1. Introduction
Photolyases (PHRs) are members of the cryptochrome/PHR protein family that utilize blue light as an energy source to facilitate the repair of ultraviolet (UV)-induced DNA damage, including cyclobutane pyrimidine dimers (CPD) and pyrimidine-pyrimidone photoproducts (6-4PP) [
1,
2,
3]. Photorepair is the mechanism through which PHRs repair DNA damage. These enzymes are found in various organisms, including photosynthetic bacteria, fungi, plants, fish, birds, and some marsupials [
4,
5,
6,
7]. In placental mammals, including humans, PHRs do not possess the ability for DNA repair [
8]. Instead, they function as a cryptochrome, acting as a blue-light receptor involved in the regulation of circadian rhythms. Placental mammals employ a distinct mechanism known as nucleotide excision repair (NER) to rectify UV-induced DNA damage [
9]. Insufficient repair of UV-induced DNA damage, particularly due to excessive sun exposure, leads to the accumulation of CPD and 6-4PP in genomic DNA. This accumulation has various consequences that increase the risk of premature aging, actinic keratosis, and skin cancer [
10].
Clinical trials are underway to demonstrate the efficacy of PHRs in preventing and repairing sun damage in human skin, with the goal of developing skincare products that can mitigate the effects of photoaging. For example, a sunscreen incorporating liposome-encapsulated PHR from the microalga
Anacystis nidulans was developed. This sunscreen was able to inhibit CPD formation in skin cells caused by UV irradiation and reduce apoptosis by 93% and 82%, respectively, compared to that by a regular sunscreen with SPF 50 [
11]. Consequently, advancements in the immobilization of PHRs on liposomes and nanomaterials facilitated their application in various products, including topical creams and sunscreens, thereby ensuring high stability. However, challenges associated with cost, safety, and quality control still need to be addressed.
We have long been interested in the significant contribution of the tropical fruit acerola to health and hypothesized that acerola may also be a valuable source of PHRs. Acerola (
Malpighia emarginata DC.) is widely recognized as one of the richest natural sources of ascorbic acid and contains various phytonutrients, such as carotenoids, phenols, flavonoids, and anthocyanins, which contribute to its remarkable antioxidant capacity [
12]. Acerola was also noted for its other intriguing biological functions, including skin whitening, anti-aging, and suppression of multidrug resistance. Furthermore, acerola juice exerts a protective effect against metal-induced DNA damage [
13,
14]; however, the role of PHRs in acerola remains unclear. Great interest persists in the scientific, cosmetic, and medical communities in studying this superfruit. If the PHR-mediated photorepair ability of acerola can be demonstrated, it would further enhance the utility of the fruit in maintaining health.
In this study, we aimed to identify a novel PHR gene in acerola. Additionally, we explored the potential for cell-to-cell transmission of PHRs through extracellular vesicles.
2. Materials and Methods
2.1. De Novo Assembly by Trinity
Acerola fruit pulp (3 g) with seeds removed and five leaves (2 g) were frozen in liquid nitrogen and ground into a fine powder using a mortar and pestle. The homogenized tissue was dissolved in QIAzol, and total RNA was extracted using an RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. RNA from acerola fruits and leaves was used to construct, assemble, and analyze de novo transcriptomes. We cloned and sequenced the cDNA encoding a PHR from acerola. Transcriptome sequences were deposited in the DDB J database and used as a proof-of-concept via the BLAST portal (version 2.14.0) to identify candidate PHR/cryptochrome genes.
2.2. cDNA Amplification by 5′- and 3′-RACE
The rapid amplification of cDNA ends (RACE) is a technique used to obtain full-length sequences of RNA transcripts. The SMARTer RACE 5′/3′ kit (Takara Bio Inc., Shiga, Japan) was used to synthesize RACE-Ready cDNA as a gene cloning template. Primers were designed using Primer 3Plus (
https://www.primer3plus.com/ (accessed on 11 December 2023)) from a part of the predicted sequence of acerola PHR (acPHR) and were PCR-amplified to obtain the acPHR gene fragment. The primer sets were as follows: F: CCTGTTTGGGTAGCTTCAGAGAAGTTGG, and R: AGCTTCTGTACTTTACGGGCCTCCA. Following the instructions of the SMARTer RACE 5′/3′ kit, we designed 5′-RACE and 3′-RACE specific primers, which are as follows: GSP1 (antisense primer for the 5′-race PCR): GATTACGCCAAGCTTAGCTTCTGTACTTTACGGGCCTCCA, and GSP2 (sence primer for 3′-race PCR): GATTACGCCAAGCTTCCTGTTTGGGTAGCTTCAGAGAAGTTGG. We performed RACE cloning and sequencing using AZENTA (Shanghai, China) to obtain the full-length putative acPHR gene.
2.3. Comparison of Amino Acid Sequence
The amino acid sequence of acPHR was predicted from the sequenced DNA data by translating the DNA sequences into their corresponding amino acid sequences. Comparison of amino acid sequences among athPHR1, potPHR1, and the putative acPHR was performed using the PROSITE database (
https://prosite.expasy.org/prosite.html).
2.4. Cell Culture
HEK293 cells and normal human dermal fibroblasts (NHDF; Lot#20TL266554) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and LONZA (Basel, Switzerland). The cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C in a humidified atmosphere containing 5% CO2, and the adherent cells were harvested through trypsinization.
2.5. Establishment of Cell Lines with Stable Expression of potPHR1 and acPHR
The fragments of the open reading frame sequence of potPHR1 and putative acPHR were purchased from IDT (Coralville, IA, USA) and amplified using PCR using the following primers, 5′-GCGGCCACCATGGACTCCAAAAAGAGGAG and 3′-TTCCCTTAATCTGCAGGGCTGA, and 5′-CCCAAGCTTATGGTGTCCCGGGACCAGGC and 3′-CGGGATCCTCAGGTCGGGTTCTCGTAGC for potPHR1 and acPHR, respectively.
The PCR products were inserted into the pcDNA3.1/Hygro (+) expression vector (Invitrogen) according to the manufacturer’s protocol. The established expression vectors were subjected to DNA sequencing to confirm the correct insertion of the full-length potPHR1 or putative acPHR genes. The expression vectors for potPHR1, acPHR, or the corresponding empty pcDNA3.1, were transfected into HEK293 cells using Lipofectamine 3000 (Invitrogen), according to the standard procedure. Forty-eight hours after transfection, the culture medium was replaced with fresh medium containing hygromycin and the cells were cultured for 2 weeks. Hygromycin-resistant clones were established and designated as HEK293/pcDNA3.1, HEK293/potPHR1, and HEK293/acPHR.
2.6. Cell Proliferation and Apoptosis Assay
HEK293/pcDNA3.1, HEK293/potPHR1, HEK293/acPHR, and NHDF were seeded at a density of 0.5 × 10
4 cells/well in 96-well plates (n = 6). NHDF was treated with or without 1.5 × 10
7 particle/well of concentrated acerola extract (CAE; For details regarding the preparation method of CAE used in this study, please refer to the following CAE section in
Section 2. Cell proliferation was assessed at 24 or 72 h of treatment using CellTiter-Glo (Promega, Madison, WI, USA), according to the manufacturer’s protocol. For cell proliferation quantification, a calibration curve was generated using a known number of control cells, and luminescence data from CellTiter-Glo were converted into cell numbers. Background subtraction was performed using cell-free wells.
NHDF was also analyzed for apoptosis induction by similar condition to those of cell proliferation assay using Caspase-Glo 3/7 reagent (Promega). After 24 or 72 h of treatment, the reagent was added to the cells. The luminescence of each sample was determined using a GloMax Multi microplate reader (Promega) according to the manufacturer’s protocol. Each experiment was repeated three times, and data were represented as the mean ± SEM.
2.7. Assay for mRNA Expression Using qRT-PCR
The acPHR and β-Actin (ACTB) expression levels were determined using qRT-PCR. Total RNA was extracted from sub-confluent HEK293/pcDNA3.1, HEK293/potPHR1, and HEK293/acPHR cells using an RNeasy MINI Kit (Qiagen). The RNA concentration was determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). The expression levels of potPHR1, acPHR, and ACTB were determined by qRT-PCR using the SYBR Green qPCR Master Mix (Thermo Fisher Scientific), assay-on-demand primers, and TaqMan Universal PCR Master Mix Reagent (Applied Biosystems). For acPHR and potPHR1, the following primers were used: F: 5′ GTGGACAAGAGGACCGGAAG 3′, R: 5′ TCTGCTTTCCGCTTCTTTCCT 3′, and F: 5′ GAGGGAGCTGGCAGACAATT 3′, R: 5′ TTCCTTCCTTCACCGTCTGC 3′, respectively. β-Actin (Hs01060665_g1) was used as normalization control. qRT-PCR was performed on a 7900HT thermal cycler (Applied Biosystems, Foster City, CA, USA). The experiment was performed in triplicate, and data are represented as the mean ± SEM.
2.8. Extraction of Extracellular Vesicle (EV) Fraction from Cell Culture Supernatant
HEK293/pcDNA3.1, HEK293/potPHR1, and HEK293/acPHR cells were grown in a 100 mm dish. Once they reached confluence (almost all cells reached 1 × 107 cells/dish), the culture medium was replaced with fresh medium (10 mL), and the cells were harvested 24 h later. According to the standard procedure of the ExoEasy Maxi kit (Qiagen), 10 mL of cell culture supernatant was filtered through a 0.45 µm PVDF filter (Millipore, Billerica, MA, USA) to remove cell debris. Equal volumes of the XBP buffer were added and mixed thoroughly. The sample was transferred to an ExoEasy spin column, to which 800 µL of Buffer XE was added, and then centrifuged at 5000× g for 5 min. The flow-through was concentrated using 100,000× g ultracentrifugation using a TLA-110 rotor 1 (Beckman Colter, Brea, CA, USA) for 70 min at 4 °C to change the elution buffer XE to 30 µL of phosphate-buffered saline (PBS).
2.9. Photorepair Activity Measurement
The photorepair activity was measured as previously described [
15], with minor modifications. Briefly, sub-confluent cells in 6-well plates were exposed to 312 nm UV-B irradiation (5 mJ/cm
2) using a UVP Crosslinker CL-1000 (Analytik JenaUS, Upland, CA, USA), followed by 500 nm blue light exposure for 40 min using an LED array system (Amuza, San Diego, CA, USA) after changing to fresh culture medium.
Genomic DNA was extracted using a DNeasy Blood & Tissue kit (Qiagen), and CPD content was measured using an OxiSelect UV-induced DNA Damage ELISA kit (Cosmo Bio, Tokyo, Japan), according to the manufacturer’s protocol (n = 6). Each experiment was repeated three times, and data are represented as the mean ± SEM.
2.10. Evaluation of Cell-to-Cell Transmission
Sub-confluent HEK293 cells in 6-well plates were treated with EV fractions from HEK293/pcDNA3.1, HEK293/potPHR1, or HEK293/acPHR cells for 3 h. The number of exogenous particles in each culture was 6 × 106. After replacing the medium with fresh medium, a CPD assay was performed. Each experiment was repeated three times, and data were represented as the mean ± SEM.
2.11. CAE
All plant experiments and collection of plant materials complied with the relevant institutional, national, and international guidelines and laws.
CAE was gifted from Nichirei Foods Inc. (Tokyo, Japan). Briefly, juice extracted from uniformly ripe acerola fruits obtained via organic cultivation on a Brazilian farm owned by Nichirei Foods, Inc. was subjected to ultrafiltration using a 100 K molecular weight cut-off filter.
The workflow is as follows: the fruit was rubbed against a stainless-steel mesh to separate the juice. The juice was centrifuged at 5150× g for 30 min at 4 °C, and the supernatant was coarsely filtered using glass filter paper (GC-90, GA-100, Advantech Toyo, Tokyo, Japan). The acerola juice was prepared by microfiltration through a cartridge filter (Polysep II Cartridge Filter, 10 in. Code 7, 1.0/0.5 μm, Merck). Subsequently, the juice was concentrated with a 100 K ultrafiltration membrane (Sartocon Slice Hydrosart Cassette 0.1 m2 100 K, 3051446801E-SW, Sartorius). After the juice was concentrated to approximately 1 L, 5 L of Dulbecco’s PBS (D5652-50L, Sigma) was added for solvent replacement and re-concentration. This process was repeated twice. The concentrate was finally passed through a 0.45-μm filter (Opticap XL2 Durapore 0.45-μm, KPHLA02FH3, Merck) to obtain the CAE. The protein concentration of CAE was determined using a Qubit Protein Assay Kit (Thermo Fisher Scientific Inc., Wilmington, NC, USA).
2.12. Nanoparticle Tracking Analysis (NTA)
NTA was performed according to the manufacturer’s instructions, using a Nanosight LM10 system (Malvern, Herrenberg, Germany) with a blue laser (405 nm). The nanoparticles in the EV fractions from HEK293/pcDNA3.1, HEK293/potPHR1, HEK293/acPHR, and CAE were illuminated by the laser, and their movement under Brownian motion was recorded in 90 s sample videos, which were analyzed using Nanoparticle Tracking Analysis 2.0 analytical software (Malvern).
Briefly, all samples were diluted with PBS to reach a particle concentration suitable for NTA (1 × 10
8–2.5 × 10
9 particles/mL). Capture (shutter and gain) and analysis settings were set manually. All settings were kept constant during each experiment. To determine the total nanoparticle concentration and size distribution profile, data were averaged for each sample across video replicates (at least three triplicate videos for each sample) [
16].
2.13. Transmission Electron Microscopy (TEM)
EV fractions from HEK293/pcDNA3.1, HEK293/potPHR1, and HEK293/acPHR were fixed with 4% paraformaldehyde and 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 37 °C and placed in a refrigerator to lower their temperature to 4 °C. The sections were fixed in osmic acid for 1 h. After washing, they were stained with 0.5% uranyl acetate solution, dehydrated in ethanol, washed, and embedded in epoxy resin. They were then sliced with a diamond cutter and observed under a transmission electron microscope (JEM-1200EX; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 80 kV, according to the standard procedure.
Negative staining was conducted. Briefly, the CAE was dropped onto a 400-mesh carbon-coated grid in the supporting film, and excess water was removed from the specimen using filter paper. Immediately, a staining solution containing heavy metals such as 2% phosphorus tungstic acid was dropped onto the specimen. Water was then removed from the specimen with a filter paper to dry the specimen. The specimen was then observed using TEM.
2.14. Photorepair Activity of CAE in NHDF
NHDF were pre-incubated with or without CAE (5 × 108 particles/well) in 6-well plates for 3 h and then exposed to UV-B irradiation at 312 nm (5 mJ/cm2) using the UVP Crosslinker CL-1000 to induce DNA damage, followed by blue light (500 nm) for 40 min. Genomic DNA was extracted 24 h after UV-B irradiation, and the CPD levels were measured using ELISA. Each experiment was repeated three times, and data are represented as the mean ± SEM.
2.15. COMET Assay
The single-cell gel electrophoresis assay called COMET was performed in NHDF using a commercial kit (OxiSelect™ Comet Assay, Cosmo Bio). NHDF were pre-incubated with or without CAE (5 × 108 particles/well) in 6-well plates for 3 h and then exposed to UV-B irradiation at 312 nm (5 mJ/cm2) using the UVP Crosslinker CL-1000 to induce DNA damage, followed by blue light exposure (500 nm) for 40 min. NHDF were removed from the culture dish 24 h after UV-B irradiation and combined with OxiSelect™ Comet Agarose at 1:10 ratio (v/v), and 75 µL/well were immediately transferred onto the OxiSelect™ Comet Slide. Slides were maintained horizontally and incubated at 4 °C for 30 min. The slides were then immersed in a lysis buffer for 60 min. The lysis buffer was removed, and the slides were immersed in an alkaline solution for 30 min. The slide was transferred to a horizontal electrophoresis chamber, and a voltage of 1 V/cm was applied for 15 min. After electrophoresis, the slides were washed with H2O and 70% ethanol and dried for 30 min. Finally, Vista Green DNA dye was added, and the mixture was incubated at room temperature for 15 min. The cells on the slides were observed under a fluorescence microscope using a fluorescein isothiocyanate filter. DNA damage was quantified by measuring the displacement between the genetic material of the head and tail of the comet. Each experiment was repeated three times, and data are represented as the mean ± SEM.
2.16. Statistical Analyses
Data are expressed as the mean ± SEM from three independent experiments. The two treatment groups were compared using Student’s t-test. Multiple group comparisons were performed using ANOVA. GraphPad Prism version 5c for Macintosh (GraphPad Inc., La Jolla, CA, USA) was used for statistical analyses. The results were considered statistically significant at p < 0.05.
4. Discussion
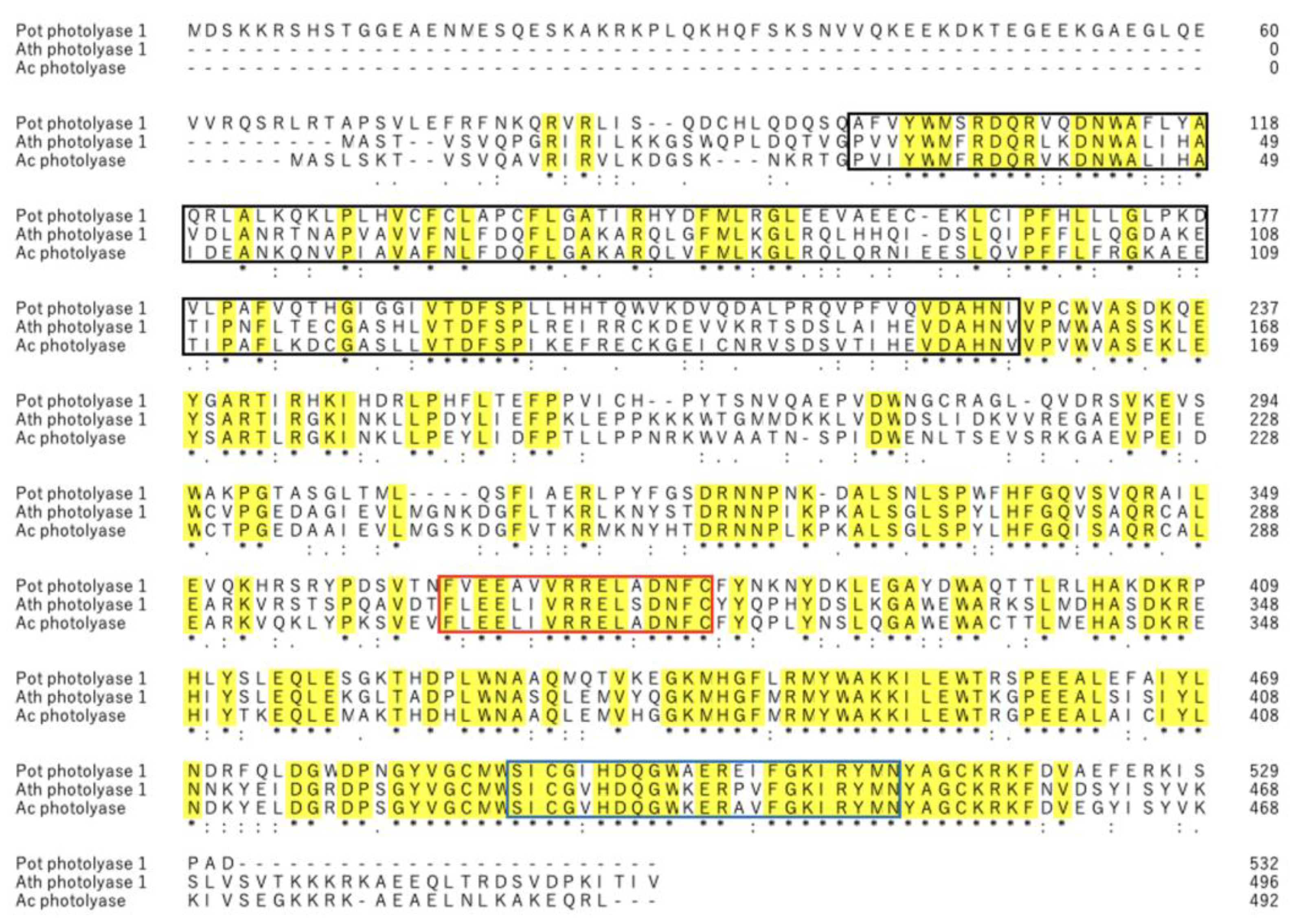
To the best of our knowledge, this is the first study identifying a novel PHR from acerola that repairs DNA damaged by UV-B irradiation. The putative mRNA and amino acid sequences of acPHR showed 72.8% and 76.8% homology to those of athPHR1, respectively (
Figure 1 and
Figure 2). The comparison of the amino acid sequences of potPHR1, a mammalian PHR, and putative acPHR exhibited only 43.8% homology. However, essential catalytic domains of PHRs, such as cryptochrome α/β domains, DNA PHR class 2 signature 1, and DNA PHR class 2 signature 2, were highly conserved among athPHR1, potPHR1, and putative acPHR (
Figure 2) [
19].
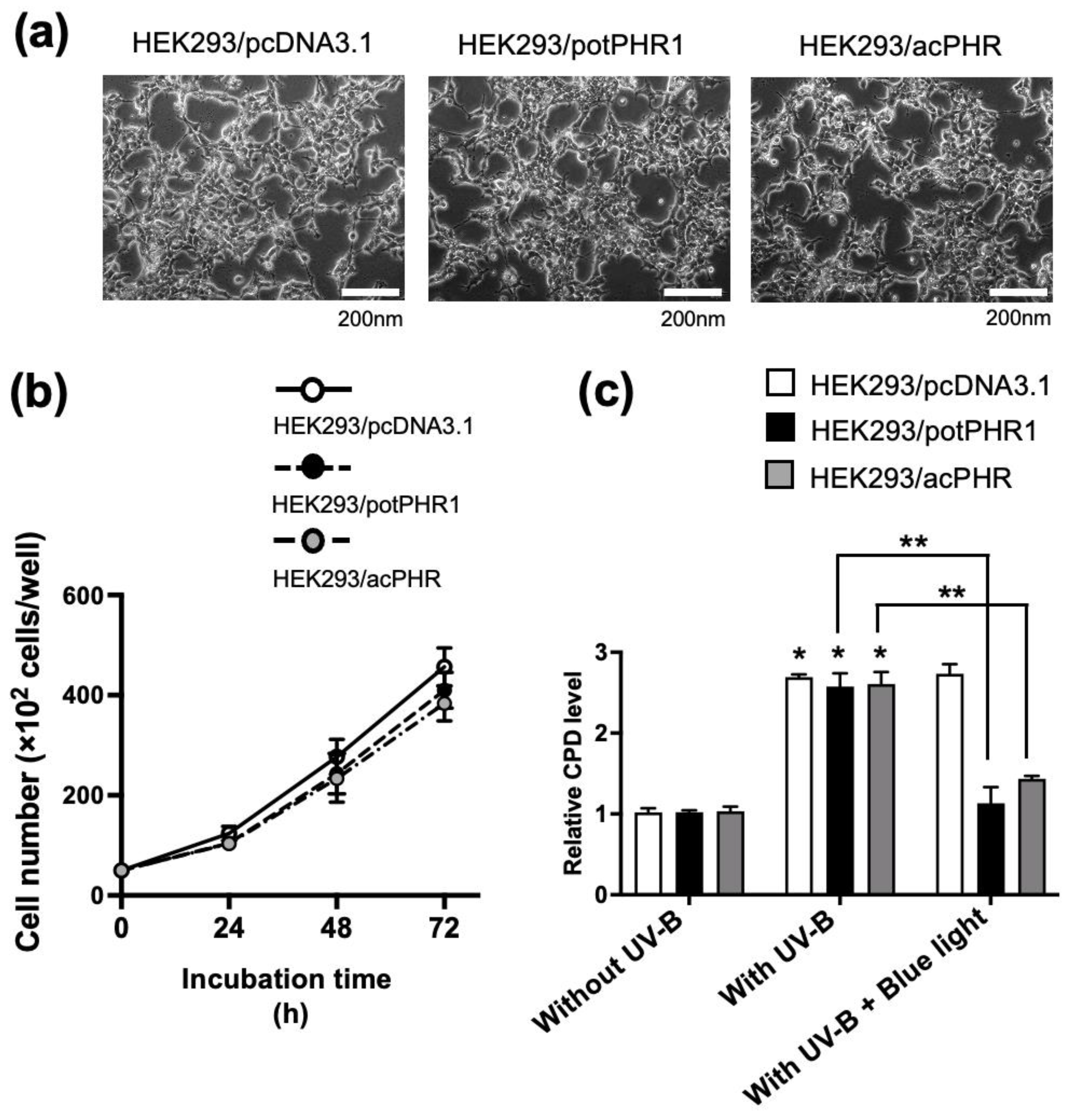
Based on the putative mRNA sequence, HEK293/acPHR cells with stable expression of the putative acPHR gene were established. HEK293/pcDNA3.1 and HEK293/potPHR1 cells were also established using the stable expression of empty vector and vector harboring potPHR1 genes as negative and positive control cell lines, respectively. During the preparation of these cell lines, specific expression of potPHR1 and acPHR mRNA was confirmed in HEK293/potPHR1 and HEK293/acPHR cells (
Table 1). No differences in cell morphology and proliferation were observed among the three cell lines, suggesting no toxicity of exogenously transfected PHR genes (
Figure 3a,b). The cellular CPD content was increased by UV-B irradiation in these cell lines to a similar extent. In HEK293/potPHR1 and HEK293/acPHR cells, CPD levels were recovered by blue light exposure, but not in HEK293/pcDNA3.1 (
Figure 3c). These data suggest that HEK293/acPHR cells possess blue light-dependent photorepair activity derived from the exogenously transfected putative acPHR gene. These results provide strong evidence for the presence of PHR in acerola. Earlier studies detected DNA repair activity in acerola juice [
20,
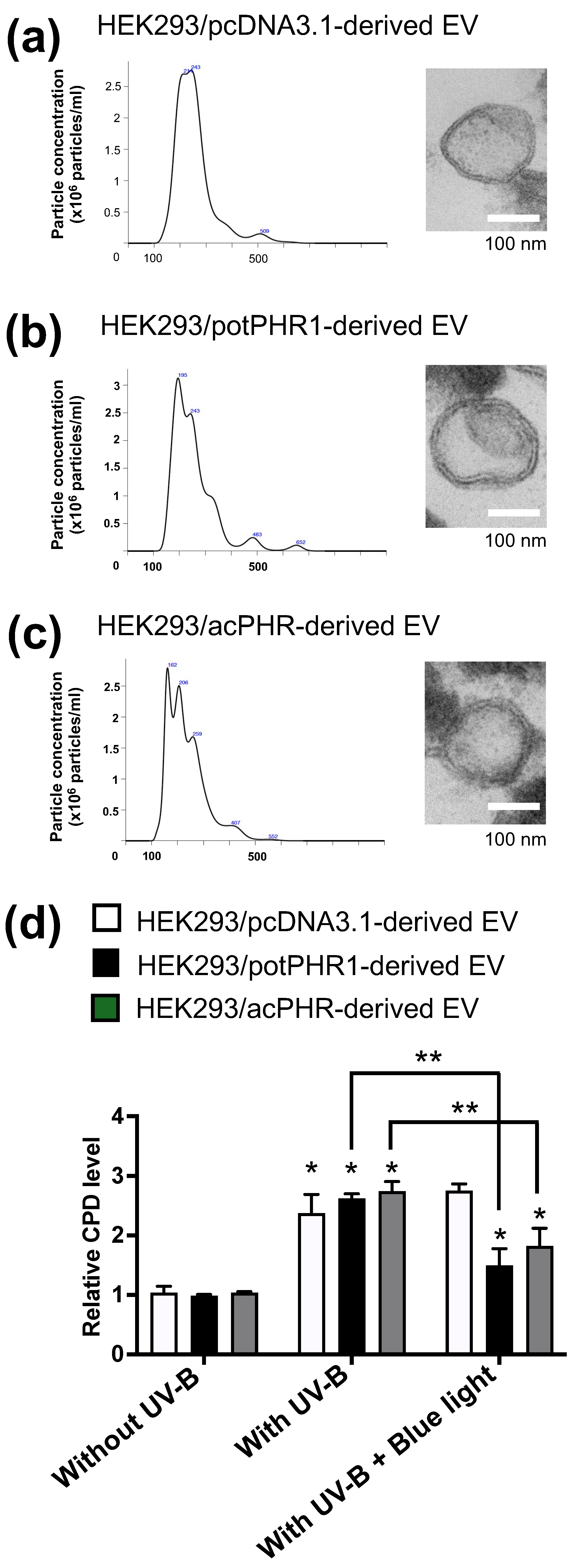
21], suggesting that the active form of PHR exists outside the cells in acerola. Therefore, we hypothesized that the acPHR protein is localized in extracellular nanovesicles, such as exosomes. As shown in
Figure 4a–c, NTA revealed that the EVs derived from HEK293/pcDNA3.1, HEK293/potPHR1, and HEK293/acPHR had similar size patterns and concentrations. Electron microscopic analysis suggested the existence of typical features of the lipid bilayer in the EVs of these three cell lines. Then, we tested whether these EVs possessed photorepair activity in intact HEK293 cells. Cellular CPD levels were significantly reduced following blue light exposure in HEK293 cells pre-incubated with EVs from HEK293/potPHR1 or HEK293/acPHR but not in cells incubated with EVs from HEK293/pcDNA3.1. (
Figure 4d). These data suggest that the PHR protein is localized not only inside cells but also outside cells, such as in EVs, and that EVs can transfer PHR activity to other cells.
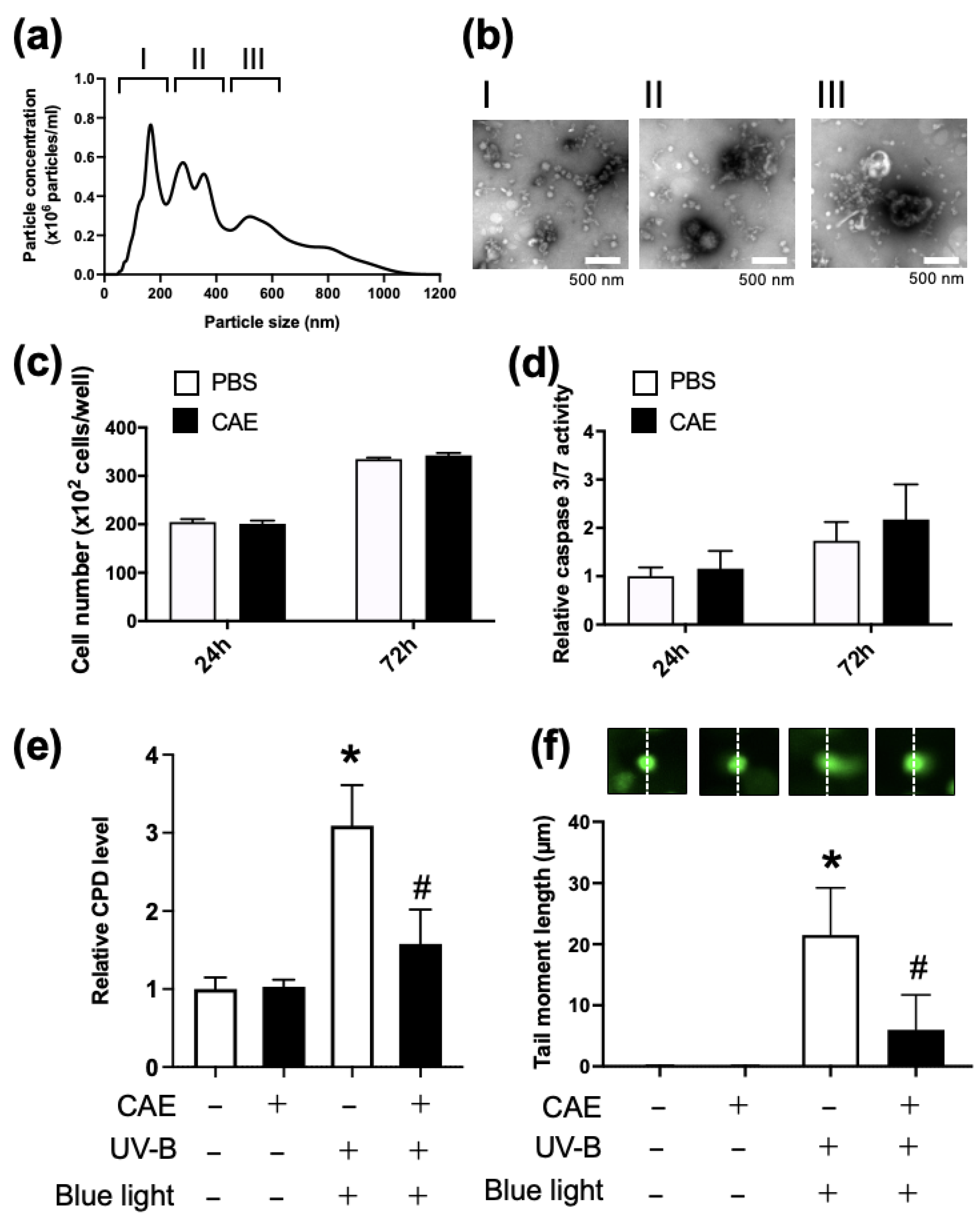
However, studies using HEK293 cell-derived cell lines were based on artificial conditions. Thus, we next evaluated the photorepair activity of the acerola extract in NHDF, which seemed to be closer in terms of physiological conditions, to humans than genetically transformed cell lines. Both NTA and electron microscopy showed vesicles of various sizes in the CAE, suggesting that they contained both extracellular and intracellular vesicles (
Figure 5a,b). We first evaluated the effect of CAE on cell proliferation and apoptosis in NHDF and found no cellular toxicity of CAE for up to 72 h (
Figure 5c,d).
Figure 5e shows the increase in cellular CPD by UV-B irradiation and restoration of CPD content by CAE treatment and blue light exposure, which suggests the susceptibility of NHDFs to DNA damage by UB-V irradiation and the possibility of blue light-dependent PHR activity in CAE. To monitor DNA damage induced by UV-B irradiation via another method other than cellular CPD content measurement, we performed COMET assay in NHDF (
Figure 5f). The COMET assay is thought to be a general method for monitoring UV-dependent cell damage, involving both thymine dimer formation and double-stranded DNA breaks. CAE and subsequent blue light exposure could restore CPD content by intrinsic PHR activity, although they seemed to fail to recover double-stranded DNA breaks and other DNA damages induced by UV-B. Therefore, CAE only partially recovered the tail length moment in the COMET assay. These findings indicate the presence of PHR in the acerola extract and the transfer of PHR-dependent photorepair activity to target cells by PHR-containing EVs.
Other studies provided preclinical and clinical evidence of reduced UV-induced DNA damage in the skin with the topical application of sunscreens containing exogenous PHR for industrial utility [
19]. Photosome-V was developed and used in industrial applications and contains PHR derived from
Anacystis nidulans, a Cyanobacteria. It has shown protective effects in human skin against CPD increase, DNA damage, inflammation, and stress signals induced by UV-B [
22]. From this perspective, PHR-liposomes were thought to be more promising than intact PHR for cosmetic and pharmaceutical preparations. However, these PHR-containing liposomes are difficult to use as commercially available products because of the numerous steps and high cost of factory-based manufacturing with adequate quality control. Contrastingly, preparing acPHR-containing CAE from acerola fruit seems to be easier and less costly than preparing PHR liposomes, for example, from Antarctic microalgae [
23]. This advantage is due to the stable supply of acerola from global agricultural markets and the development of a simple method for preparing CAE without special reagents or equipment, as described in the Materials and Methods. Additionally, concerns regarding the safety of acPHR and CAE are low because acerola is widely consumed globally. Thus, acPHR may have powerful cosmetic, industrial, and aesthetic medicinal applications.