Effects of Lebanese Folk Herbs on Adult Male Rats: Hepatic and Renal Toxicity, Histological, and Biochemical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Extract
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection
2.5. Biochemical Analysis
2.6. Liver and Kidney Lipid Peroxidation and Antioxidant Enzyme
2.6.1. Superoxide Dismutase (SOD)
2.6.2. Reduced Glutathione (GSH)
2.6.3. Lipid Peroxidation
2.7. Histological Observation
2.8. Statistical Analysis
3. Results
3.1. Total Body Weight, Organ Weight, and Organs Indices
3.2. Serum Hepatic and Renal Functional Markers
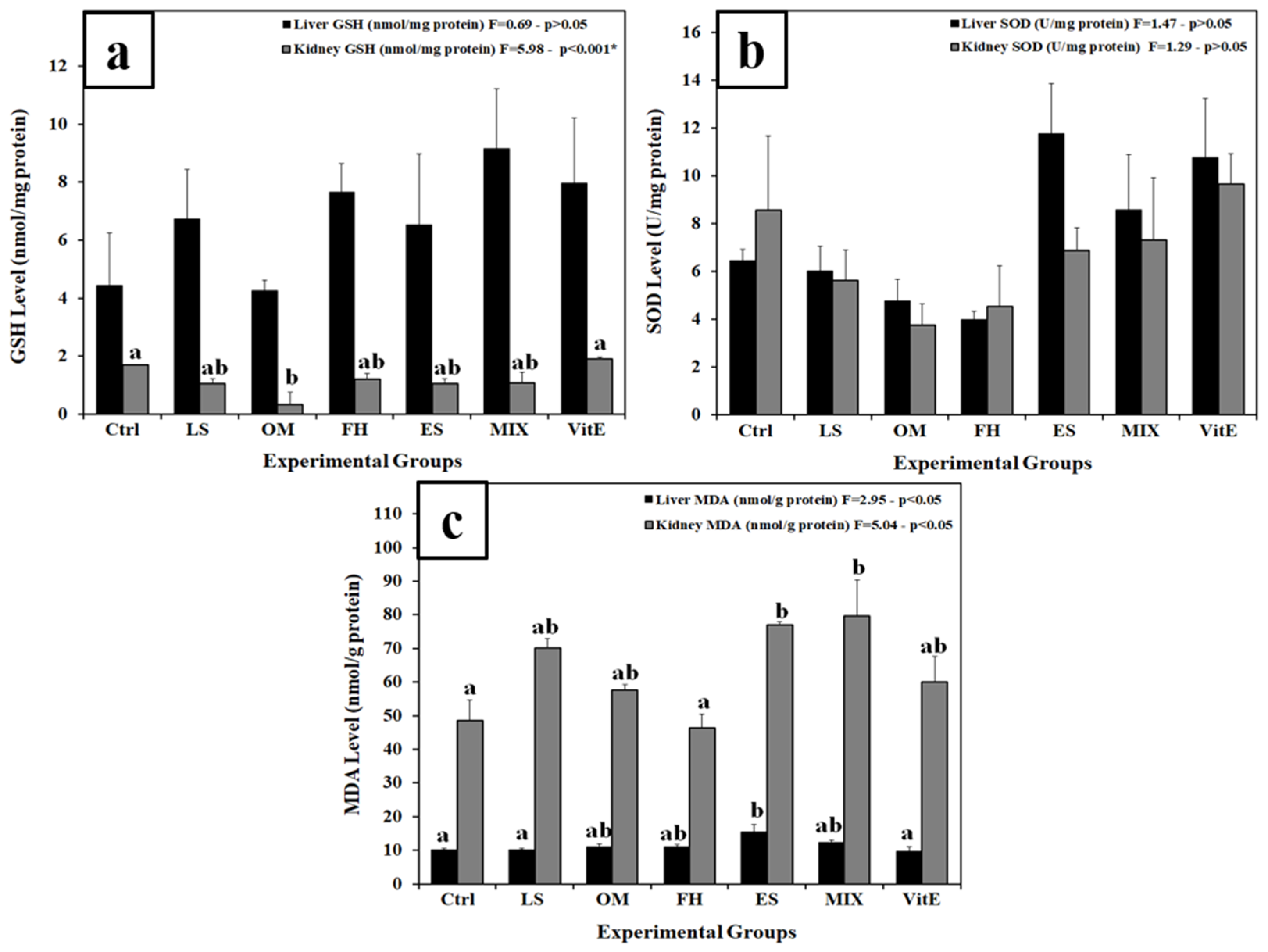
3.3. Changes in Lipid Peroxidation and Antioxidant Markers
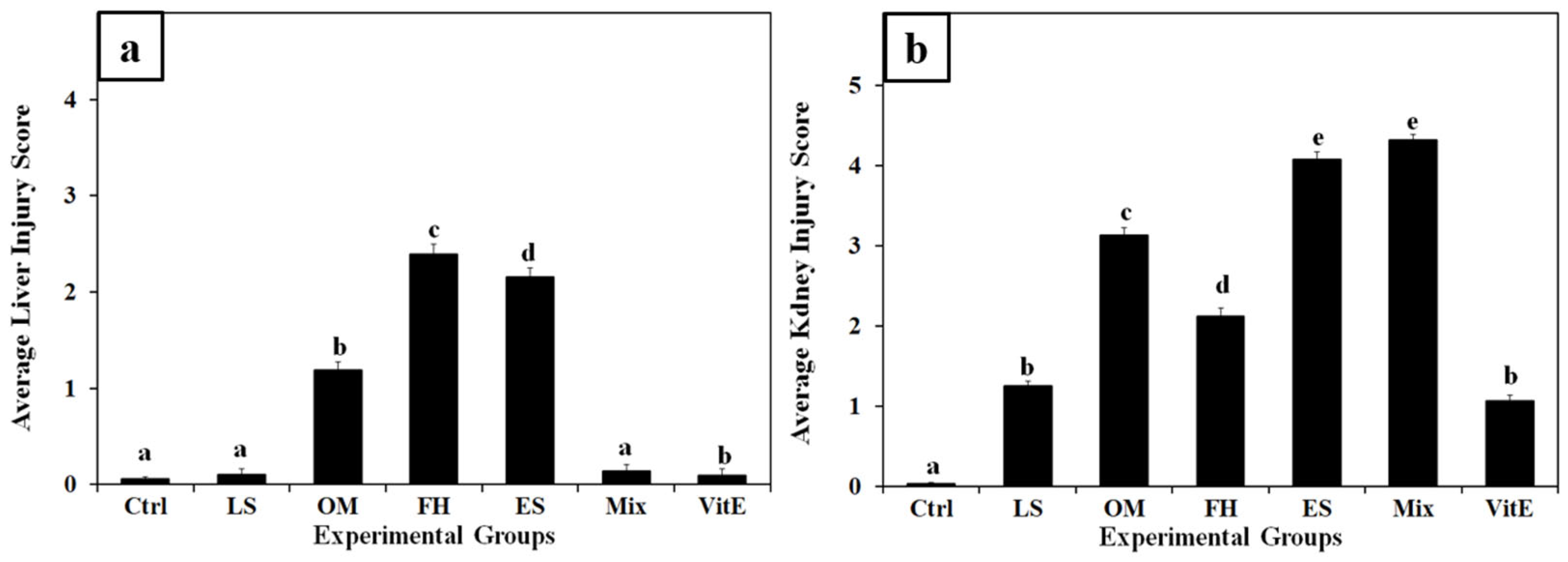
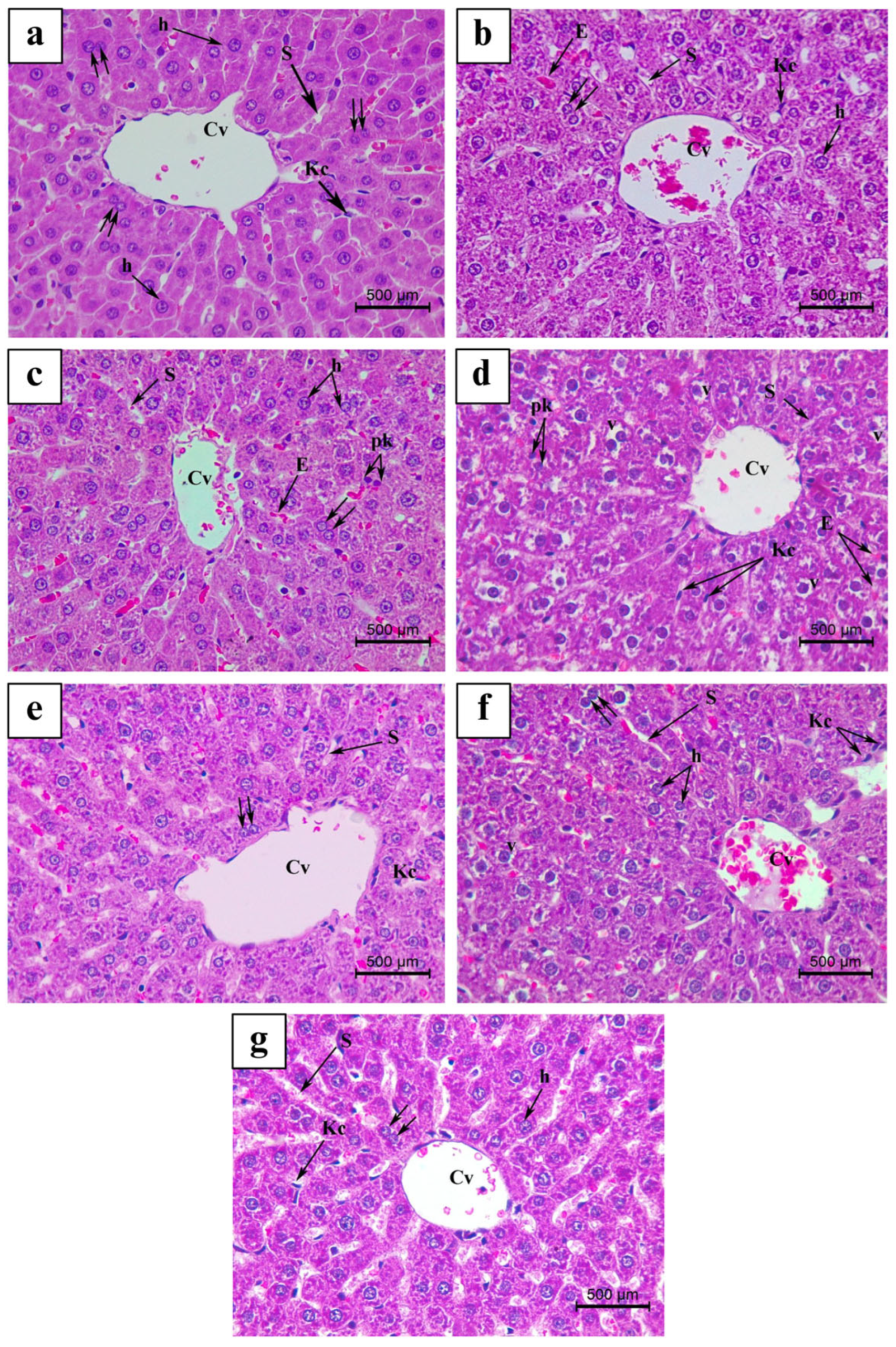
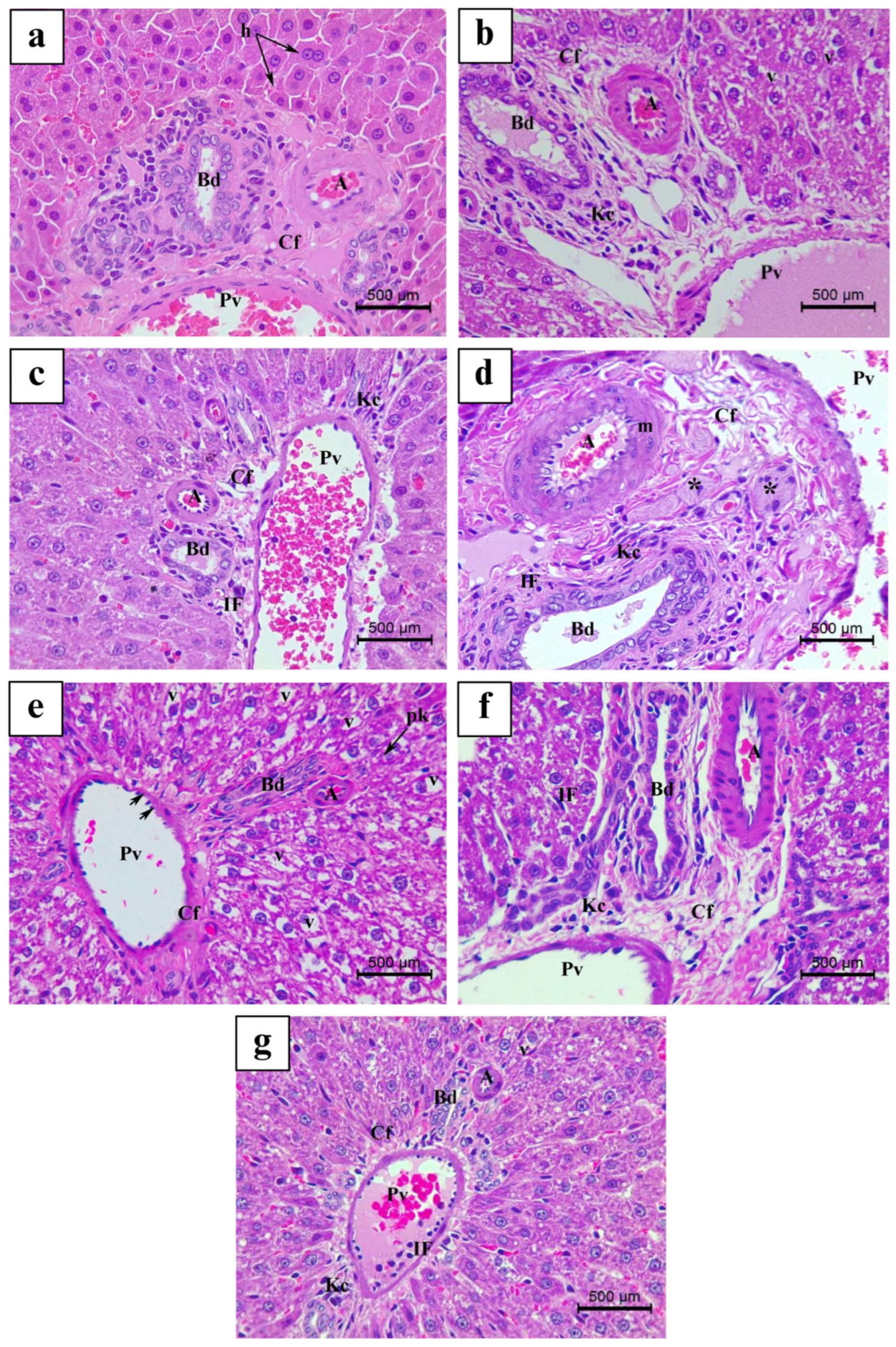
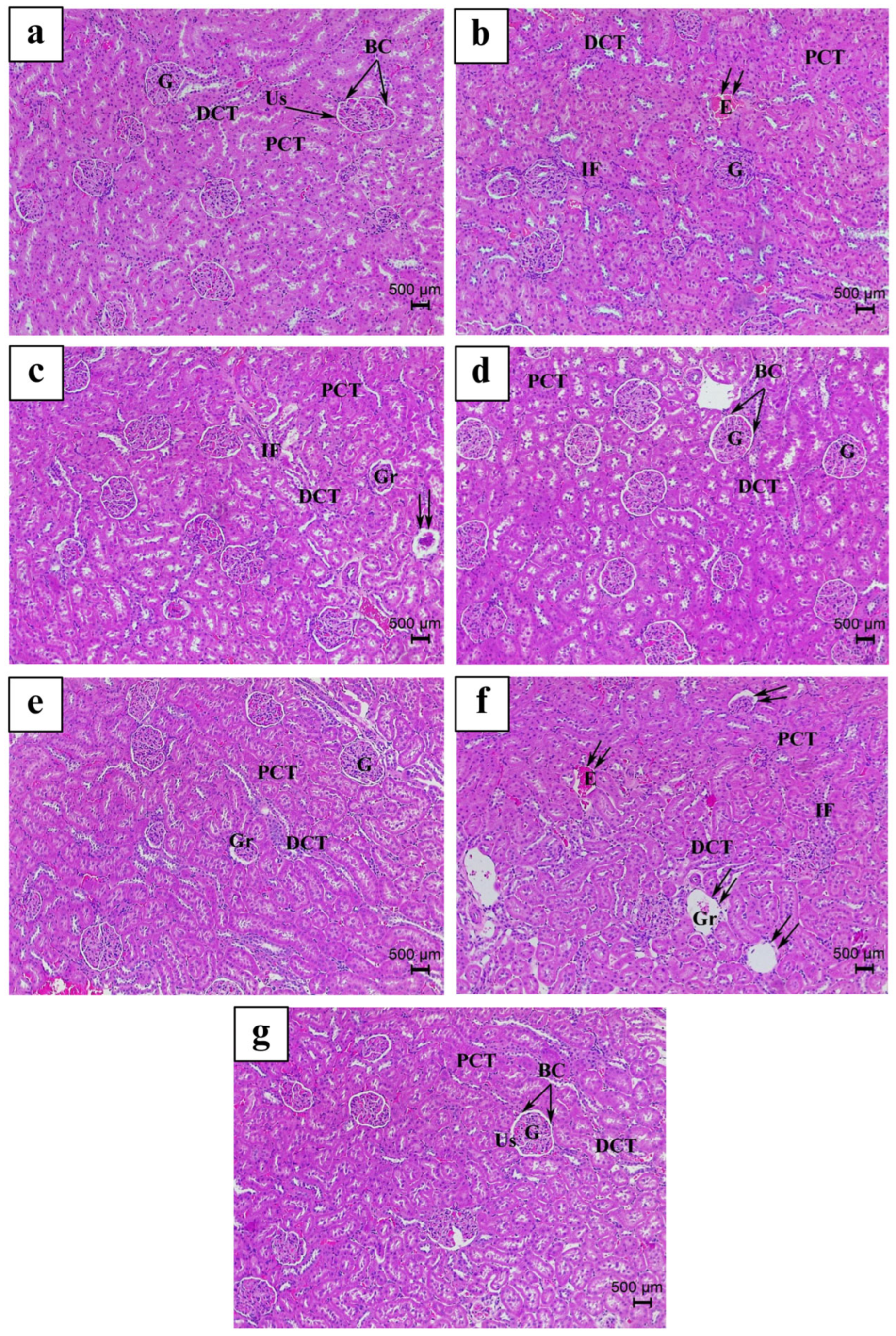
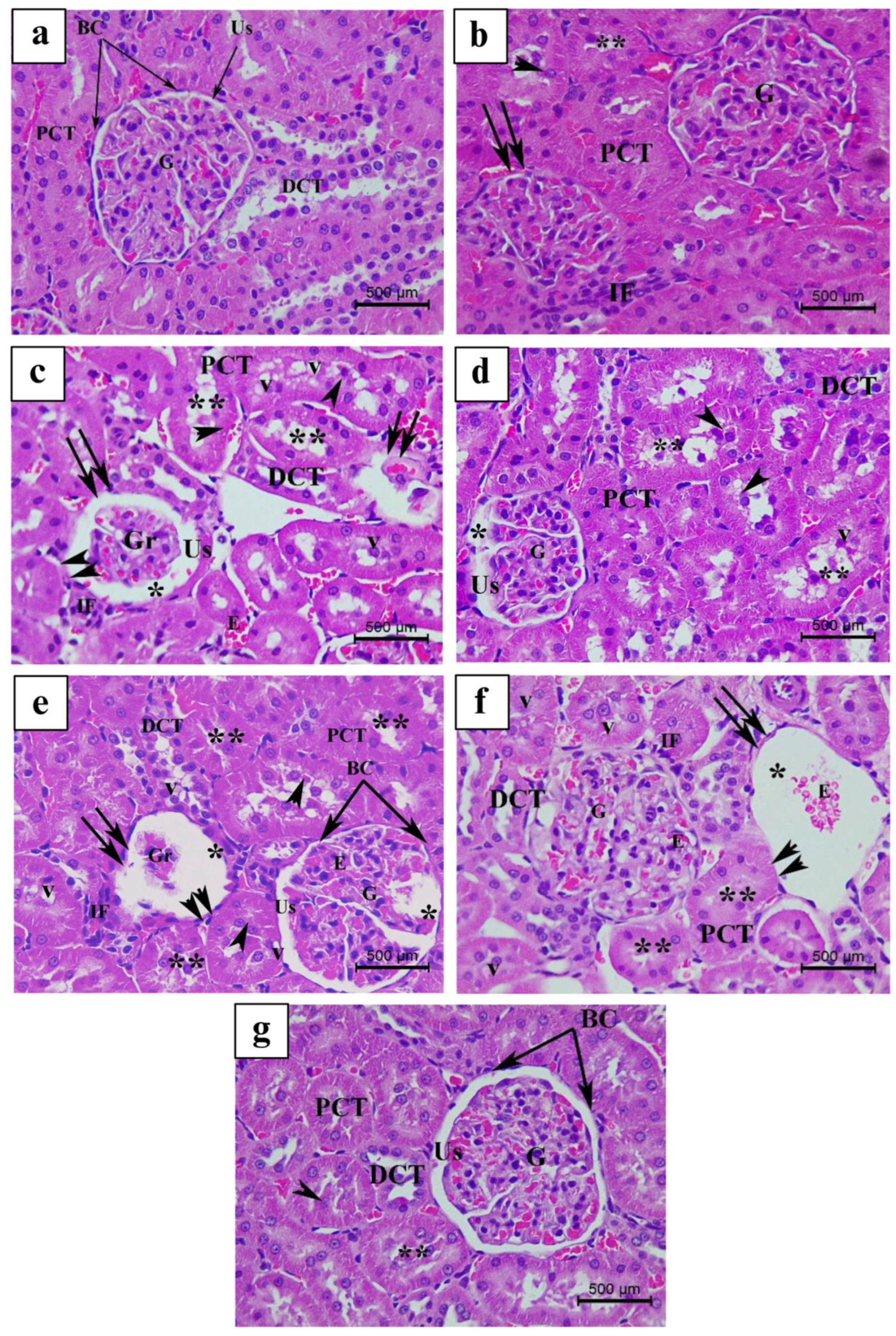
3.4. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OM | Origanum majorana |
| LS | Lepidium sativum |
| ES | Eruca sativa |
| FH | Ferula hermonis |
| PA | pyrrolizidine alkaloid |
| SD | Sprague Dawley |
| VitE | vitamin E |
| ALT | alanine transaminase |
| AST | aspartate aminotransferase |
| GSH | glutathione |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| NBT | nitro blue tetrazolium |
| TBA | thiobarbituric acid |
| DTNB | 5,5’-dithio-bis(2-nitrobenzoic acid) |
| H&E | hematoxylin and eosin |
| CREA | creatinine |
| PCTs | proximal convoluted tubfules |
| DCTs | distal convoluted tubules |
| BC | Bowman’s capsule |
| SEM | standard error of the mean |
| BW | body weight |
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.H.U.; Saleem, U.; Ahmad, B.; Rashid, M. Phytochemical and toxicological evaluation of Zephyranthes citrina. Front. Pharmacol. 2022, 13, 1007310. [Google Scholar] [CrossRef] [PubMed]
- Dixit JR, V., III; Kumar, I.; Palandurkar, K.; Giri, R.; Giri, K. Lepidium sativum: Bone healer in traditional medicine, an experimental validation study in rats. J. Fam. Med. Prim. Care 2020, 9, 812–818. [Google Scholar] [CrossRef]
- Safi, R.; El-Sabban, M.; Najjar, F. Ferula hermonis: A Review of Current Use and Pharmacological Studies of its Sesquiterpene Ester Ferutinin. Curr. Drug Targets 2020, 21, 499–508. [Google Scholar] [CrossRef]
- Bina, F.; Rahimi, R. Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J. Evid.-Based Complement. Altern. Med. 2017, 22, 175–185. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquò, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Özkan, T.; Rahbar Saadat, Y. Evidence for Health-Promoting Properties of Lepidium sativum L.: An Updated Comprehensive Review. Turk. J. Pharm. Sci. 2022, 19, 714–723. [Google Scholar] [CrossRef]
- Vazifeh, S.; Kananpour, P.; Khalilpour, M.; Eisalou, S.V.; Hamblin, M.R. Anti-inflammatory and Immunomodulatory Properties of Lepidium sativum. BioMed Res. Int. 2022, 2022, 3645038. [Google Scholar] [CrossRef]
- Fuentes, E.; Alarcón, M.; Fuentes, M.; Carrasco, G.; Palomo, I. A novel role of Eruca sativa Mill. (rocket) extract: Antiplatelet (NF-κB inhibition) and antithrombotic activities. Nutrients 2014, 6, 5839–5852. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; ANasr, F.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Sattar, Z.; Iranshahi, M. Phytochemistry and Pharmacology of Ferula hermonis Boiss.—A Review. Drug Res. 2017, 67, 437–446. [Google Scholar] [CrossRef]
- El-Wakf, A.M.; El-Habibi, E.M.; Ali, D.A.; Abd El-Ghany, E.; Elmougy, R. Marjoram and sage oils protect against testicular apoptosis, suppressed Ki-67 expression and cell cycle arrest as a therapy for male infertility in the obese rats. J. Food Biochem. 2020, 44, e13080. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Shieh, A.; Ghalenoei, J.A.; Yadegari, M.; Alborzi, N. Review of potential spermatogenic and aphrodisiac effects of the Ferula genus. Clin. Exp. Reprod. Med. 2023, 50, 143–153. [Google Scholar] [CrossRef]
- Al-Qudah, M.M.A. Effects of Eruca sativa Leaves Extracts on Testes, Fertility Potential and Testosterone Concentration in Male Rats. Annu. Res. Rev. Biol. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Razaq, S.; R.Ibraheem, M.; Hashim, S. Effect of Lepidium sativum Aqueous Crude Extract in Some Fertility Parameters in Mice. Int. J. Sci. Res. (IJSR) 2015, 6, 260. [Google Scholar]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. CB 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Frenzel, C.; Teschke, R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. Int. J. Mol. Sci. 2016, 17, 588. [Google Scholar] [CrossRef]
- Lei, X.; Chen, J.; Ren, J.; Li, Y.; Zhai, J.; Mu, W.; Zhang, L.; Zheng, W.; Tian, G.; Shang, H. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evid.-Based Complement. Altern. Med. Ecam 2015, 2015, 459749. [Google Scholar] [CrossRef]
- Larrey, D.; Vial, T.; Pauwels, A.; Castot, A.; Biour, M.; David, M.; Michel, H. Hepatitis after germander (Teucrium chamaedrys) administration: Another instance of herbal medicine hepatotoxicity. Ann. Intern. Med. 1992, 117, 129–132. [Google Scholar] [CrossRef]
- Lin, G.; Wang, J.Y.; Li, N.; Li, M.; Gao, H.; Ji, Y.; Zhang, F.; Wang, H.; Zhou, Y.; Ye, Y.; et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Gueutin, V.; Deray, G.; Isnard-Bagnis, C. [Renal physiology]. Bull. Cancer 2012, 99, 237–249. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, R.; Ying, J.; Zhao, M.; Wu, X.; Cao, G.; Wang, K. Nephrotoxicity of Herbal Medicine and Its Prevention. Front. Pharmacol. 2020, 11, 569551. [Google Scholar] [CrossRef]
- Khalaf, R.R.; Khazaal, S.; Abouzeinab, N.S.; Khalil, M.I. Evaluation of Selected Folk Herbs on the Fertility of Sprague Dawley Male Rats: Biochemical, Histological, and Molecular Investigations. Life 2024, 14, 1620. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Vaez, A.; Mardani, M.; Razavi, S. Impact of saffron on rat sperm membrane integrity and spermatogenesis status. Adv. Biomed. Res. 2014, 3, 146. [Google Scholar] [CrossRef]
- Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Ibrahim, F.M.; Farag, O.M.; Ahmed, K.A. Eruca sativa seed extract modulates oxidative stress and apoptosis and up-regulates the expression of Bcl-2 and Bax genes in acrylamide-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 53249–53266. [Google Scholar] [CrossRef] [PubMed]
- Geroushi, A.; Auzi, A.A.; Elhwuegi, A.S.; Elzawam, F.; Elsherif, A.; Nahar, L.; Sarker, S.D. Antiinflammatory sesquiterpenes from the root oil of Ferula hermonis. Phytother. Res. PTR 2011, 25, 774–777. [Google Scholar] [CrossRef]
- Ramadan, G.; El-Beih, N.M.; Zahra, M.M. Egyptian sweet marjoram leaves protect against genotoxicity, immunosuppression and other complications induced by cyclophosphamide in albino rats. Br. J. Nutr. 2012, 108, 1059–1068. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Park, Y.; Hirose, R.; Dang, K.; Xu, F.; Behrends, M.; Tan, V.; Roberts, J.P.; Niemann, C.U. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 2008, 143, 243–251. [Google Scholar] [CrossRef]
- Schafer, K.A.; Eighmy, J.; Fikes, J.D.; Halpern, W.G.; Hukkanen, R.R.; Long, G.G.; Meseck, E.K.; Patrick, D.J.; Thibodeau, M.S.; Wood, C.E.; et al. Use of Severity Grades to Characterize Histopathologic Changes. Toxicol. Pathol. 2018, 46, 256–265. [Google Scholar] [CrossRef]
- Guan, Y.S.; He, Q. Plants Consumption and Liver Health. Evid.-Based Complement. Altern. Med. Ecam 2015, 2015, 824185. [Google Scholar] [CrossRef]
- Rusli, R.N.M.; Naomi, R.; Yazid, M.D.; Embong, H.; Perumal, K.; Othman, F.; Jasni, A.S.; Jumidil, S.H.; Balan, S.S.; Abidin, A.Z.; et al. Phytochemical Analysis and Toxicity Assessment of Bouea macrophylla Yoghurt. Toxins 2023, 15, 125. [Google Scholar] [CrossRef]
- Fan, Q.; Zhao, B.; Wang, C.; Zhang, J.; Wu, J.; Wang, T.; Xu, A. Subchronic Toxicity Studies of Cortex Dictamni Extracts in Mice and Its Potential Hepatotoxicity Mechanisms in Vitro. Molecules 2018, 23, 2486. [Google Scholar] [CrossRef]
- Gebrezgi, E.M.; Hiben, M.G.; Kidanu, K.G.; Tsegay, A.T. Subacute Hepatotoxicity of Extracts of Senna occidentalis Seeds in Swiss Albino Mice. J. Toxicol. 2020, 2020, 8843044. [Google Scholar] [CrossRef]
- Abebe, M.S.; Asres, K.; Bekuretsion, Y.; Abebe, A.; Bikila, D.; Seyoum, G. Sub-chronic toxicity of ethanol leaf extract of Syzygium guineense on the biochemical parameters and histopathology of liver and kidney in the rats. Toxicol. Rep. 2021, 8, 822–828. [Google Scholar] [CrossRef]
- Singh, T.; Sinha, N.; Singh, A. Biochemical and histopathological effects on liver due to acute oral toxicity of aqueous leaf extract of Ecliptaalba on female Swiss albino mice. Indian J. Pharmacol. 2013, 45, 61–65. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Kasarala, G.; Tillmann, H.L. Standard liver tests. Clin. Liver Dis. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Sikander, M.; Malik, S.; Parveen, K.; Ahmad, M.; Yadav, D.; Hafeez, Z.B.; Bansal, M. Hepatoprotective effect of Origanum vulgare in Wistar rats against carbon tetrachloride-induced hepatotoxicity. Protoplasma 2013, 250, 483–493. [Google Scholar] [CrossRef]
- Bafeel, S.O.; Ali, S.S. The Potential Liver Toxicity of Lepidium sativum Seeds in Albino Rats. Res. J. Biol. Sci. 2009, 4, 1250–1258. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Dasgupta, A.; Wahed, A. Chapter 11—Renal function tests. In Clinical Chemistry, Immunology and Laboratory Quality Control, 2nd ed.; Dasgupta, A., Wahed, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 229–252. [Google Scholar]
- Francoz, C.; Glotz, D.; Moreau, R.; Durand, F. The evaluation of renal function and disease in patients with cirrhosis. J. Hepatol. 2010, 52, 605–613. [Google Scholar] [CrossRef]
- Balgoon, M.J. Assessment of the Protective Effect of Lepidium sativum against Aluminum-Induced Liver and Kidney Effects in Albino Rat. BioMed Res. Int. 2019, 2019, 4516730. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1444–1458. [Google Scholar] [CrossRef]
- Imo, C.; Arowora, K.A.; Ezeonu, C.S.; Yakubu, O.E.; Nwokwu, C.D.; Azubuike, N.C.; Sallah, Y.G. Effects of ethanolic extracts of leaf, seed and fruit of Datura metel L. on kidney function of male albino rats. J. Tradit. Complement. Med. 2019, 9, 271–277. [Google Scholar] [CrossRef]
- Ogundipe, D.J.; Akomolafe, R.O.; Sanusi, A.A.; Imafidon, C.E.; Olukiran, O.S.; Oladele, A.A. Ocimum gratissimum Ameliorates Gentamicin-Induced Kidney Injury but Decreases Creatinine Clearance Following Sub-Chronic Administration in Rats. J. Evid.-Based Complement. Altern. Med. 2017, 22, 592–602. [Google Scholar] [CrossRef]
- Bahmani, M.; Rafieian, M.; Baradaran, A.; Rafieian, S.; Rafieian-Kopaei, M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol. 2014, 3, 81–85. [Google Scholar] [CrossRef]
- Sarwar Alam, M.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem. Toxicol. 2007, 45, 910–920. [Google Scholar] [CrossRef]
- Kiliś-Pstrusińska, K.; Wiela-Hojeńska, A. Nephrotoxicity of Herbal Products in Europe—A Review of an Underestimated Problem. Int. J. Mol. Sci. 2021, 22, 4132. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Aouachria, S.; Boumerfeg, S.; Benslama, A.; Benbacha, F.; Guemmez, T.; Khennouf, S.; Arrar, L.; Baghiani, A. Acute, sub-acute toxicity and antioxidant activities (in vitro and in vivo) of Reichardia picroide crude extract. J. Ethnopharmacol. 2017, 208, 105–116. [Google Scholar] [CrossRef]
- Refaie, A.A.; Ramadan, A.; Mossa, A.T. Oxidative damage and nephrotoxicity induced by prallethrin in rat and the protective effect of Origanum majorana essential oil. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. S1), S506–S513. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef]
- El-Bakry, H.A.; El-Sherif, G.; Rostom, R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. = Biomed. Pharmacother. 2017, 96, 798–811. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Gao, X.; Lv, J.; Bian, X.; Lv, J.; Sun, J.; Luo, G.; Zhang, H. Effects of medicine food Fructus Gardeniae on liver and kidney functions after oral administration to rats for 12 weeks. J. Food Biochem. 2021, 45, e13752. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and necrosis in the liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Walsh, S.B.; Unwin, R.J. Renal tubular disorders. Clin. Med. 2012, 12, 476–479. [Google Scholar] [CrossRef]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Chen, D.; Weng, H.; Li, H.; Lu, Y. Oral subacute nephrotoxicity of aristololactam I in rats. Toxicology 2022, 475, 153228. [Google Scholar] [CrossRef]

| Group | Number of Rats | Name | Treatment |
|---|---|---|---|
| 1 | 4 | Ctrl | Normal saline |
| 2 | 4 | LS | Lepidium sativum extract |
| 3 | 4 | OM | Origanum majorana extract |
| 4 | 4 | FH | Ferula hermonis extract |
| 5 | 4 | ES | Eruca sativa extract |
| 6 | 4 | Mix | Mixture of all herbs |
| 7 | 4 | VitE (positive control) | Vitamin E |
| Experimental Groups | Body Weight (g) | Kidney Weight (g) | Kidney Index | Liver Weight (g) | Liver Index |
|---|---|---|---|---|---|
| Ctrl | 348.75 ± 17.96 | 2.50 ± 0.29 | 0.74 ± 0.09 | 9.25 ± 0.48 a | 2.27 ± 0.50 a |
| LS | 378.75 ± 12.65 | 2.46 ± 0.28 | 0.65 ± 0.02 | 14.18 ± 0.69 b | 3.74 ± 0.11 b |
| OM | 368.75 ± 12.14 | 2.46 ± 0.10 | 0.66 ± 0.003 | 12.78 ± 1.56 ab | 3.44 ± 0.33 ab |
| FH | 393.75 ±8.75 | 2.35 ± 0.88 | 0.60 ± 0.03 | 11.78 ± 1.20 ab | 2.97 ± 0.25 ab |
| ES | 397.50 ±19.84 | 2.59 ± 0.05 | 0.65 ±0.04 | 14.61 ± 1.06 b | 3.66 ± 0.15 b |
| Mix | 371.25 ± 11.43 | 2.53 ± 0.09 | 0.68 ± 0.04 | 11.17 ± 0.24 ab | 3.01 ± 0.03 ab |
| VitE | 408.75 ± 11.43 | 2.69 ± 0.08 | 0.66 ± 0.01 | 14.89 ± 0.90 b | 3.64 ± 0.16 b |
| Results of one-way ANOVA (group) | F = 2.138 p > 0.05 | F = 0.698 p > 0.05 | F = 1.057 p > 0.05 | F = 4.585 p < 0.05 | F = 4.121 p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, R.R.; Abouzeinab, N.S.; Khalil, M.I. Effects of Lebanese Folk Herbs on Adult Male Rats: Hepatic and Renal Toxicity, Histological, and Biochemical Studies. Nutrients 2025, 17, 875. https://doi.org/10.3390/nu17050875
Khalaf RR, Abouzeinab NS, Khalil MI. Effects of Lebanese Folk Herbs on Adult Male Rats: Hepatic and Renal Toxicity, Histological, and Biochemical Studies. Nutrients. 2025; 17(5):875. https://doi.org/10.3390/nu17050875
Chicago/Turabian StyleKhalaf, Rana R., Noura S. Abouzeinab, and Mahmoud I. Khalil. 2025. "Effects of Lebanese Folk Herbs on Adult Male Rats: Hepatic and Renal Toxicity, Histological, and Biochemical Studies" Nutrients 17, no. 5: 875. https://doi.org/10.3390/nu17050875
APA StyleKhalaf, R. R., Abouzeinab, N. S., & Khalil, M. I. (2025). Effects of Lebanese Folk Herbs on Adult Male Rats: Hepatic and Renal Toxicity, Histological, and Biochemical Studies. Nutrients, 17(5), 875. https://doi.org/10.3390/nu17050875








