Anserine, Balenine, and Ergothioneine: Impact of Histidine-Containing Compounds on Exercise Performance—A Narrative Review
Abstract
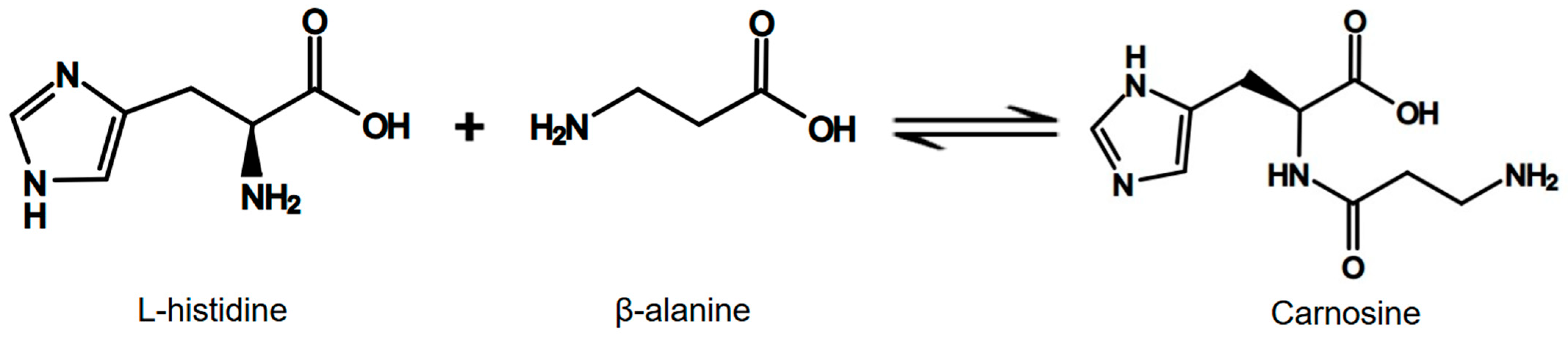
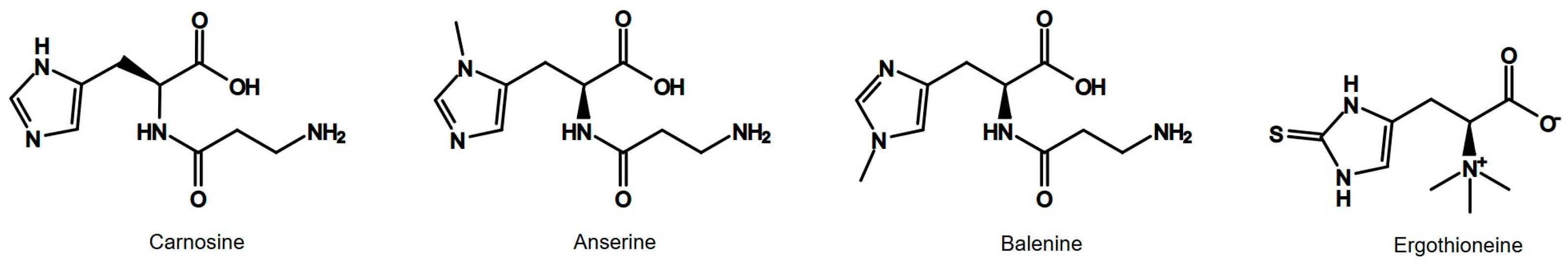
1. Introduction
2. Research Selection Criteria
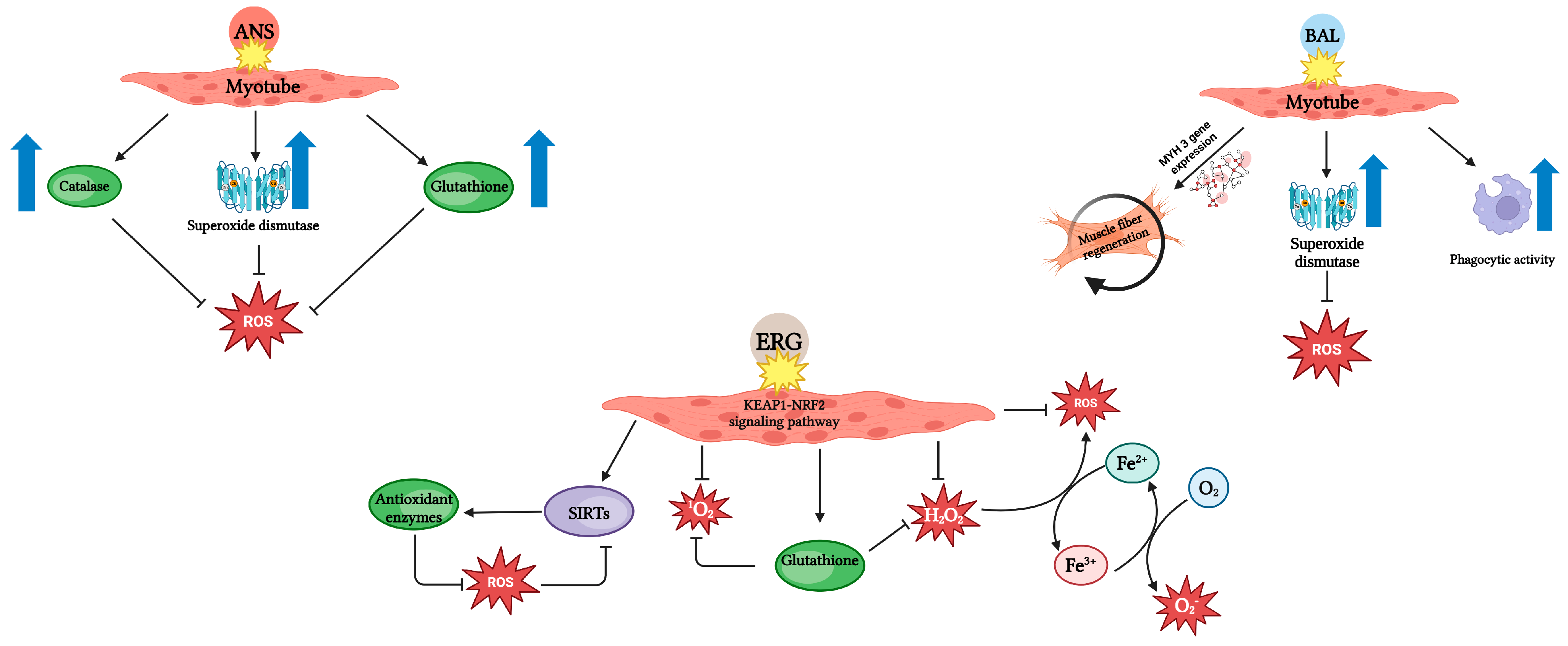
3. Impact of Anserine on Exercise Performance
4. Impact of Balenine on Exercise Performance
5. Impact of Ergothioneine on Exercise Performance
6. Studies’ Limitations
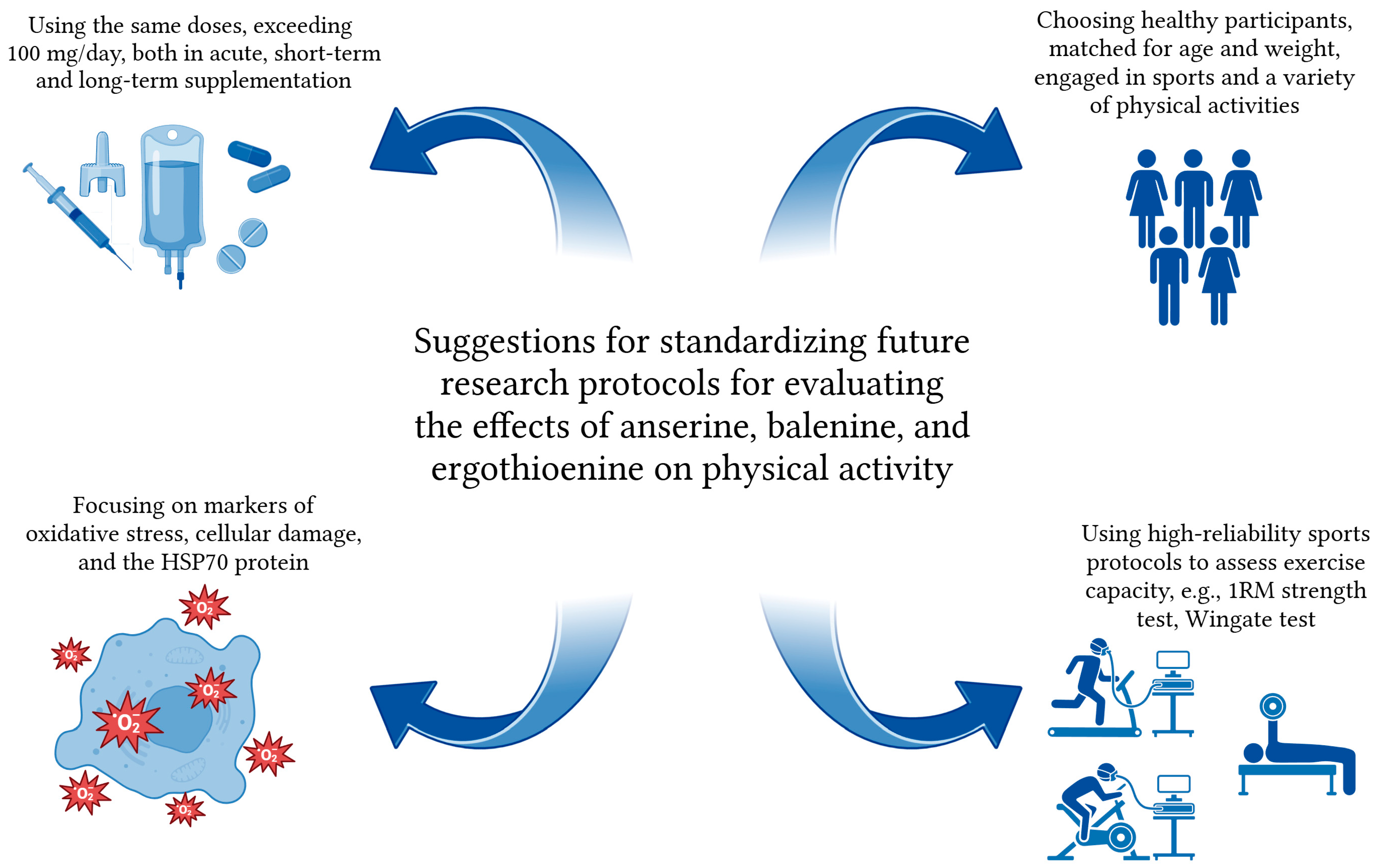
7. Implications for Future Studies on Anserine, Balenine, and Ergothioneine
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhagavan, N.V. Protein and Amino Acid Metabolism. In Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; pp. 331–363. [Google Scholar]
- Brosnan, M.E.; Brosnan, J.T. Histidine Metabolism and Function. J. Nutr. 2020, 150, 2570S–2575S. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.-A.; Bendsen, N.T.; Tremblay, A.; Astrup, A. Effect of Proteins from Different Sources on Body Composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 2), B16–B31. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, I.; Fujimura, N.; Nozawa, Y.; Furuhata, Y.; Sato, H. The Effect of Histidine on Mental Fatigue and Cognitive Performance in Subjects with High Fatigue and Sleep Disruption Scores. Physiol. Behav. 2015, 147, 238–244. [Google Scholar] [CrossRef]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From Exercise Performance to Health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Church, D.D.; Hoffman, J.R.; Varanoske, A.N.; Wang, R.; Baker, K.M.; La Monica, M.B.; Beyer, K.S.; Dodd, S.J.; Oliveira, L.P.; Harris, R.C.; et al. Comparison of Two β-Alanine Dosing Protocols on Muscle Carnosine Elevations. J. Am. Coll. Nutr. 2017, 36, 608–616. [Google Scholar] [CrossRef]
- Varanoske, A.N.; Hoffman, J.R.; Church, D.D.; Coker, N.A.; Baker, K.M.; Dodd, S.J.; Oliveira, L.P.; Dawson, V.L.; Wang, R.; Fukuda, D.H.; et al. β-Alanine Supplementation Elevates Intramuscular Carnosine Content and Attenuates Fatigue in Men and Women Similarly but Does Not Change Muscle L-Histidine Content. Nutr. Res. 2017, 48, 16–25. [Google Scholar] [CrossRef]
- Varanoske, A.N.; Hoffman, J.R.; Church, D.D.; Coker, N.A.; Baker, K.M.; Dodd, S.J.; Harris, R.C.; Oliveira, L.P.; Dawson, V.L.; Wang, R.; et al. Comparison of Sustained-Release and Rapid-Release β-Alanine Formulations on Changes in Skeletal Muscle Carnosine and Histidine Content and Isometric Performance Following a Muscle-Damaging Protocol. Amino Acids 2019, 51, 49–60. [Google Scholar] [CrossRef]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Orioli, M.; Regazzoni, L.; Carini, M.; Rasmussen, H.; Russell, R.M.; Aldini, G. Profiling Histidine Dipeptides in Plasma and Urine after Ingesting Beef, Chicken or Chicken Broth in Humans. Amino Acids 2010, 38, 847–858. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L. State of the Art in the Development of Human Serum Carnosinase Inhibitors. Molecules 2024, 29, 2488. [Google Scholar] [CrossRef] [PubMed]
- Yurievich Egorov, S.; Krasnovsky, A.A., Jr. Quenching of Singlet Molecular Oxygen by Components of the Media Used for Isolation of Chloroplasts and Testing Their Photosynthetic Activity. Russ. J. Plant Physiol. 1986, 33, 10–14. [Google Scholar]
- Hartman, P.E.; Hartman, Z.; Citardi, M.J. Ergothioneine, Histidine, and Two Naturally Occurring Histidine Dipeptides as Radioprotectors against Gamma-Irradiation Inactivation of Bacteriophages T4 and P22. Radiat. Res. 1988, 114, 319–330. [Google Scholar] [CrossRef]
- Boldyrev, A.; Abe, H. Metabolic Transformation of Neuropeptide Carnosine Modifies Its Biological Activity. Cell. Mol. Neurobiol. 1999, 19, 163–175. [Google Scholar] [CrossRef]
- Cordell, G.A.; Lamahewage, S.N.S. Ergothioneine, Ovothiol A, and Selenoneine-Histidine-Derived, Biologically Significant, Trace Global Alkaloids. Molecules 2022, 27, 2673. [Google Scholar] [CrossRef]
- Caruso, G.; Privitera, A.; Saab, M.W.; Musso, N.; Maugeri, S.; Fidilio, A.; Privitera, A.P.; Pittalà, A.; Jolivet, R.B.; Lanzanò, L.; et al. Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions. Biomedicines 2023, 11, 474. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Baguet, A.; Bex, T.; Barbaresi, S.; de Jager, S.; Lievens, E.; Stautemas, J.; De Smet, S.; Baron, G.; et al. Acute Preexercise Supplementation of Combined Carnosine and Anserine Enhances Initial Maximal Power of Wingate Tests in Humans. J. Appl. Physiol. 2021, 130, 1868–1878. [Google Scholar] [CrossRef]
- Barbaresi, S.; Blancquaert, L.; Nikolovski, Z.; de Jager, S.; Wilson, M.; Everaert, I.; De Baere, S.; Croubels, S.; De Smet, S.; Cable, N.T.; et al. Ergogenic Effect of Pre-Exercise Chicken Broth Ingestion on a High-Intensity Cycling Time-Trial. J. Int. Soc. Sports Nutr. 2021, 18, 15. [Google Scholar] [CrossRef]
- Maemura, H.; Goto, K.; Yoshioka, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Takamatsu, K. Effects of Carnosine and Anserine Supplementation on Relatively High Intensity Endurance Performance. Int. J. Sport Health Sci. 2006, 4, 86–94. [Google Scholar] [CrossRef][Green Version]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and Carnosine Supplementation in the Elderly: Effects on Cognitive Functioning and Physical Capacity. Arch. Gerontol Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Maemura, H.; Takamatsu, K.; Ishii, N. Hormonal Responses to Resistance Exercise after Ingestion of Carnosine and Anserine. J. Strength Cond. Res. 2011, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Dolan, E.; Saunders, B.; Harris, R.C.; Bicudo, J.E.P.W.; Bishop, D.J.; Sale, C.; Gualano, B. Comparative Physiology Investigations Support a Role for Histidine-Containing Dipeptides in Intracellular Acid–Base Regulation of Skeletal Muscle. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 234, 77–86. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Severin, S.E. The Histidine-Containing Dipeptides, Carnosine and Anserine: Distribution, Properties and Biological Significance. Adv. Enzym. Regul. 1990, 30, 175–194. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Nikawa, T.; Hirasaka, K. Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Mar. Drugs 2022, 20, 313. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Jiang, T.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Taniyama, S.; Tachibana, K.; Hirasaka, K. Safety Evaluation and Physiological Function of Dietary Balenine Derived from Opah Lampris guttatus on Skeletal Muscle of Mice. Int. J. Pept. Res. Ther. 2021, 27, 2083–2089. [Google Scholar] [CrossRef]
- Leinsoo, T.A.; Abe, H.; Boldyrev, A.A. Carnosine and Related Compounds Protect the Double-Chain DNA from Oxidative Damages. J. Evol. Biochem. Physiol. 2006, 42, 570–574. [Google Scholar] [CrossRef]
- Ames, B.N. Prolonging Healthy Aging: Longevity Vitamins and Proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Ye, X.; Deng, Z.; Zhao, C. Ergothioneine and Its Congeners: Anti-Ageing Mechanisms and Pharmacophore Biosynthesis. Protein Cell 2024, 15, 191–206. [Google Scholar] [CrossRef]
- Dare, A.; Channa, M.L.; Nadar, A. L-Ergothioneine and Its Combination with Metformin Attenuates Renal Dysfunction in Type-2 Diabetic Rat Model by Activating Nrf2 Antioxidant Pathway. Biomed. Pharmacother. 2021, 141, 111921. [Google Scholar] [CrossRef] [PubMed]
- Apparoo, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and Its Prospects as an Anti-Ageing Compound. Exp. Gerontol. 2022, 170, 111982. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. Chapter 16. Anserine as a Suppressor of Fatigue; Royal Society of Chemistry: London, UK, 2015; pp. 313–324. [Google Scholar]
- Muneda, H. Effects of Salmon Extract Containing Anserine on Exercise Performance of Student Athlete. Aminosan Kenkyu 2012, 6, 61–63. [Google Scholar]
- Kishi, H.; Kubomura, D.; Sugiura, T. Verification of Anti-Fatigue Effect of Anserine by Angle Fatigue Indicator Based on Median Frequency Changes of Electromyograms. Funct. Foods Health Dis. 2013, 3, 389. [Google Scholar] [CrossRef][Green Version]
- Terasawa, N.; Muneda, H.; Takahashi, Y.; Koo, A.; Shiina, Y. Effect of a Salmon Extract Containing Anserine on Exercise Performance and Anti-Lassitude of Student Athletes. Nippon Suisan Gakkaishi 2014, 80, 601–609. [Google Scholar] [CrossRef][Green Version]
- Alkhatib, A.; Feng, W.-H.; Huang, Y.-J.; Kuo, C.-H.; Hou, C.-W. Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men. Nutrients 2020, 12, 1146. [Google Scholar] [CrossRef]
- de Jager, S.; Van Damme, S.; De Baere, S.; Croubels, S.; Jäger, R.; Purpura, M.; Lievens, E.; Bourgois, J.G.; Derave, W. No Effect of Acute Balenine Supplementation on Maximal and Submaximal Exercise Performance in Recreational Cyclists. Int. J. Sport Nutr. Exerc. Metab. 2023, 33, 84–92. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Gajewski, M.; Naczk, M.; Siatkowski, I. Effect of Shiitake (Lentinus Edodes) Extract on Antioxidant and Inflammatory Response to Prolonged Eccentric Exercise. J. Physiol. Pharmacol. 2013, 64, 249–254. [Google Scholar]
- Adah, A.S.; Ayo, J.O.; Rekwot, P.I.; Aluwong, T.; Adah, D.A. Haematological Profiles and Erythrocyte Fragility of Arabian Stallions Subjected to a 2000-m Maximal Race Following Administration of Ergothioneine. Comp. Clin. Pathol. 2021, 30, 641–646. [Google Scholar] [CrossRef]
- Adah, A.; Ayo, J.; Rekwot, P.; Aluwong, T.; Adah, D. Ergothioneine Modulates Biomarkers of Oxidative Stress, Biochemical Profiles and Rectal Temperatures of Arabian Stallions Following Exercise in A Hot-Humid Environment. Media Kedokt. Hewan 2022, 33, 163–176. [Google Scholar] [CrossRef]
- Fovet, T.; Guilhot, C.; Delobel, P.; Chopard, A.; Py, G.; Brioche, T. Ergothioneine Improves Aerobic Performance Without Any Negative Effect on Early Muscle Recovery Signaling in Response to Acute Exercise. Front. Physiol. 2022, 13, 834597. [Google Scholar] [CrossRef] [PubMed]
- Adah, A.S.; Ayo, J.O.; Adah, D.A.; Nwonuma, C.O.; Lawal, T.A. Molecular Docking and Experimental Validation of the Effect of Ergothioneine on Heat Shock Protein-70 Following Endurance Exercise by Arabian Stallions. BMC Vet. Res. 2023, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, H.-G.; Mittenbühler, M.J.; Sun, Y.; Van Vranken, J.G.; Schindler, S.; Jayaraj, A.; Khetarpal, S.A.; Vargas-Castillo, A.; Puszynska, A.M.; Spinelli, J.B.; et al. Ergothioneine Boosts Mitochondrial Respiration and Exercise Performance via Direct Activation of MPST. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kaneko, J.; Enya, A.; Enomoto, K.; Ding, Q.; Hisatsune, T. Anserine (Beta-Alanyl-3-Methyl-L-Histidine) Improves Neurovascular-Unit Dysfunction and Spatial Memory in Aged AβPPswe/PSEN1dE9 Alzheimer’s-Model Mice. Sci. Rep. 2017, 7, 12571. [Google Scholar] [CrossRef]
- Kikuchi, K.; Matahira, Y.; Sakai, K. Separation and Physiological Functions of Anserine from Fish Extract. Dev. Food Sci. 2004, 42, 97–105. [Google Scholar]
- Muneta, Y. Effect of Anserine-Containing Salmon Extract (SEAns) on Eye Fatigue and Presbyopia. Amino Acid Rep. 2010, 4, 75–77. [Google Scholar]
- Khaitin, V.Y.; Matveev, S.V.; Grishin, M.Y. The Level of Serum Creatine Phosphokinase as a Criterion of Recovery in Professional Soccer Players during the Competitive Period. Sports Med. Res. Pract. 2019, 4, 22–27. [Google Scholar] [CrossRef]
- Hackney, A.C.; Walz, E.A. Hormonal Adaptation and the Stress of Exercise Training: The Role of Glucocorticoids. Trends Sport Sci. 2013, 20, 165–171. [Google Scholar]
- Doan, B.; Newton, R.; Kraemer, W.; Kwon, Y.-H.; Scheet, T. Salivary Cortisol, Testosterone, and T/C Ratio Responses during a 36-Hole Golf Competition. Int. J. Sports Med. 2007, 28, 470–479. [Google Scholar] [CrossRef]
- Lautenbach, F.; Laborde, S.; Achtzehn, S.; Raab, M. Preliminary Evidence of Salivary Cortisol Predicting Performance in a Controlled Setting. Psychoneuroendocrinology 2014, 42, 218–224. [Google Scholar] [CrossRef]
- Viru, A.; Viru, M. Cortisol—Essential Adaptation Hormone in Exercise. Int. J. Sports Med. 2004, 25, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, L.; Yang, S.; Wang, H.; Yao, J.; Dai, Q.; Chang, S. Contributions of Visuo-Oculomotor Abilities to Interceptive Skills in Sports. Optom. Vis. Sci. 2015, 92, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Iehisa, I.; Negishi, K.; Ayaki, M.; Tsubota, K. Kinetic Visual Acuity Is Correlated with Functional Visual Acuity at Higher Speeds. BMJ Open Ophthalmol. 2019, 4, e000383. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, K.; Kohmura, Y.; Aoki, K.; Nakamura, M.; Murakami, S.; Suzuki, Y. Sports and Kinetic Visual Acuity. Juntendo Iji Zasshi Juntendo Med. J. 2022, 68, 387–392. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sato, M.; Morimatsu, F.; Takamatsu, K. Effect of CBEXTM Supplementation on High-Intensity Intermittent Exercise. Taiikugaku Kenkyu (Jpn. J. Phys. Educ. Health Sport Sci.) 2004, 49, 159–169. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Cramer, J.T.; Weir, J.P.; Coburn, J.W.; Malek, M.H. Does the Frequency Content of the Surface Mechanomyographic Signal Reflect Motor Unit Firing Rates? A Brief Review. J. Electromyogr. Kinesiol. 2007, 17, 1–13. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Joubert, J.E.; Diefenthaeler, F.; Trinity, J.D. Changes in Muscle Activity and Kinematics of Highly Trained Cyclists during Fatigue. IEEE Trans. Biomed. Eng. 2008, 55, 2666–2674. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Fu, W. Changes in Impact Signals and Muscle Activity in Response to Different Shoe and Landing Conditions. J. Hum. Kinet. 2017, 56, 5–18. [Google Scholar] [CrossRef]
- Valdersnes, S.; Birkenes, A.; Froyland, L. Validation of a Method for the Determination of Balenine/Ophidine in Whale. J. AOAC Int. 2017, 100, 1814–1818. [Google Scholar] [CrossRef]
- de Jager, S.; Vermeulen, A.; De Baere, S.; Van der Stede, T.; Lievens, E.; Croubels, S.; Jäger, R.; Purpura, M.; Bourgois, J.G.; Derave, W. Acute Balenine Supplementation in Humans as a Natural Carnosinase-Resistant Alternative to Carnosine. Sci. Rep. 2023, 13, 6484. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The Biology of Ergothioneine, an Antioxidant Nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Hartmann, L.; Flögel, S. The Ergothioneine Transporter (ETT): Substrates and Locations, an Inventory. FEBS Lett. 2022, 596, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I. Ergothioneine, Where Are We Now? FEBS Lett. 2022, 596, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Feng, L.; Tang, R.M.Y.; Lim, K.H.C.; Halliwell, B. Ergothioneine Levels in an Elderly Population Decrease with Age and Incidence of Cognitive Decline; a Risk Factor for Neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Statement on the Safety of Synthetic L-ergothioneine as a Novel Food—Supplementary Dietary Exposure and Safety Assessment for Infants and Young Children, Pregnant and Breastfeeding Women. EFSA J. 2017, 15, e05060. [Google Scholar] [CrossRef]
- Chen, B.-X.; Xue, L.-N.; Wei, T.; Ye, Z.-W.; Li, X.-H.; Guo, L.-Q.; Lin, J.-F. Enhancement of Ergothioneine Production by Discovering and Regulating Its Metabolic Pathway in Cordyceps Militaris. Microb. Cell Factories 2022, 21, 169. [Google Scholar] [CrossRef]
- Tian, X.; Thorne, J.L.; Moore, J.B. Ergothioneine: An Underrecognised Dietary Micronutrient Required for Healthy Ageing? Br. J. Nutr. 2023, 129, 104–114. [Google Scholar] [CrossRef]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The Bioavailability of Ergothioneine from Mushrooms (Agaricus bisporus) and the Acute Effects on Antioxidant Capacity and Biomarkers of Inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef]
- Geiger, P.C.; Gupte, A.A. Heat Shock Proteins Are Important Mediators of Skeletal Muscle Insulin Sensitivity. Exerc. Sport Sci. Rev. 2011, 39, 34–42. [Google Scholar] [CrossRef]
- Tukaj, S. Immunoregulatory Properties of Hsp70. Postep. Hig. Med. Dosw. 2014, 68, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Fräsdorf, B.; Radon, C.; Leimkühler, S. Characterization and Interaction Studies of Two Isoforms of the Dual Localized 3-Mercaptopyruvate Sulfurtransferase TUM1 from Humans. J. Biol. Chem. 2014, 289, 34543–34556. [Google Scholar] [CrossRef] [PubMed]
- Toviwek, B.; Suwanasopee, T.; Koonawootrittriron, S.; Jattawa, D.; Pongprayoon, P. Binding Modes of Carnostatine, Homocarnosine, and Ophidine to Human Carnosinase 1. ACS Omega 2023, 8, 42966–42975. [Google Scholar] [CrossRef] [PubMed]
- Puntschart, A.; Vogt, M.; Widmer, H.R.; Hoppeler, H.; Billeter, R. Hsp70 Expression in Human Skeletal Muscle after Exercise. Acta Physiol. Scand. 1996, 157, 411–417. [Google Scholar] [CrossRef]
- Grgic, J.; Lazinica, B.; Schoenfeld, B.J.; Pedisic, Z. Test–Retest Reliability of the One-Repetition Maximum (1RM) Strength Assessment: A Systematic Review. Sports Med. Open 2020, 6, 31. [Google Scholar] [CrossRef]
- Castañeda-Babarro, A. The Wingate Anaerobic Test, a Narrative Review of the Protocol Variables That Affect the Results Obtained. Appl. Sci. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Williams, J.D.; Abt, G.; Kilding, A.E. Ball-Sport Endurance and Sprint Test (BEAST90): Validity and Reliability of a 90-Minute Soccer Performance Test. J. Strength Cond. Res. 2010, 24, 3209–3218. [Google Scholar] [CrossRef]
- Podstawski, R.; Choszcz, D.; Konopka, S. The Impact of Training on the 500 M Rowing Ergometer Time and the Assessment of the Applied Test’s Relevancy. Hum. Mov. 2011, 12, 264–272. [Google Scholar] [CrossRef]
- Beato, M.; Fleming, A.; Coates, A.; Dello Iacono, A. Validity and Reliability of a Flywheel Squat Test in Sport. J. Sports Sci. 2021, 39, 482–488. [Google Scholar] [CrossRef]
- Bohlooli, S.; Barmaki, S.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The Effect of Spinach Supplementation on Exercise-Induced Oxidative Stress. J. Sports Med. Phys. Fit. 2014, 55, 609–614. [Google Scholar]
- Trexler, E.T.; Keith, D.S.; Schwartz, T.A.; Ryan, E.D.; Stoner, L.; Persky, A.M.; Smith-Ryan, A.E. Effects of Citrulline Malate and Beetroot Juice Supplementation on Blood Flow, Energy Metabolism, and Performance During Maximum Effort Leg Extension Exercise. J. Strength Cond. Res. 2019, 33, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Candow, D.G.; Forbes, S.C.; Neary, J.P.; Ormsbee, M.J.; Antonio, J. Effects of Creatine Supplementation during Resistance Training Sessions in Physically Active Young Adults. Nutrients 2020, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Margaritelis, N.V.; Kyparos, A.; Nikolaidis, M.G. N-Acetylcysteine Supplementation Increases Exercise Performance and Reduces Oxidative Stress Only in Individuals with Low Levels of Glutathione. Free Radic. Biol. Med. 2018, 115, 288–297. [Google Scholar] [CrossRef] [PubMed]
| References | Ingredient | Participants | Dose | Duration Time | Exercise Protocols | Investigated Parameters | Results |
|---|---|---|---|---|---|---|---|
| In vivo human studies | |||||||
| [34] | Anserine (SEAns) | 10 healthy male individuals n = 10 | 400 mg (2 g SEAns) | Acute dosage | 3 × 4 physical fatigue-inducing tasks | CPK TTE Cortisol level | ↓ CPK ↓ TTE ↓ Cortisol level |
| [35] | Anserine (SEAns) | 20 male table tennis players n = 20 | 120 mg (400 mg SEAns) | 8 weeks | Alternate bounce of the served ball in pairs | KVA The accuracy of the rebound of the counterattack serve | ↓ KVA More points of improvement in return accuracy |
| [36] | Anserine (from fish) | 17 healthy male individuals n = 17 Aged 35–40 Mean body weight 75.5 ± 5.0 kg | 11 mg/kg body weight | Acute dose | Isometric ETT m. rectus femoris | MDF | ↓ MDF |
| [37] | Anserine (SEAns) Placebo | 9 healthy male individuals n = 9 Aged 19–21 | 120 mg (400 mg SEAns) | 7 days | 3 × 1500 m running TT | CPK WBCs count FFA Blood glucose Total ketone bodies Time for the 1500 m trial | ↓ CPK ↓ WBCs ↓ FFA ↔ for all investigated parameters Shortening of TT in compare to baseline value, and placebo group |
| [38] | Anserine (chicken broth) Placebo | 10 healthy male individuals n = 10 Aged 20–23 Mean body weight 69.5 ± 2.5 kg | 15 or 30 mg/kg body weight | Acute dosage | TTE test on treadmill | Exercise performance (TTE) Oxidative stress markers (SOD, CAT, GSSG, GSH) Biomarkers of cell damage (GOT, GPT, CMKB) Hematology biomarkers of blood | ↔ TTE ↔ H2O2 ↑ SOD, GSSG ↓ CAT, GSH ↑ CKMB, GPT ↓ GOT ↔ for all investigated parameters |
| [39] | Balenine Placebo | 20 healthy individuals (6 female, 14 male) n = 20 Aged 27–31 Mean body weight 69.0 ± 9.2 kg | 10 mg/kg body weight | Acute dosage | 3 × 3 s MVC with 20 s rest 3 × 6 s maximal cycling sprints with 2 min rest 4 and 20 km TT | Peak power Peak Torque TTC 4 and 20 km pH, glucose, bicarbonate, and lactate concentration | ↔ for all investigated parameters |
| [40] | Ergothioneine (Lentinus edodes extract/shiitake mushrooms) Placebo | 14 healthy male individuals n = 14 Aged 21–22 | 1400 mg/day of L. edodes extract (2.77 mg of ergothioneine) | 10 days | Exercises on treadmill: 90 min run | CK Immune cells (LC, MONO, NC, LYM) Thiol redox status Inflammatory cytokines Reactive oxygen and nitrogen species | ↔ CK ↔ Immune cells ↔ GSHt ↓ GSSG ↓ IL-10 ↔ IL-1β, IL-6, TNF-α ↔ H2O2 ↑ NO (postexercise 20 min) ↓ 8-iso (postexercise 20 min and 24 h) |
| In vivo animal studies | |||||||
| [41] | Ergothioneine Placebo | 20 stallions n = 20 | 0.2 mg/kg body weight | Acute dosage | Maximal race of 2000 m on a standard racetrack | Hematological parameters | ↓ LC, NC, NLR ↑ EC, PCV |
| [42] | Ergothioneine Placebo | 12 stallions n = 12 | 0.5 mg/kg body weight | 2 months | Race of 1800 m on a standard racetrack | Body temperature Oxidative stress markers (AST, LDH, CREAT, SOD, CAT, GPx, MDA) | ↓ Rectal temperature ↓ Oxidative stress markers |
| [43] | Ergothioneine Placebo | 18 female mice n = 18 | 70 mg/kg bodyweight/day | 7 days | TTE test on treadmill 10 min of warm-up Increase in speed run by 2 m/min every minute till 70% MAS | MAS TTE Inflammation markers (TNF-α) Markers of global protein synthesis (PI, RPS6p) Muscle protein breakdown markers Metabolic stress markers (AMPKαp) Oxidative stress markers Muscle satellite cells | ↑ MAS ↑ TTE ↓ TNF-α ↑ Markers of global protein synthesis ↔ for all investigated parameters ↓ Metabolic stress markers ↔ for all investigated parameters ↑ m. soleus satellite cells |
| [44] | Ergothioneine Placebo | 18 stallions n = 18 | 0.02 mg/kg body weight | 4 weeks | Endurance exercise of 30 km distance Each 10 km was followed by a 15 min break and consumption of cold water | Heat Shock Protein-70 Oxidative stress markers (SOD, CAT, GPx, GR, MDA) | ↑ Heat Shock Protein-70 ↑ GR, GR ↓ SOD, CAT, MDA |
| [45] | Ergothioneine (from diet) | 15 mice n = 15 | 209 ng/mg body weight | 8 weeks | VWR | Training parameters (exercise performance, running speed, total distance) | ↑ for all investigated parameters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrejko, M.; Kała, K.; Muszyńska, B. Anserine, Balenine, and Ergothioneine: Impact of Histidine-Containing Compounds on Exercise Performance—A Narrative Review. Nutrients 2025, 17, 828. https://doi.org/10.3390/nu17050828
Jędrejko M, Kała K, Muszyńska B. Anserine, Balenine, and Ergothioneine: Impact of Histidine-Containing Compounds on Exercise Performance—A Narrative Review. Nutrients. 2025; 17(5):828. https://doi.org/10.3390/nu17050828
Chicago/Turabian StyleJędrejko, Maciej, Katarzyna Kała, and Bożena Muszyńska. 2025. "Anserine, Balenine, and Ergothioneine: Impact of Histidine-Containing Compounds on Exercise Performance—A Narrative Review" Nutrients 17, no. 5: 828. https://doi.org/10.3390/nu17050828
APA StyleJędrejko, M., Kała, K., & Muszyńska, B. (2025). Anserine, Balenine, and Ergothioneine: Impact of Histidine-Containing Compounds on Exercise Performance—A Narrative Review. Nutrients, 17(5), 828. https://doi.org/10.3390/nu17050828







