Abstract
Background/Objectives: The skin, being the body’s outermost organ, plays a vital role in protecting against various external stimuli. Ultraviolet generates reactive oxygen species (ROS), promoting the secretion of matrix metalloproteinases (MMPs) and inducing collagen degradation. Many studies have been conducted to identify natural substances that can prevent or delay the harmful effects of UV. Methods: A wound healing assay, DCF-DA reactive oxygen species (ROS) assay, and JC-1 assay were performed to assess the effects of bio-converted eggplant peels (BEPs) on human dermal fibroblasts (HDFs). Western blot analysis was also conducted to understand the underlying mechanisms for their effects. Finally, hematoxylin–eosin staining and immunohistochemistry were also performed in animal studies. Results: Our study evaluated the antioxidant efficacy of BEPs fermented with Lactobacillus plantarum in hydrogen peroxide (H2O2)-HDFs and UVB-induced skin damage in hairless mice. We demonstrated that BEPs exhibited enhanced antioxidant properties compared to non-fermented eggplant peels (EPs). BEPs facilitated wound healing in H2O2-damaged HDFs, reduced ROS levels, and restored mitochondrial membrane potential. BEPs suppressed the phosphorylation of ERK, p38, and JNK as their underlying mechanism. We further demonstrated that dietary supplementation of BEPs also downregulated matrix metalloproteinase 1 (MMP1) expression and upregulated collagen I (COL1) in UVB-damaged hairless mice, indicating that BEPs were more effective compared to EPs. Conclusions: Our studies suggest that BEPs fermented with Lactobacillus plantarum hold significant potential as a protective agent for mitigating UVB-induced damage and promoting skin health.
1. Introduction
The skin is constantly exposed to various external stimuli that influence its morphology and function [1]. It plays a vital role in safeguarding the body from external insults and preserving homeostasis [2]. Structurally, the skin consists of the epidermis, dermis, and subcutaneous tissue. The epidermis serves as a physical barrier, preventing water loss and protecting against environmental damage. The dermis contains fibroblasts and the extracellular matrix (ECM), which are essential for maintaining skin elasticity and strength [3,4,5]. The subcutaneous tissue, primarily composed of adipose cells, serves as a cushion and energy reservoir [6].
The skin is subjected to various external stressors, including ultraviolet (UV) radiation, pollutants, and chemical irritants. Ultraviolet (UV) radiation causes oxidative stress [7,8]. Excessive ROS production leads to oxidative damage, targeting proteins and DNA, and ultimately impairing cellular function and survival [9,10]. Beyond oxidative stress, chronic UV exposure causes skin carcinogenesis by inducing direct DNA damage. UV radiation generates 6-4 photoproducts (6-4PPs) and cyclobutane pyrimidine dimers (CPDs), which induce mutations in key regulatory genes. The severity of UV-induced damage depends on multiple factors, including age at exposure, frequency, duration, geographic location, and individual skin phototype [11,12,13].
Excessive ROS levels trigger the activation of the mitogen-activated protein kinase (MAPK) pathway, which promotes the expression of matrix metalloproteinases (MMPs) and accelerates collagen degradation, resulting in structural damage [14,15,16]. Moreover, ROS-induced mitochondrial dysfunction disrupts energy metabolism, exacerbating oxidative stress and further compromising skin health [17]. These processes collectively undermine the structural and functional integrity of the skin, resulting in visible signs of aging.
To counteract the detrimental effects of ROS, extensive research has focused on identifying natural antioxidants capable of mitigating oxidative stress. Plant-derived compounds, in particular, have demonstrated significant potential due to their potent antioxidant properties [18]. Solanum melongena L. (eggplant) reduces chronic degenerative diseases when included in the diet, primarily owing to the bioactive ingredients present in its peel [19,20,21]. Furthermore, the peel of eggplant is especially concentrated with these bioactive compounds, exhibiting potent antioxidant activity [22,23].
Probiotics are live microorganisms that, when taken in sufficient quantities, offer health advantages to the host and contribute to immune system regulation [24,25,26]. Recent investigations into probiotic microorganisms as natural antioxidants have shown promising results. Lactobacillus, a major constituent of probiotics, has demonstrated significant antioxidant potential in various studies on lactic acid bacteria [27,28,29,30]. Additionally, certain bacterial products with antioxidant activity have been identified as key players in skincare [31]. Recent studies highlighted the beneficial effects of Lactobacillus plantarum to alleviate symptoms of diabetes in human subjects [32,33] while exhibiting notable anti-inflammatory effects [34,35].
In this study, we selected Lactobacillus plantarum for the fermentation of eggplant peel extracts (BEPs) due to its well-documented antioxidant and anti-inflammatory properties. Previous studies have reported that fermentation using L. plantarum enhances the bioavailability of polyphenols and other bioactive compounds, potentially augmenting their protective effects against oxidative stress [36,37]. Thus, we hypothesized that fermenting eggplant peel with L. plantarum would enhance its antioxidant capacity and improve its protective effects against ROS-induced skin damage.
Based on these findings, this study evaluated whether eggplant peel extracts fermented with Lactobacillus plantarum (BEPs) could mitigate skin damage in human dermal fibroblasts (HDFs) caused by H2O2.
2. Materials and Methods
2.1. Preparation of Fermented Solanum melongena L. (Eggplant) Peel Extracts
Solanum melongena L. (eggplant) peel was dried at 40 °C in an oven for 3 days to remove moisture and then subjected to triple extraction with 70% ethanol at room temperature for 2 h each time, using 30 times the dry weight. The evaporation process was carried out using a rotary evaporator, and fermentation was conducted using Lactiplantibacillus plantarum. Non-fermented eggplant peel extracts (EPs) and fermented eggplant peels, referred to as bio-converted eggplant peels (BEPs), were prepared and used for the experiments, respectively. Furthermore, EPs without incubation were labeled as EP-not Inc, while the remaining extracts subjected to incubation were designated as EP-Inc, BEPs 48-h, and BEPs 72-h. In addition, the extraction yield was determined to be 22% relative to the dry weight. These samples were dissolved in cell/tissue culture-grade water and then stored at −20 °C.
2.2. Cell Culture
Human dermal fibroblasts (HDFs) (PromoCell (Heidelberg, Germany)) were cultured in 10% fetal bovine serum (FBS)-DMEM (Welgene, Gyeongsan-si, Republic of Korea) in a 5% CO2 incubator at 37 °C.
2.3. Measurement of Cytotoxicity
Cell proliferation was detected with a cell proliferation kit (MTT) (Roche, Mannheim, Germany). HDFs were seeded on a 96-well plate. EPs were applied to the cells at various concentrations (0, 10, 50, and 100 ppm) and incubated for 24 h. After incubation, the DMEM containing EPs or BEPs was removed and washed with DPBS. Subsequently, the MTT reagent was incubated for 4 h. Following the reaction, 100 µL of solubilization buffer was incubated for 30 min. The absorbance was read using a microplate reader (BioTek Multi-Mode Microplate Reader, Winooski, VT, USA) at 570 nm.
2.4. Wound-Healing Analysis
HDFs were seeded in a 60 mm dish and cultured until over 80% confluence was reached. A scratch was then created at the center of the dish using a 200 μL pipette tip. After removing the culture medium, fresh medium was added, and various concentrations of Eps, BEPs (0, 50, and 100 ppm), and H2O2 (200 μM) were treated for 24 h, and images were obtained using phase-contrast microscopy (Microscope ECLIPSE Ts2, Nikon, Tokyo, Japan) at 0 and 24 h for comparison; % Migration area = (A0 − An)/A0 × 100 (A0: the initial area, An: the remaining area) [38].
2.5. Assessment of Intracellular ROS
The fluorescent marker 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) (Sig-ma-Aldrich, St. Louis, MO, USA) was used for detecting ROS from the H2O2. HDFs were seeded on a 96-well black plate and cultured for 24 h. Subsequently, EPs and BEPs were applied to the cells at various concentrations (0, 50, and 100 ppm) and incubated for 24 h. Cells were stained with 20 mM DCF-DA for 45 min. Fluorescence intensity was detected at 485/528 nm using a BioTek Synergy HTX multimode reader (Winooski, VT, USA).
Additionally, fluorescence analysis was performed to observe DCF-DA staining. HDFs were plated on a confocal dish. The cells were treated with EPs and BEPs (0, 50, and 100 ppm) for 24 h. Cells were stained with 10 μM DCF-DA for 15 min for imaging analysis. Each image was visualized using a Nikon Eclipse Ti2 fluorescence live-cell imaging microscope (Nikon, Tokyo, Japan).
2.6. Membrane Potential of Mitochondria
The mitochondrial membrane potential was measured with a JC-1 assay kit (Abcam, Cambridge, UK). HDFs were plated on confocal dishes and treated with EPs and BEPs (100 ppm) for 24 h. Cells were washed with DPBS and exposed to H2O2 (200 μM). Cells were washed again and incubated with JC-1 solution at 5 μM for 15 min. Fluorescence images were acquired using a Nikon Eclipse Ti2 microscope (Nikon, Tokyo, Japan).
2.7. Western Blot Analysis
HDFs were seeded in a 100 mm dish and cultured in a CO2 incubator. Cells were treated with EPs and BEPs at a concentration of 100 ppm for 24 h, followed by an additional 2 h treatment with H2O2 (200 μM). Cells were lysed using RIPA buffer containing a phosphatase inhibitor cocktail. The lysates were centrifuged at 12,000, and protein levels were determined by the BCA assay. Proteins (40 μg/μL) were resolved by SDS-PAGE and transferred onto PVDF membranes. Following blocking with 5% skim milk, the membranes were incubated with primary antibodies against p-ERK, total ERK, p-p38, total p38, p-JNK, and total JNK, followed by secondary antibody incubation at room temperature for 2 h, and were washed with TBS-T. The membranes were reacted with an ECL reagent, and images were captured using the Invitrogen iBright 1500 imaging system (Waltham, MA, USA). The results were analyzed using ImageJ software version 2.9.0 (National Institutes of Health, Bethesda, MD, USA).
2.8. In Vivo Antioxidant Effect of BEPs
Male hairless mice (15–20 g, 4 weeks old) were purchased from Samtaco (Osan, Pyeongtaek, Republic of Korea). They were adapted for one week before the experiment. All animal experiments were performed following the standard guidelines of the Institutional Animal Care and Use Committee (IACUC) at Konkuk University (reference number: KU23213). The mice group were divided into six groups (n = 7 per group): (1) control (no UVB, 0.9% saline, 200 µL), (2) UVB-only (100–120 mJ/cm2), (3) UVB + EPs (200 mg/kg), and (4) UVB + BEPs (200 mg/kg). UV irradiation (Vilber, Collégien, France) was applied every two days (1.5 cm2, 100–120 mJ/cm2) for approximately 10 sessions until visible wrinkling appeared. Samples (200 µL) were orally administered using a gavage needle 5 days a week for a duration of 4 weeks. Skin wrinkle progression was monitored weekly to determine the experimental endpoint.
2.9. Histological Analysis
Dorsal skin tissues were harvested for analysis. The excised tissues were fixed in 10% formalin (HuBENTech, Damyang, Republic of Korea). Hematoxylin and Eosin (H&E), immunohistochemistry (IHC), and Masson’s trichrome (MT) were performed in KP&T Technology Company (Osong, Cheongju-si, Republic of Korea). IHC staining was assessed for the expression of collagen I (COL1) and matrix metalloproteinase 1 (MMP1). Quantification of the MT, COL1, and MMP1-stained tissues was carried out using ImageJ software version 2.9.0
2.10. Statistical Analysis
The results are presented as the mean ± standard deviation (SD). Statistical analyses were conducted using GraphPad Prism 5.0 software (San Diego, CA, USA) employing one-way analysis of variance (ANOVA) with Tukey’s Multiple Comparison Test. Statistical significance was considered for p values < 0.05.
3. Result
3.1. Effect of EPs and BEPs on the Viability of HDFs
The potential cytotoxicity of EPs and BEPs on HDFs was evaluated using the MTT assay. HDFs were administered with each extract at varying concentrations (10, 50, and 100 ppm). Cell viability exceeded 90% across all concentrations, indicating no significant cytotoxic effects of EPs or BEPs on HDFs (Figure 1). Based on these findings, 50 ppm and 100 ppm concentrations of EPs and BEPs were selected for further efficacy analysis.
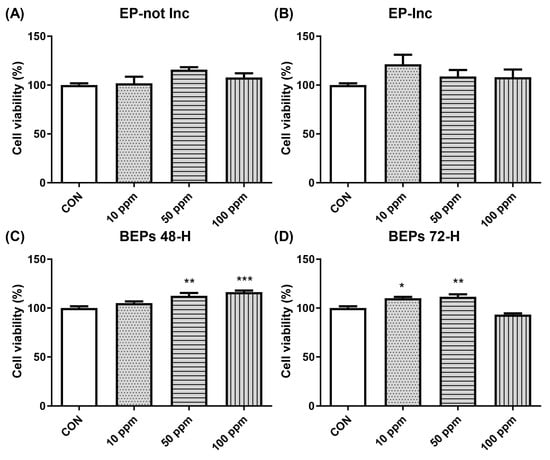
Figure 1.
The cell viability of both EPs and BEPs in HDFs. (A) EP-not Inc, (B) EP-Inc, (C) BEPs 48-h, and (D) BEPs 72-h at various concentrations were assessed for their effects on cell viability (n = 4). Data are presented as mean values ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, compared to the control group.
3.2. Wound-Healing Effects of BEPs in H2O2-Damaged HDFs
The migration capacity of HDFs plays a pivotal role in skin regeneration and wound-healing processes [39]. To evaluate this, a scratch assay was performed to investigate the effects of EPs and BEPs on cell migration in H2O2-damaged HDFs. HDFs were scratched and treated with H2O2 (200 μM), followed by the application of EPs and BEPs at concentrations of 100 ppm (Figure 2A), respectively. Wound-healing efficiency was assessed over time. After 24 h, all EPs and BEPs exhibited wound-healing activity, as evidenced by a graphical analysis of cell migration (Figure 2B). Among the treatments, BEPs 72-h demonstrated the highest wound-healing efficacy compared to other samples.
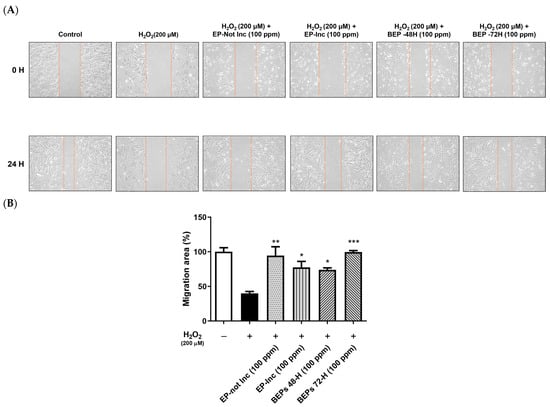
Figure 2.
BEPs promoted wound healing in H2O2-damaged HDFs. (A) The wound-healing effects of EPs and BEPs were assessed (n = 3). (B) The wound closure area corresponding to EP and BEP treatments is presented (n = 3). Data are presented as mean values ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, compared to the H2O2-damaged group.
3.3. Inhibitory Effect of BEPs on ROS Generation in H2O2-Damaged HDFs
Excessive oxidative stress causes cellular damage, particularly in the skin, where it accelerates aging, inflammation, and the formation of wrinkles [40,41]. ROS are byproducts of normal cellular metabolism. However, under stress conditions, their accumulation induces oxidative damage to cellular structures [42,43]. The effects of EPs and BEPs inhibiting ROS production in H2O2-damaged HDFs were evaluated using a DCF-DA assay. The results demonstrated an elevated level of ROS levels in the H2O2-damaged group, whereas the BEP group exhibited a marked reduction in ROS levels compared to the EP-not Inc group. Among the treatments, the BEPs 72-h group demonstrated the most effective inhibition of ROS production (Figure 3A). Additionally, DCF-DA fluorescence was observed in HDFs using fluorescence microscopy. The elevated ROS levels in the H2O2-damaged group were significantly reduced in the BEPs 72-h group (Figure 3B), suggesting that the BEPs 72-h sample possesses higher potential activity in reducing H2O2-induced ROS compared to EP samples.
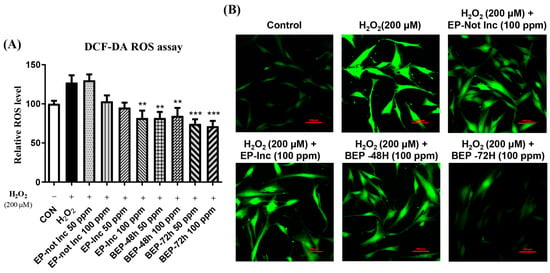
Figure 3.
BEPs decreased ROS levels in H2O2-damaged HDFs. (A) The antioxidant capacity of EPs and BEPs (50, 100 ppm) was evaluated using the DCF-DA assay (n = 3). (B) DCF-DA fluorescence images of EPs and BEPs were captured using fluorescence microscopy (n = 3). Data were presented as mean values ± SD. ** p < 0.05, *** p < 0.001, compared to the H2O2-damaged group.
3.4. Effects of BEPs on the Membrane Potential of Mitochondria in H2O2-Damaged HDFs
Mitochondria are essential for regulating cellular metabolism and homeostasis by generating ATP and defending against oxidative stress [44,45]. In the skin, mitochondria support critical processes such as aging prevention, wound healing, and cellular repair, thereby preserving structural and functional integrity [46]. Mitochondrial membrane potential (ΔΨm) serves as a vital indicator of mitochondrial health, regulating ATP production and cellular redox balance. Loss of ΔΨm is associated with increased ROS generation and exacerbation of skin damage [47]. The JC-1 mitochondrial membrane potential assay was performed to assess the effects of EPs and BEPs on changes in ΔΨm in H2O2-damaged HDFs. A polarized mitochondrial membrane potential is signified by the red fluorescence of JC-1 dimers, whereas depolarized membrane potential is represented by the green fluorescence of JC-1 monomers [48]. Treatment with EPs or BEPs at a concentration of 100 ppm in H2O2-damaged HDFs resulted in a reduction in green fluorescence and an increase in red fluorescence, similar to the control group (Figure 4). These findings suggest that EPs and BEPs effectively restore mitochondrial membrane potential in H2O2-damaged HDFs.
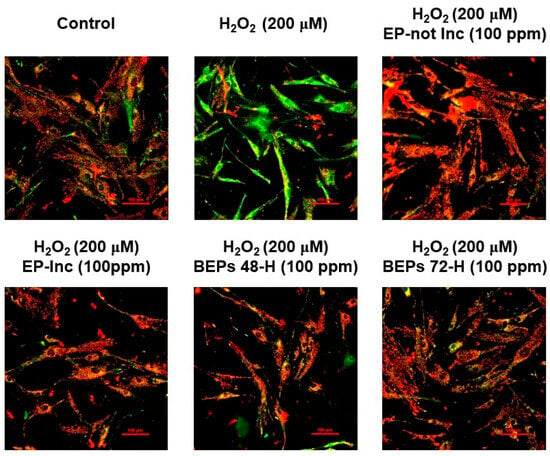
Figure 4.
Effects of EPs and BEPs on the mitochondrial membrane potential in H2O2-damaged HDFs. The membrane potential of mitochondria was assessed using JC-1 in response to treatment with EPs and BEPs (n = 3). These images were representative images of three independent experiments (scale bars, 100 µm).
3.5. Effect of BEPs on the Phosphorylation Levels of MAPKs in H2O2-Damaged HDFs
In the MAP kinase (MAPK) signaling pathway, membrane receptors are activated in response to ROS, leading to the phosphorylation of MAPKs, which triggers inflammatory responses and oxidative stress [49,50]. Changes in MAPK phosphorylation were analyzed using a Western blot analysis. In H2O2-damaged HDFs, an increase in the phosphorylation levels of ERK, p38, and JNK was observed. Importantly, the BEPs 72-h treatment group exhibited a significant decrease in the phosphorylation levels of ERK, p38, and JNK compared to the EPs-treated group (Figure 5). These findings indicate that BEPs, particularly after fermentation, exhibit superior efficacy in suppressing MAPK phosphorylation.
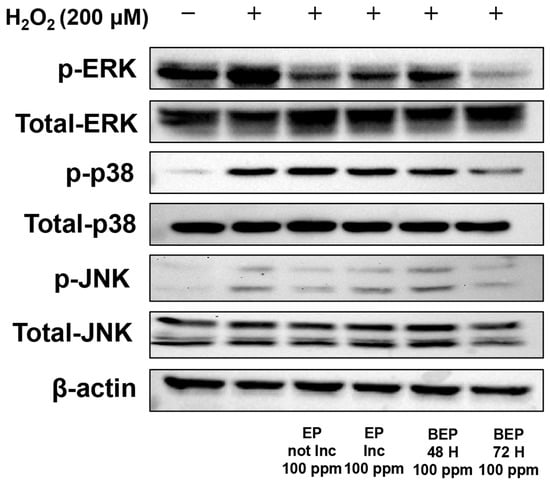
Figure 5.
Effect of BEPs on the phosphorylation levels of MAPKs in H2O2-damaged HDFs. Western blot analysis was performed to assess the inhibitory effects of EPs and BEPs on MAPKs. This image was a representative image from 3 independent experiments.
3.6. Effect of BEPs on UVB-Induced Skin Damage
We further investigated whether EPs and BEPs have beneficial effects on UVB-induced skin damage using hairless mice. All treatment groups, except for the control group, were orally administered the test substances at 200 mg/kg (200 μL) five times per week for three weeks. Skin moisture content was measured weekly throughout the experimental period. Compared to the UVB group, the BEPs group demonstrated significant improvements in skin health, as confirmed by H&E staining (Figure 6). Moisture content measurements further supported these findings, indicating that the fermented extracts contributed to the maintenance of skin hydration. Moreover, the inhibition of MMP1 observed in the fermented extracts group was associated with increased collagen production. Our results suggest that BEPs remarkably mitigate UVB-induced skin damage by improving skin hydration, inhibiting MMP1, and enhancing collagen production, highlighting its potential as a promising agent for improving skin health under UVB-induced stress.
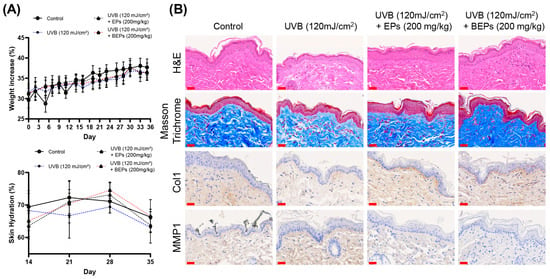
Figure 6.
BEPs improve on UVB-induced skin damage. (A) Body weights were recorded for each experimental group (n = 7). Skin moisture content was assessed on days 14, 21, 28, and 35 during the experimental period (n = 7). (B) Histological analysis was performed on tissue sections using H&E staining, Masson staining, and immunohistochemical analysis. These images are one of three independent experiments (scale bars, 30 µm).
4. Discussion
The skin serves as a protective barrier, covering the body’s exterior and playing a vital role in maintaining homeostasis [51]. Beyond its physical barrier function, the skin is critical for thermoregulation, moisture retention, and immune defense, contributing to overall health and functionality. However, the skin is exposed to external factors, such as ultraviolet (UV) radiation, which can induce structural and functional alterations. These changes highlight the significance of understanding the skin’s protective mechanisms to mitigate damage and preserve its integrity [52,53].
UV radiation is known to generate ROS, leading to various detrimental changes in the skin [54,55]. ROS activate MAPKs, which increase MMPs, resulting in the degradation of collagen and subsequent loss of skin elasticity [56,57,58]. Furthermore, mitochondrial dysfunction, a critical regulator of cellular energy metabolism, disrupts energy homeostasis and exacerbates oxidative stress [59,60]. These detrimental effects collectively compromise the structural integrity of the skin.
To counteract the adverse effects of ROS, significant research efforts have been directed toward identifying natural antioxidants. Plant-derived compounds are particularly noteworthy for their potent antioxidant properties [18]. Plants belonging to the Solanum genus, such as eggplants, are predominantly found in warm regions worldwide and have been traditionally utilized in both medicine and food applications. These plants are rich in bioactive compounds, including phenols, saponins, flavonoids, and carotenoids, which contribute to their health-promoting properties [61,62]. Eggplants, in particular, are recognized for their high nutritional value and potent antioxidant activity, positioning them as a promising source of antioxidants beneficial for human health [63,64]. These attributes emphasize a potential therapeutic agent for alleviating oxidative stress, reducing inflammation, and promoting skin health.
In this study, we fermented eggplant peel using Lactobacillus plantarum to investigate changes in its efficacy. ROS generation was induced in HDFs through H2O2 treatment, and the antioxidant potential of fermented BEPs was evaluated. BEPs effectively suppressed ROS production and inhibited the activity of MAPKs. Additionally, BEPs improved mitochondrial dysfunction and demonstrated significant wound-healing effects. In UVB-induced hairless mice models, BEPs reduced MMP1 expression and enhanced collagen production. These findings suggest that BEPs possess superior antioxidant properties compared to non-fermented eggplant peel extracts (EPs) and play a critical role in protecting and improving skin health by promoting collagen synthesis and reducing oxidative stress (Figure 7).
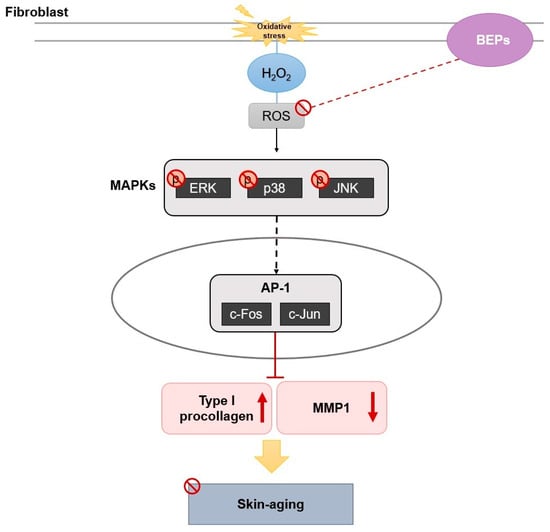
Figure 7.
The mechanism of BEPs in UVB-induced skin damage.
Collectively, the results indicate that fermentation with Lactobacillus plantarum enhances the antioxidant and therapeutic properties of eggplant peel, highlighting its potential as a protective and functional food agent against UVB-induced skin damage.
Although the antioxidant and anti-inflammatory properties of BEPs were studied, more research is needed to investigate other biological pathways, including immune modulation and interactions with the gut microbiota. Additionally, the bioavailability and long-term stability of BEPs are not yet fully characterized and require further assessment. Further studies will lead to a deeper understanding of BEPs and broaden their potential applications.
5. Conclusions
In conclusion, BEPs effectively reduced ROS levels and the activity of MAPKs, improved mitochondrial dysfunction, and suppressed MMP1 expression while promoting collagen production and wound healing. These results suggest that fermentation with Lactobacillus plantarum enhances the antioxidant and protective properties of eggplant peels. Specifically, BEPs demonstrated significant efficacy in mitigating UVB-induced oxidative stress by inhibiting ROS accumulation, downregulating MAPK signaling, and promoting collagen synthesis, thereby contributing to skin protection and repair.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17050847/s1.
Author Contributions
Conceptualization, J.H.L., J.K. and D.W.S.; methodology, J.H.L. and J.K.; validation, J.H.L. and J.K.; investigation, J.H.L., J.K. and Y.H.J. (Yun Hoo Jo); resources, Y.C.J., Y.H.J. (Yeong Hwan Jeong) and S.A.J.; data curation, J.K., J.H.L.; writing—original draft preparation, J.H.L. and J.K.; writing—review and editing, B.O.L. and D.W.S.; administration, D.W.S.; funding acquisition, B.O.L. and D.W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Konkuk University (reference number: KU23213, 13 October 2023) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by the Technology Development Program (RS-2023-00226342) and funded by the Ministry of SMEs and Startups (MSS, Korea).
Conflicts of Interest
B.O.L. and S.A.J were employed by Human Bioscience Corp. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BEPs | Bio-converted eggplant peels |
| COL1 | Collagen I |
| Eps | Eggplant peels |
| HDFs | Human dermal fibroblasts |
| MMPs | Matrix metallopeptidase |
| MAPK | Mitogen-activated protein kinase |
| UVB | Ultraviolet B |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| HDFs | Human dermal fibroblasts |
| DCFDA | 2′, 7′ -dichlorodihydrofluorescein diacetate |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| FBS | Fetal bovine serum |
| DMEM | High-glucose Dulbecco’s modified Eagle medium |
| IHC | Immunohistochemistry |
| H&E | Hematoxylin and eosin |
| MT | Masson’s trichrome |
| CPDs | Cyclobutane pyrimidine dimers |
| 6-4PPs | 6-4 photoproducts |
References
- Csekes, E.; Rackova, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, J.; Kim, J.; Jung, T.; Cho, B.; Choi, S.; Lee, D.; Kim, J.; Kim, J.; Lee, O. Ulmus Macrocarpa Hance Extracts Attenuated H(2)O(2) and UVB-Induced Skin Photo-Aging by Activating Antioxidant Enzymes and Inhibiting MAPK Pathways. Int. J. Mol. Sci. 2017, 18, 1200. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kwon, S.; Choi, J.; Na, J.; Huh, C.; Choi, H.; Park, K. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Philippeos, C.; Telerman, S.B.; Oules, B.; Pisco, A.O.; Shaw, T.J.; Elgueta, R.; Lombardi, G.; Driskell, R.R.; Soldin, M.; Lynch, M.D.; et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J. Investig. Dermatol. 2018, 138, 811–825. [Google Scholar] [CrossRef]
- Harper, R.A.; Grove, G. Human Skin Fibroblasts Derived from Papillary and Reticular Dermis: Differences in Growth Potential in Vitro. Science 1979, 204, 526–527. [Google Scholar] [CrossRef]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H.; Rho, N.; Park, K.Y. Skin Aging from Mechanisms to Interventions: Focusing on Dermal Aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV Irradiation and Dermal Photoaging. J. Photochem. Photobiol. B. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Rysava, A.; Vostalova, J.; Rajnochova Svobodova, A. Effect of Ultraviolet Radiation on the Nrf2 Signaling Pathway in Skin Cells. Int. J. Radiat. Biol. 2021, 97, 1383–1403. [Google Scholar] [CrossRef]
- Brenneisen, P.; Briviba, K.; Wlaschek, M.; Wenk, J.; Scharffetter-Kochanek, K. Hydrogen Peroxide (H2O2) Increases the Steady-State mRNA Levels of Collagenase/MMP-1 in Human Dermal Fibroblasts. Free Radic. Biol. Med. 1997, 22, 515–524. [Google Scholar]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-Induced Skin Damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [PubMed]
- Kim, J.; Kim, S.Y.; Noh, E.; Song, H.; Lee, G.; Kwon, K.; Lee, Y. Reversine Inhibits MMP-1 and MMP-3 Expressions by Suppressing of ROS/MAPK/AP-1 Activation in UV-Stimulated Human Keratinocytes and Dermal Fibroblasts. Exp. Dermatol. 2018, 27, 298–301. [Google Scholar] [CrossRef]
- Jang, H.; Kim, G.; Kim, J.; Kang, S.Y.; Youn, H.; Park, J.; Ro, S.Y.; Chung, E.; Park, K.; Kim, J. Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes. Int. J. Mol. Sci. 2023, 24, 17358. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in Skin Health, Aging, and Disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Kim, J.; Go, M.Y.; Jeon, C.Y.; Shin, J.U.; Kim, M.; Lim, H.W.; Shin, D.W. Pinitol Improves Diabetic Foot Ulcers in Streptozotocin-Induced Diabetes Rats through Upregulation of Nrf2/HO-1 Signaling. Antioxidants 2024, 14, 15. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Tapia-Hernandez, J.A.; Ruiz-Cruz, S.; Marquez-Rios, E.; Ornelas-Paz, J.d.J.; Del-Toro-Sanchez, C.L.; Ocano-Higuera, V.M.; Rodriguez-Felix, F.; Estrada-Alvarado, M.I.; Cira-Chavez, L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and in Vitro Release. Nanomaterials 2022, 12, 2303. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of Ultrasound-Assisted Extraction of Total Monomeric Anthocyanin (TMA) and Total Phenolic Content (TPC) from Eggplant (Solanum melongena L.) Peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef]
- Docimo, T.; Francese, G.; Ruggiero, A.; Batelli, G.; De Palma, M.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Mennella, G.; Tucci, M. Phenylpropanoids Accumulation in Eggplant Fruit: Characterization of Biosynthetic Genes and Regulation by a MYB Transcription Factor. Front. Plant Sci. 2016, 6, 1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shen, L.; Yu, J.; Wan, H.; Guo, A.; Chen, J.; Long, Y.; Zhao, J.; Pei, G. Rapamycin and Other Longevity-Promoting Compounds Enhance the Generation of Mouse Induced Pluripotent Stem Cells. Aging Cell 2011, 10, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Total Monomeric Anthocyanin, Total Phenolic Content and Antioxidant Activity of Extracts from Eggplant (Solanum melongena L.) Peel using Ultrasonic Treatments. J. Food Process Eng. 2015, 40, e12312. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, J.; Lee, N.; Paik, H. Antioxidant and Anti-Inflammatory Effect of Probiotic Lactobacillus Plantarum KU15149 Derived from Korean Homemade Diced-Radish Kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus Plantarum and its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Hong, S.M.; Kang, M.C.; Jin, M.; Lee, T.H.; Lim, B.O.; Kim, S.Y. Fermented Blueberry and Black Rice Containing Lactobacillus Plantarum MG4221: A Novel Functional Food for Particulate Matter (PM(2.5))/Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis. Food Funct. 2021, 12, 3611–3623. [Google Scholar] [CrossRef]
- Rwubuzizi, R.; Kim, H.; Holzapfel, W.H.; Todorov, S.D. Beneficial, Safety, and Antioxidant Properties of Lactic Acid Bacteria: A Next Step in their Evaluation as Potential Probiotics. Heliyon 2023, 9, e15610. [Google Scholar] [CrossRef]
- DUz, M.; DoGan, Y.N.; DoGan, I. Antioxidant Activitiy of Lactobacillus Plantarum, Lactobacillus Sake and Lactobacillus Curvatus Strains Isolated from Fermented Turkish Sucuk. An. Acad. Bras. Cienc. 2020, 92, e20200105. [Google Scholar] [CrossRef]
- AlKalbani, N.S.; Turner, M.S.; Ayyash, M.M. Isolation, Identification, and Potential Probiotic Characterization of Isolated Lactic Acid Bacteria and in Vitro Investigation of the Cytotoxicity, Antioxidant, and Antidiabetic Activities in Fermented Sausage. Microb. Cell Factories 2019, 18, 188. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Taneyo Saa, D.L.; Gianotti, A. Changes in Carotenoids, Phenolic Acids and Antioxidant Capacity in Bread Wheat Doughs Fermented with Different Lactic Acid Bacteria Strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef]
- Tsai, W.; Chou, C.; Chiang, Y.; Lin, C.; Lee, C. Regulatory Effects of Lactobacillus Plantarum-GMNL6 on Human Skin Health by Improving Skin Microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus Plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Tsai, W.; Jheng, Y.; Su, S.; Wang, S.; Lin, C.; Chen, Y.; Chang, W. The Beneficial Effects of Lactobacillus Reuteri ADR-1 Or ADR-3 Consumption on Type 2 Diabetes Mellitus: A Randomized, Double-Blinded, Placebo-Controlled Trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]
- Chang, C.; Lin, T.; Tsai, Y.; Wu, T.; Lai, W.; Lu, C.; Lai, H. Next Generation Probiotics in Disease Amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Efficacy of Lactobacillus Plantarum in Prevention of Inflammatory Bowel Disease. Toxicol. Rep. 2018, 5, 314–317. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic Fermentation of Polyphenols: Potential Sources of Novel Functional Foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus Plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.; Wang, Z.; Cao, J.; Tan, Y.; Luo, J.; Li, H.; Zhang, W.; Chen, C.; Xie, H. Exosomes from Human Umbilical Cord Blood Accelerate Cutaneous Wound Healing through miR-21-3p-Mediated Promotion of Angiogenesis and Fibroblast Function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, L.; Hui, L.; Lu, Y. Exploring the Role of Dermal Sheath Cells in Wound Healing and Fibrosis. Wound Repair Regen. 2024, 32, 735–745. [Google Scholar] [CrossRef]
- Ma, W.; Wlaschek, M.; Tantcheva-Poor, I.; Schneider, L.A.; Naderi, L.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Chronological Ageing and Photoageing of the Fibroblasts and the Dermal Connective Tissue. Clin. Exp. Dermatol. 2001, 26, 592–599. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the Skin from Phenotype to Mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of Metabolic H2O2 Generation: Redox Signaling and Oxidative Stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Aflatooni, S.; Bhatia, S.; Sivamani, R.K. Dermatologic Manifestations of Mitochondrial Dysfunction: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 3303. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 21, 49. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.G.; Lee, H.; Lim, S.J.; Nho, C.W. Youngiasides A and C Isolated from Youngia Denticulatum Inhibit UVB-Induced MMP Expression and Promote Type I Procollagen Production Via Repression of MAPK/AP-1/NF-kappaB and Activation of AMPK/Nrf2 in HaCaT Cells and Human Dermal Fibroblasts. J. Agric. Food Chem. 2015, 63, 5428–5438. [Google Scholar] [CrossRef]
- Bonifant, H.; Holloway, S. A Review of the Effects of Ageing on Skin Integrity and Wound Healing. Br. J. Community Nurs. 2019, 24, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.B.; Gibbs, N.K.; Griffiths, C.E.M.; Sherratt, M.J. Damage to Skin Extracellular Matrix Induced by UV Exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-Light-Induced Signal Cascades and Skin Aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Fisher, G.J.; Voorhees, J.J. Molecular Mechanisms of Photoaging and its Prevention by Retinoic Acid: Ultraviolet Irradiation Induces MAP Kinase Signal Transduction Cascades that Induce Ap-1-Regulated Matrix Metalloproteinases that Degrade Human Skin in Vivo. J. Investig. Dermatol. Symp. Proc. 1998, 3, 61–68. [Google Scholar] [PubMed]
- Curran, S.; Murray, G.I. Matrix Metalloproteinases in Tumour Invasion and Metastasis. J. Pathol. 1999, 189, 300–308. [Google Scholar] [CrossRef]
- Kahari, V.M.; Saarialho-Kere, U. Matrix Metalloproteinases in Skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E.; Kim, J.H.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Kim, J.; Kim, S.H.; Park, C.; Seo, D.B.; et al. Antimelanogenesis and Skin-Protective Activities of Panax Ginseng Calyx Ethanol Extract. J. Ginseng Res. 2018, 42, 389–399. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Trifunovic, A.; Larsson, N. Mitochondrial Dysfunction as a Cause of Ageing. J. Intern. Med. 2008, 263, 167–178. [Google Scholar] [CrossRef]
- Kaunda, J.S.; Zhang, Y. The Genus Solanum: An Ethnopharmacological, Phytochemical and Biological Properties Review. Nat. Prod. Bioprospect 2019, 9, 77–137. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Romero, C.A.; Montoya-Inzunza, L.A.; Contreras-Angulo, L.A.; Heredia, J.B.; Gutierrez-Grijalva, E.P. Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and their Relationship with their Health-Promoting Effects. Front. Nutr. 2021, 8, 790582. [Google Scholar] [CrossRef] [PubMed]
- Sembring, H.S.B.; Chin, K.B. Antioxidant Activities of Eggplant (Solanum melongena) Powder with Different Drying Methods and Addition Levels to Pork Sausages. Food Sci. Anim. Resour. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.M.; Yang, R.; Tsou, S.C.S.; Ledesma, D.; Engle, L.; Lee, T. Diversity in Eggplant (Solanum melongena) for Superoxide Scavenging Activity, Total Phenolics, and Ascorbic Acid. J. Food Compos. Anal. 2006, 19, 594–600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).