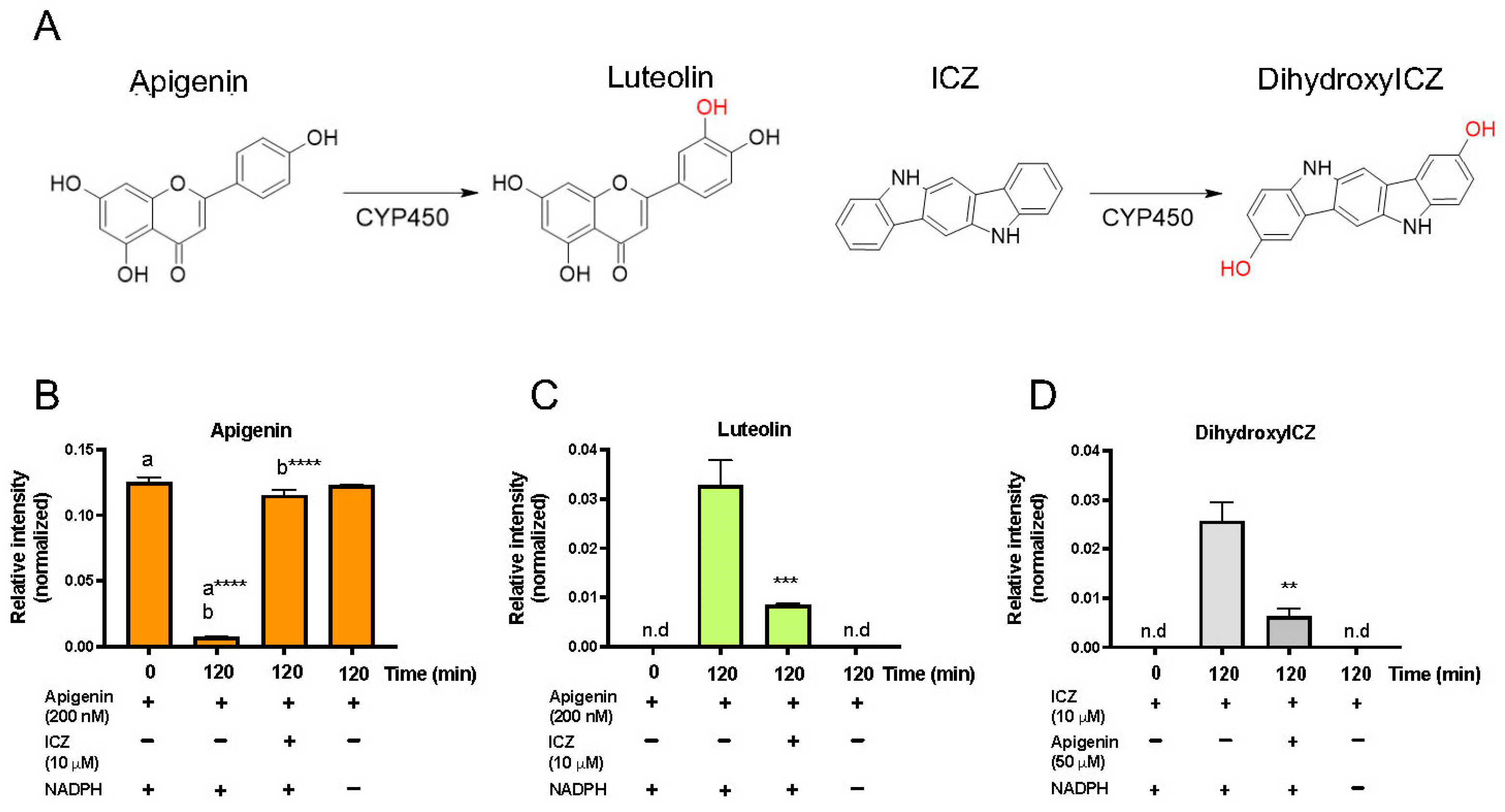
3.8. Apigenin Is Metabolized by CYP1A1/1B1 to Luteolin
3.9. Apigenin Inhibits ICZ Metabolism
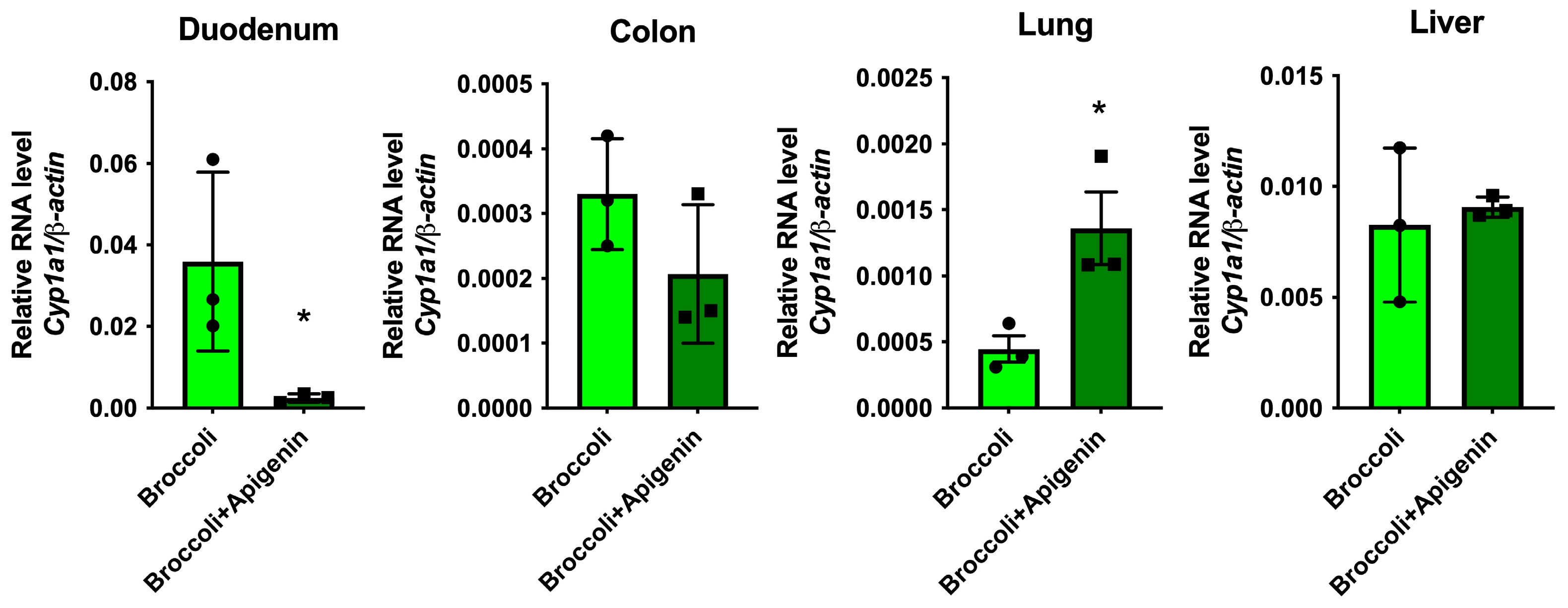
3.10. Apigenin Increases AHR Activation in the Lung
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Constant Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| dihydroxyICZ | 5H,11H-indolo[3,2-b]carbazole-2,8-diol |
| ICZ | Indolo[3,2-b]carbazole |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
References
- Piwarski, S.A.; Salisbury, T.B. The effects of environmental aryl hydrocarbon receptor ligands on signaling and cell metabolism in cancer. Biochem. Pharmacol. 2023, 216, 115771. [Google Scholar] [CrossRef] [PubMed]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.R.; Schiering, C.; Stockinger, B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018, 49, 353–362.e355. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Nakamura, M.; Yoshida, T.; Maitani, T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry 2008, 69, 3117–3130. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Preobrazhenskaya, M.N.; Bukhman, V.M.; Korolev, A.M.; Efimov, S.A. Ascorbigen and other indole-derived compounds from Brassica vegetables and their analogs as anticarcinogenic and immunomodulating agents. Pharmacol. Ther. 1993, 60, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Tsai, C.H.; Kulp, S.K.; Chen, C.S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Kim, J.Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef]
- Gillner, M.; Bergman, J.; Cambillau, C.; Fernstrom, B.; Gustafsson, J.A. Interactions of indoles with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol. Pharmacol. 1985, 28, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jeuken, A.; Keser, B.J.; Khan, E.; Brouwer, A.; Koeman, J.; Denison, M.S. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J. Agric. Food Chem. 2003, 51, 5478–5487. [Google Scholar] [CrossRef]
- Yoshida, K.; Satsu, H.; Mikubo, A.; Ogiwara, H.; Yakabe, T.; Inakuma, T.; Shimizu, M. 6-shogaol, a major compound in ginger, induces aryl hydrocarbon receptor-mediated transcriptional activity and gene expression. J. Agric. Food Chem. 2014, 62, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Nichols, R.G.; Cassel, K.; Podolsky, M.; Kuzu, G.; Tian, Y.; Smith, P.; Kennett, M.J.; Patterson, A.D.; et al. Dietary Broccoli Impacts Microbial Community Structure and Attenuates Chemically Induced Colitis in Mice in an Ah receptor dependent manner. J. Funct. Foods 2017, 37, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Murray, I.A.; Annalora, A.; Coslo, D.M.; Desai, D.; Gowda, K.; Yang, J.; Wang, D.; Koo, I.; Hao, F.; et al. Complex chemical signals dictate Ah receptor activation through the gut-lung axis. FASEB J. 2023, 37, e23010. [Google Scholar] [CrossRef]
- Muku, G.E.; Murray, I.A.; Espin, J.C.; Perdew, G.H. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites 2018, 8, 86. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef]
- Wang, A.; Savas, U.; Stout, C.D.; Johnson, E.F. Structural characterization of the complex between alpha-naphthoflavone and human cytochrome P450 1B1. J. Biol. Chem. 2011, 286, 5736–5743. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic. Acids. Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Luthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.I. ISOLDE: A physically realistic environment for model building into low-resolution electron-density maps. Acta. Crystallogr. D Struct. Biol. 2018, 74, 519–530. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic. Acids. Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Annalora, A.J.; Goodin, D.B.; Hong, W.X.; Zhang, Q.; Johnson, E.F.; Stout, C.D. Crystal structure of CYP24A1, a mitochondrial cytochrome P450 involved in vitamin D metabolism. J. Mol. Biol. 2010, 396, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L.; DeLano, W. PyMOL 2.5; Schrödinger, Inc.: New York, NY, USA, 2020. [Google Scholar]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1; U.S. Department of Agriculture; Agricultural Research Service: Beltsville, MD, USA, 2018.
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef]
- Gronke, K.; Hernandez, P.P.; Zimmermann, J.; Klose, C.S.N.; Kofoed-Branzk, M.; Guendel, F.; Witkowski, M.; Tizian, C.; Amann, L.; Schumacher, F.; et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566, 249–253. [Google Scholar] [CrossRef]
- Faust, D.; Nikolova, T.; Watjen, W.; Kaina, B.; Dietrich, C. The Brassica-derived phytochemical indolo[3,2-b]carbazole protects against oxidative DNA damage by aryl hydrocarbon receptor activation. Arch. Toxicol. 2017, 91, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Corre, S.; Tardif, N.; Mouchet, N.; Leclair, H.M.; Boussemart, L.; Gautron, A.; Bachelot, L.; Perrot, A.; Soshilov, A.; Rogiers, A.; et al. Sustained activation of the Aryl hydrocarbon Receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat. Commun. 2018, 9, 4775. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Stanford Zulick, E.; Novikov, O.; Parks, A.J.; Schlezinger, J.J.; Wang, Z.; Laroche, F.; Feng, H.; Mulas, F.; Monti, S.; et al. Towards Resolving the Pro- and Anti-Tumor Effects of the Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2018, 19, 1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Zhang, L.; Zhao, X.C.; Gao, S.H.; Qu, L.W.; Yu, H.; Fang, W.F.; Zhou, Y.C.; Liang, F.; Zhang, C.; et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Congues, F.; Wang, P.; Lee, J.; Lin, D.; Shahid, A.; Xie, J.; Huang, Y. Targeting aryl hydrocarbon receptor to prevent cancer in barrier organs. Biochem. Pharmacol. 2024, 223, 116156. [Google Scholar] [CrossRef]
- Mukai, R.; Fukuda, I.; Nishiumi, S.; Natsume, M.; Osakabe, N.; Yoshida, K.; Ashida, H. Cacao polyphenol extract suppresses transformation of an aryl hydrocarbon receptor in C57BL/6 mice. J. Agric. Food Chem. 2008, 56, 10399–10405. [Google Scholar] [CrossRef]
- Dong, F.; Annalora, A.J.; Murray, I.A.; Tian, Y.; Marcus, C.B.; Patterson, A.D.; Perdew, G.H. Endogenous Tryptophan-Derived Ah Receptor Ligands are Dissociated from CYP1A1/1B1-Dependent Negative-Feedback. Int. J. Tryptophan Res. 2023, 16, 11786469231182508. [Google Scholar] [CrossRef]
- Griffiths, L.A.; Smith, G.E. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 128, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Chiaro, C.R.; Patel, R.D.; Marcus, C.B.; Perdew, G.H. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol. Pharmacol. 2007, 72, 1369–1379. [Google Scholar] [CrossRef]


| Substrate | Computational Docking Program | ||||||
|---|---|---|---|---|---|---|---|
| Autodock 4.2 | Autodock Vina | ||||||
| Human CYP1A1 | Mouse CYP1A1 | Human CYP1A1 | Mouse CYP1A1 | ||||
| Dissociation Constant a (KD) in nM | Binding Energy b (kcal/mole) | Dissociation Constant (KD) in nM | Binding Energy (kcal/mole) | Binding Energy c (kcal/mole) [KD] | Binding Energy (kcal/mole) [KD] | ||
| Apigenin | Max d | 107 | −9.5 | 90 | −9.6 | −11.2 [6.3 nM] | −11.1 [7.5 nM] |
| Avg e | 125 ± 27 | −9.4 ± 0.1 | 110 ± 21 | −9.5 ± 0.1 | −10.4 ± 0.8 | −10.3 ± 0.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Annalora, A.J.; Murray, I.A.; Chakraborty, D.; Coslo, D.M.; Marcus, C.; Patterson, A.D.; Perdew, G.H. Phytochemical-Mediated Ah Receptor Activity Is Dependent on Dietary Context. Nutrients 2025, 17, 876. https://doi.org/10.3390/nu17050876
Dong F, Annalora AJ, Murray IA, Chakraborty D, Coslo DM, Marcus C, Patterson AD, Perdew GH. Phytochemical-Mediated Ah Receptor Activity Is Dependent on Dietary Context. Nutrients. 2025; 17(5):876. https://doi.org/10.3390/nu17050876
Chicago/Turabian StyleDong, Fangcong, Andrew J. Annalora, Iain A. Murray, Debopriya Chakraborty, Denise M. Coslo, Craig Marcus, Andrew D. Patterson, and Gary H. Perdew. 2025. "Phytochemical-Mediated Ah Receptor Activity Is Dependent on Dietary Context" Nutrients 17, no. 5: 876. https://doi.org/10.3390/nu17050876
APA StyleDong, F., Annalora, A. J., Murray, I. A., Chakraborty, D., Coslo, D. M., Marcus, C., Patterson, A. D., & Perdew, G. H. (2025). Phytochemical-Mediated Ah Receptor Activity Is Dependent on Dietary Context. Nutrients, 17(5), 876. https://doi.org/10.3390/nu17050876







