Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sources and Search
2.2. Study Selection
2.3. Data Extraction
- -
- κ < 0.20: poor agreement;
- -
- κ = 0.21–0.40: fair agreement;
- -
- κ = 0.41–0.60: moderate agreement;
- -
- κ = 0.61–0.80: substantial agreement;
- -
- κ = 0.81–1.00: almost perfect agreement.
2.4. Statistical Analysis
3. Results
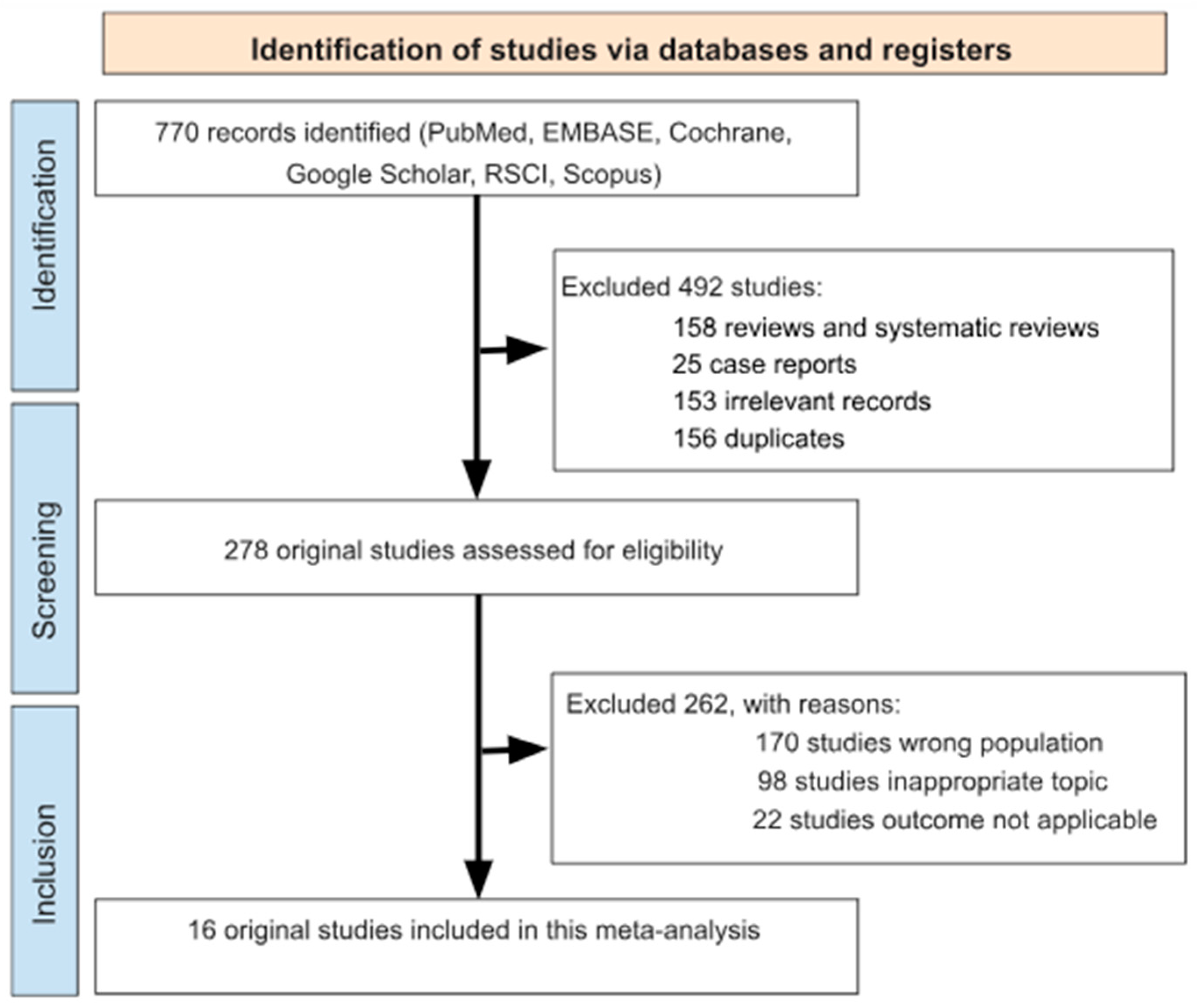
3.1. Search Results
3.2. Prevalence of Sarcopenia in CP Patients
3.3. Risk of Sarcopenia in CP Patients
3.4. Subgroup Analysis
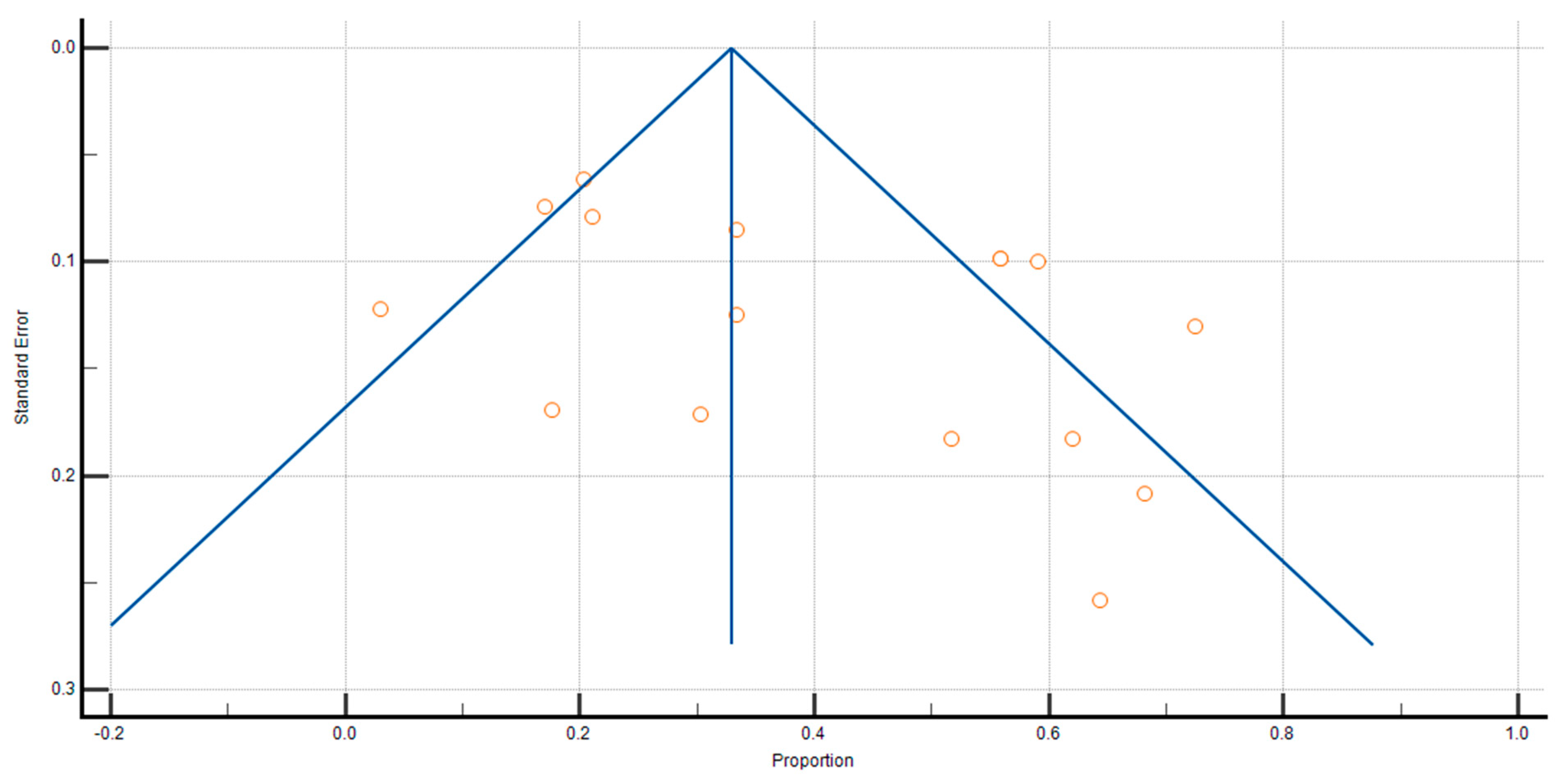
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RSCI | Russian Science Citation Index |
| NOS | Newcastle–Ottawa Scale |
| CP | Chronic Pancreatitis |
| OR | Odds Ratio |
| SMI | Skeletal Muscle Index |
| EPI | Exocrine Pancreatic Insufficiency |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin-6 |
| PERT | Pancreatic Enzyme Replacement Therapy |
| BMI | Body Mass Index |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| RT | Resistance Training |
| CI | Confidence Interval |
| RR | Relative Risk |
| HR | Hazard Ratio |
| UEG | United European Gastroenterology |
| EPC | European Pancreatic Club |
| EDS | European Digestive Surgery |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| ESDO | European Society of Digestive Oncology |
| ESPCG | European Society for Primary Care Gastroenterology |
References
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J. Associations Between Handgrip Strength and Disease-Specific Mortality Including Cancer, Cardiovascular, and Respiratory Diseases in Older Adults: A Meta-Analysis. J. Aging Phys. Act. 2020, 28, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 115–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasullo, M.; Omer, E.; Kaspar, M. Sarcopenia in Chronic Pancreatitis—Prevalence, Diagnosis, Mechanisms and Potential Therapies. Curr. Gastroenterol. Rep. 2022, 24, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez-Sousa, M.A.; Venegas-Sanabria, L.C.; Chavarro-Carvajal, D.A.; Cano-Gutierrez, C.A.; Izquierdo, M.; Correa-Bautista, J.E.; Ramírez-Vélez, R. Gait speed as a mediator of the effect of sarcopenia on dependency in activities of daily living. J. Cachexia Sarcopenia Muscle 2019, 10, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardeljan, A.D.; Hurezeanu, R. Sarcopenia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560813/ (accessed on 4 July 2023).
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, S.M.; Kehayias, J.J. Sarcopenia and the analysis of body composition. Adv. Nutr. 2014, 5, 260–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steffl, M.; Sima, J.; Shiells, K.; Holmerova, I. The increase in health care costs associated with muscle weakness in older people without long-term illnesses in the Czech Republic: Results from the Survey of Health, Ageing and Retirement in Europe (SHARE). Clin. Interv. Aging 2017, 12, 2003–2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Westbury, L.D.; Beaudart, C.; Bruyère, O.; Cauley, J.A.; Cawthon, P.; Cruz-Jentoft, A.J.; Curtis, E.M.; Ensrud, K.; Fielding, R.A.; Johansson, H.; et al. International Musculoskeletal Ageing Network. Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: Findings from population-based cohorts. J. Cachexia Sarcopenia Muscle 2023, 14, 565–575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, M.; Patel, B.; Han, S.; Bocelli, L.; Kheder, J.; Vaze, A.; Wassef, W. Exocrine Pancreatic Insufficiency and Malnutrition in Chronic Pancreatitis: Identification, Treatment, and Consequences. Pancreas 2018, 47, 1015–1018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bideeva, T.V.; Maev, I.V.; Kucheryavyy, Y.A.; Andreev, D.N.; Shah, Y.S.; Lobanova, E.G.; Zaborovskiy, A.V.; Levchenko, A.I. The effectiveness of pancreatic enzyme replacement therapy using microencapsulated pancreatin preparations in the correction of nutritional status in patients with chronic pancreatitis: A prospective observational study. Ter. Arkh. 2020, 92, 30–35. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Supriya, R.; Singh, K.P.; Gao, Y.; Gu, Y.; Baker, J.S. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bundred, J.; Thakkar, R.G.; Pandanaboyana, S. Systematic review of sarcopenia in chronic pancreatitis: Prevalence, impact on surgical outcomes, and survival. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Andreev, D.N.; Kucheryavyy, Y.A.; Levchenko, A.I. The prevalence of sarcopenia in patients with chronic pancreatitis: A meta-analysis. Ter. Arkh. 2020, 92, 43–47. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kasatkina, E.; Lyadov, V.K.; Mershina, E.A.; Sinitsyn, V. CT assessment of sarcopenia in patients with pancreatic cancer and chronic pancreatitis. In Proceedings of the European Congress of Radiology, Vienna, Austria, 1–5 March 2012; p. B-0563. [Google Scholar] [CrossRef]
- Lyadov, V.K.; Bulanova, E.A.; Sinitsyn, V.E. Possibilities of CT in detecting sarcopenia in patients with tumor and inflammatory diseases of the pancreas. Diagn. Interv. Radiol. 2012, 6, 13–18. (In Russia) [Google Scholar]
- OConnor, D.; Kok, T.; Purcell, C.; Duggan, S.; Conlon, K. Investigating the prevalence of sarcopenia in chronic pancreatitis in an irsih cohort: A CT-scan based pilot study. Pancreatology 2014, 14, 74. [Google Scholar] [CrossRef]
- El Amrani, M.; Vermersch, M.; Fulbert, M.; Prodeau, M.; Lecolle, K.; Hebbar, M.; Ernst, O.; Pruvot, F.R.; Truant, S. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: A retrospective analysis of 107 patients. Medicine 2018, 97, e12076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bieliuniene, E.; Brøndum Frøkjær, J.; Pockevicius, A.; Kemesiene, J.; Lukosevičius, S.; Basevicius, A.; Atstupenaite, V.; Barauskas, G.; Ignatavicius, P.; Gulbinas, A.; et al. CT- and MRI-Based Assessment of Body Composition and Pancreatic Fibrosis Reveals High Incidence of Clinically Significant Metabolic Changes That Affect the Quality of Life and Treatment Outcomes of Patients with Chronic Pancreatitis and Pancreatic Cancer. Medicina 2019, 55, 649. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.S.; Büyükuslu, A.; Køhler, M.; Rasmussen, H.H.; Drewes, A.M. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology 2019, 19, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ozola-Zālīte, I.; Frøkjær, J.B.; Mark, E.B.; Gudauskas, T.; Gudauskas, L.; Dedelaite, M.; Bieliuniene, E.; Ignatavicius, P.; Pukitis, A.; Drewes, A.M.; et al. A Clinical Feasible Method for Computed Tomography-Based Assessment of Sarcopenia in Patients With Chronic Pancreatitis. Pancreas 2019, 48, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Shahbazov, R.; Pattarabanjird, O.; Brayman, K.L.; Alekberzade, A.V.; Krylov, N.N. Morphometry of adipose tissue for prediction of the outcomes of total pancreatectomy with pancreatic islets autotransplantation in patients with chronic pancreatitis. Pirogov Russ. J. Surg. 2020, 5, 12–19. (In Russia) [Google Scholar] [CrossRef] [PubMed]
- Trikudanathan, G.; Feussom, G.; Teigen, L.; Munigala, S.; Price, K.; Dirweesh, A.; Wilhelm, J.J.; Hering, B.J.; Kirchner, V.; Chinnakotla, S.; et al. Pre-operative Sarcopenia Predicts Low Islet Cell Yield Following Total Pancreatectomy with Islet Autotransplantation for Chronic Pancreatitis. J. Gastrointest. Surg. 2020, 24, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, I.V.; Bykova, A.P. Osteosarcopenia in chronic pancreatitis. Ter. Arkhiv. 2021, 93, 869–875. [Google Scholar] [CrossRef]
- Jalal, M.; Rosendahl, J.; Campbell, J.A.; Vinayagam, R.; Al-Mukhtar, A.; Hopper, A.D. Identification of “Digital Sarcopenia” Can Aid the Detection of Pancreatic Exocrine Insufficiency and Malnutrition Assessment in Patients with Suspected Pancreatic Pathology. Dig Dis. 2022, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.L.; Gärtner, S.; von Essen, N.; Doller, J.; Frost, F.; Tran, Q.T.; Weiss, F.U.; Meyer, F.; Valentini, L.; Garbe, L.A.; et al. Malnutrition Is Highly Prevalent in Patients With Chronic Pancreatitis and Characterized by Loss of Skeletal Muscle Mass but Absence of Impaired Physical Function. Front Nutr. 2022, 9, 889489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parhiala, M.; Ukkonen, M.; Sand, J.; Laukkarinen, J. Osteoporosis and sarcopenia are common and insufficiently diagnosed among chronic pancreatitis patients. BMC Gastroenterol. 2023, 23, 124. [Google Scholar] [CrossRef]
- Oyebola, T.; Mavilakandy, A.; Stephenson, J.A.; Boyce, R.; Bhardwaj, N.; Garcea, G. Sarcopenia: An Assessment into the Prevalence and Disease Burden in Chronic Pancreatitis Patients. J. Frailty Sarcopenia Falls 2023, 8, 38–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raman, R.; Shiran, S. Anthropometric Measurements as Clinical Indicators of Sarcopenia in Chronic Pancreatitis. Pancreatology 2023, 23, e17. [Google Scholar] [CrossRef]
- Matsumoto, R.; Kikuta, K.; Takikawa, T.; Sano, T.; Hamada, S.; Sasaki, A.; Sakano, M.; Hayashi, H.; Manaka, T.; Ikeda, M.; et al. Skeletal muscle mass and function are affected by pancreatic atrophy, pancreatic exocrine insufficiency and poor nutritional status in patients with chronic pancreatitis. Pancreatology 2024, 24, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Kuan, L.L.; Dennison, A.R.; Garcea, G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis: A Review of the Literature. World J. Surg. 2021, 45, 590–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shintakuya, R.; Uemura, K.; Murakami, Y.; Kondo, N.; Nakagawa, N.; Urabe, K.; Okano, K.; Awai, K.; Higaki, T.; Sueda, T. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology 2017, 17, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J. Gastroenterol. 2013, 19, 7258–7266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Girish, B.N.; Rajesh, G.; Vaidyanathan, K. Deficiency of folate and vitamin B12 increases oxidative stress in chronic pancreatitis patients. Indian J. Gastroenterol. 2022, 41, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Vujasinovic, M.; Hedström, A.; Maisonneuve, P.; Valente, R.; von Horn, H.; Löhr, J.M.; Haas, S.L. Zinc deficiency in patients with chronic pancreatitis. World J. Gastroenterol. 2019, 25, 600–607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babinets, L.; Palykhata, M. Pathogenetic and clinical features of iron deficiency anemia on the background of chronic pancreatitis. Bull. Club Pancreatol. 2017, 37, 36–39. [Google Scholar] [CrossRef]
- Ul Ain, Q.; Bashir, Y.; Kelleher, L.; Bourne, D.M.; Egan, S.M.; McMahon, J.; Keaskin, L.; Griffin, O.M.; Conlon, K.C.; Duggan, S.N. Dietary intake in patients with chronic pancreatitis: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 5775–5792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lankisch, M.R.; Imoto, M.; Layer, P.; DiMagno, E.P. The effect of small amounts of alcohol on the clinical course of chronic pancreatitis. Mayo Clin. Proc. 2001, 76, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Surinlert, P.; Thitiphatphuvanon, T.; Khimmaktong, W.; Pholpramoo, C.; Tipbunjong, C. Hyperglycemia induced C2C12 myoblast cell cycle arrest and skeletal muscle atrophy by modulating sirtuins gene expression in rats. Pol. J. Vet. Sci. 2021, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhullar, F.A.; Faghih, M.; Akshintala, V.S.; Ahmed, A.I.; Lobner, K.; Afghani, E.; Phillips, A.E.; Hart, P.A.; Ramsey, M.L.; Bick, B.L.; et al. Prevalence of primary painless chronic pancreatitis: A systematic review and meta-analysis. Pancreatology 2022, 22, 20–29, Erratum in Pancreatology 2022, 22, 448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, F.; Li, N.; Yang, J.; Yang, J.; Yang, W.; Ran, J.; Sun, P.; Liao, Y. The effect of resistance training on patients with secondary sarcopenia: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 28784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitcomb, D.C.; Buchner, A.M.; Forsmark, C.E. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatic Insufficiency: Expert Review. Gastroenterology 2023, 165, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klapdor, S.; Richter, E.; Klapdor, R. Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer. Res. 2012, 32, 1991–1998. [Google Scholar] [PubMed]
- Yamada, M.; Arai, H.; Yoshimura, K.; Kajiwara, Y.; Sonoda, T.; Nishiguchi, S.; Aoyama, T. Nutritional supplementation during resistance training improved skeletal muscle mass in community-dwelling frail older adults. J. Frailty Aging 2012, 1, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Amaral, T.F. Sarcopenia and length of hospital stay. Eur. J. Clin. Nutr. 2016, 70, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Kucheryavyy, Y.A.; Andreev, D.N. Exocrine pancreas insufficiency: Clinical significance and approaches to correction from evidence medicine. Ter. Arkh. 2021, 93, 509–515. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Chen, Y.H.; Liu, L.; Gao, J.H.; Tong, H.; Tang, C.W.; Liu, R. Efficacy and safety of pancreatic enzyme replacement therapy on exocrine pancreatic insufficiency: A meta-analysis. Oncotarget 2017, 8, 94920–94931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de la Iglesia-García, D.; Huang, W.; Szatmary, P.; Baston-Rey, I.; Gonzalez-Lopez, J.; Prada-Ramallal, G.; Mukherjee, R.; Nunes, Q.M.; Domínguez-Muñoz, J.E.; Sutton, R.; et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: Systematic review and meta-analysis. Gut 2017, 66, 1354–1355. [Google Scholar] [CrossRef]
- Iglesia, D.; Avci, B.; Kiriukova, M.; Panic, N.; Bozhychko, M.; Sandru, V.; de-Madaria, E.; Capurso, G. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 1115–1125. [Google Scholar] [CrossRef]
- Dominguez-Muñoz, J.E.; Vujasinovic, M.; de la Iglesia, D.; Cahen, D.; Capurso, G.; Gubergrits, N.; Hegyi, P.; Hungin, P.; Ockenga, J.; Paiella, S.; et al. European guidelines for the diagnosis and treatment of pancreatic exocrine insufficiency: UEG, EPC, EDS, ESPEN, ESPGHAN, ESDO, and ESPCG evidence-based recommendations. United Eur. Gastroenterol. J. 2025, 13, 125–172. [Google Scholar] [CrossRef]
- Barkin, J.A.; Westermann, A.; Hoos, W.; Moravek, C.; Matrisian, L.; Wang, H.; Shemanski, L.; Barkin, J.S.; Rahib, L. Frequency of appropriate use of pancreatic enzyme replacement therapy and symptomatic response in pancreatic cancer patients. Pancreas 2019, 48, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 January 2024).
- McPheeters, M.L.; Kripalini, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.C. Quality Improvement Interventions To Address Health Disparities. Evidence Report/Technology Assessment; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK117083/ (accessed on 20 January 2024).
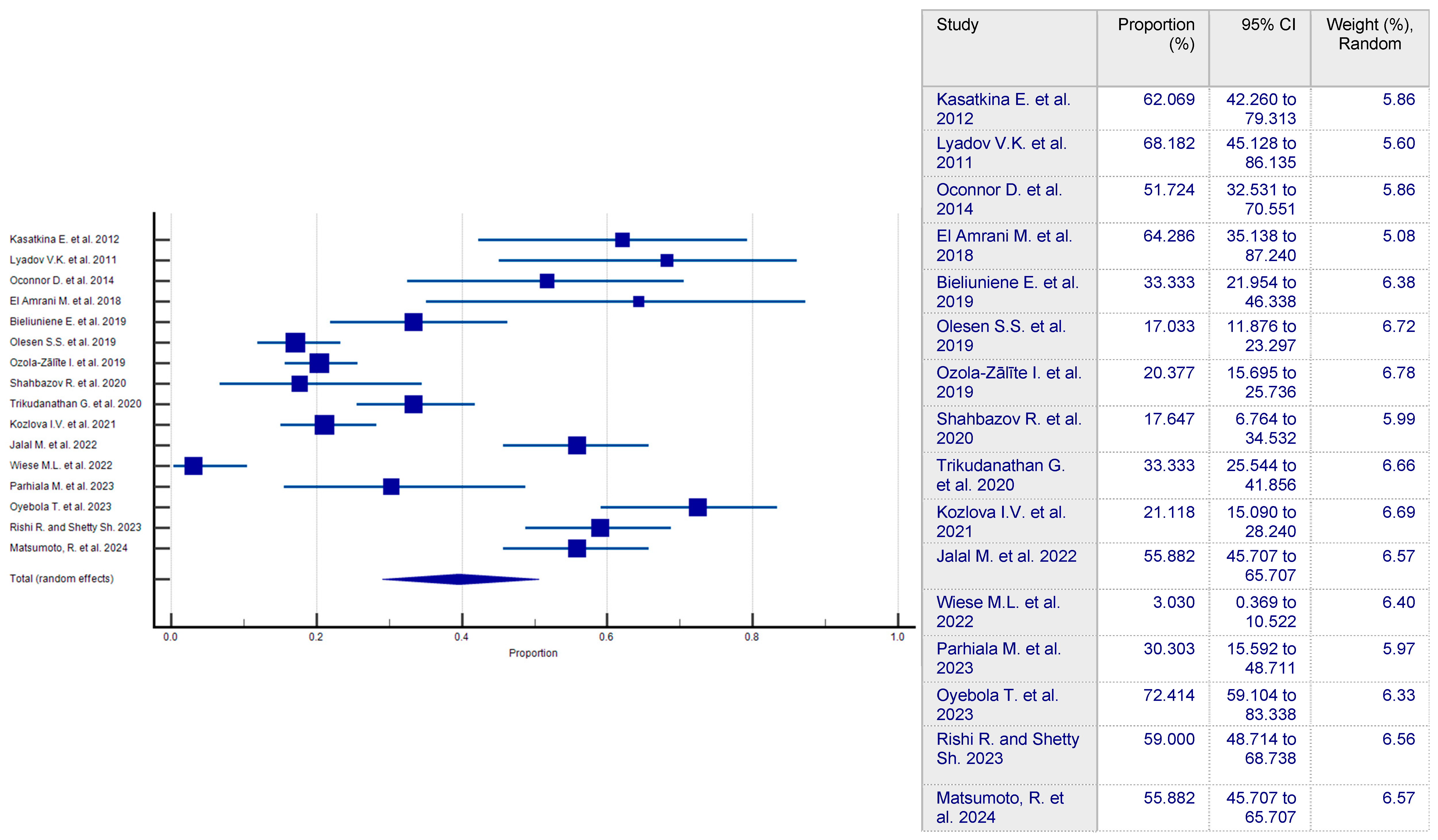
| Authorship and Year | Country | Approach to Sarcopenia Diagnosis | Number of CP Patients | Number of Sarcopenic Patients in CP Group | Number of Patients in Control Group | Number of Sarcopenic Patients in Control Group | Evaluation of Articles According to NOS Criteria |
|---|---|---|---|---|---|---|---|
| Kasatkina E. et al., 2012 [24] | Russia | SMI measured by abdominal CT scan L3 (<52.4 cm2/m2 for men and <38.5 cm2/m2 for women) | 29 | 18 | 0 | 0 | 4 |
| Lyadov V.K. et al., 2012 [25] | Russia | SMI measured by abdominal CT scan L3 (<52.4 cm2/m2 for men and <38.5 cm2/m2 for women) | 22 | 15 | 0 | 0 | 6 |
| Oconnor D. et al., 2014 [26] | Ireland | SMI measured by abdominal CT scan L3 | 29 | 15 | 0 | 0 | 4 |
| El Amrani M. et al., 2018 [27] | France | SMI measured by abdominal CT scan L3 (<52.4 cm2/m2 for men and <38.5 cm2/m2 for women) | 14 | 9 | 0 | 0 | 7 |
| Bieliuniene E. et al., 2019 [28] | Lithuania | SMI measured by abdominal CT/MRI/DEXA scan L3 (<45.4 cm2/m2 for men and <34.4 cm2/m2 for women) | 63 | 21 | 0 | 0 | 6 |
| Olesen S.S. et al., 2019 [29] | Denmark | Bioimpedance analysis with determination of the index of muscle (lean) mass (<10.76 kg/m2 for men; <6.76 kg/m2 for women) | 182 | 31 | 0 | 0 | 6 |
| Ozola-Zālīte I. et al., 2019 [30] | Lithuania | SMI measured by abdominal CT scan L4 (SMI < 41.3 cm2/m2 for men; <34.2 cm2/m2 for women) | 265 | 54 | 0 | 0 | 7 |
| Shahbazov R. et al., 2020 [31] | United States of America | CT scan to determine the thickness of the lumbar muscle (<492 mm2/m2 for men and <362 mm2/m2 for women) | 34 | 6 | 0 | 0 | 7 |
| Trikudanathan G. et al., 2020 [32] | United States of America | SMI measured by abdominal CT scan L3 (<52.4 cm2/m2 for men and <38.5 cm2/m2 for women) | 138 | 46 | 0 | 0 | 6 |
| Kozlova I.V. et al., 2021 [33] | Russia | The short physical performance battery—SPPB + handgrip strength (SPPB: ≤11 points in men and ≤10 points in women; handgrip strength: <27 kg for men and <16 kg for women) | 161 | 34 | 30 | 0 | 6 |
| Jalal M. et al., 2022 [34] | Great Britain | SMI measured by abdominal CT scan L3 (<41 cm2/m2 for women and <43 cm2/m2 if BMI 25 kg/m2 or 53 cm2/m2 if BMI ≥ 25 kg/m2 for men) | 102 | 57 | 0 | 0 | 6 |
| Wiese M.L. et al., 2022 [35] | Germany | SMI measured by abdominal CT scan L3 + handgrip strength (for men < 8.97 kg/m2, for women < 6.68 kg/m2, handgrip strength: <27 kg for men and <16 kg for women) | 66 | 2 | 66 | 0 | 8 |
| Parhiala M. et al., 2023 [36] | Finland | CT/MRI calculation of the Psoas muscle area (average area of both left and right lumbar muscles at the level of the middle of L3)—less than 800 mm2 for men and less than 550 mm2 for women | 33 | 10 | 0 | 0 | 8 |
| Oyebola T. et al., 2023 [37] | Great Britain | CT scan with determination of the psoas musculoskeletal index: total psoas muscle cross-sectional area at L3 level (cm2)/the patient’s height squared (m2) | 58 | 42 | 62 | 28 | 7 |
| Rishi R. and Shetty Sh. 2023 [38] | India | SMI measured by abdominal CT scan L3 | 100 | 59 | 0 | 0 | 4 |
| Matsumoto R. et al., 2024 [39] | Japan | SMI measured by abdominal CT scan L3 (42 cm2/m2 for men and 38 cm2/m2) + handgrip strength (for men less than 28 kg and less than 18 kg for women) | 102 | 57 | 0 | 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurmatullina, A.R.; Andreev, D.N.; Maev, I.V.; Kucheryavyy, Y.A.; Beliy, P.A.; Dzhafarova, A.R.; Cherenkova, V.V.; Sokolov, F.S. Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis. Nutrients 2025, 17, 870. https://doi.org/10.3390/nu17050870
Khurmatullina AR, Andreev DN, Maev IV, Kucheryavyy YA, Beliy PA, Dzhafarova AR, Cherenkova VV, Sokolov FS. Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis. Nutrients. 2025; 17(5):870. https://doi.org/10.3390/nu17050870
Chicago/Turabian StyleKhurmatullina, Alsu R., Dmitrii N. Andreev, Igor V. Maev, Yury A. Kucheryavyy, Petr A. Beliy, Aida R. Dzhafarova, Valeriya V. Cherenkova, and Filipp S. Sokolov. 2025. "Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis" Nutrients 17, no. 5: 870. https://doi.org/10.3390/nu17050870
APA StyleKhurmatullina, A. R., Andreev, D. N., Maev, I. V., Kucheryavyy, Y. A., Beliy, P. A., Dzhafarova, A. R., Cherenkova, V. V., & Sokolov, F. S. (2025). Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis. Nutrients, 17(5), 870. https://doi.org/10.3390/nu17050870






