The Potential Role of Phytochemicals in Alzheimer’s Disease
Abstract
1. Introduction
2. Herbs in the Prevention of AD
2.1. Ayurveda Herbs in AD Treatment
2.2. Traditional Chinese Medicine in AD Treatment
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Siu, W.; Li, L.; Jin, Y.; Linag, S.; Cao, M.; Ma, M.; Wu, Z. Autophagy in Alzheimer’s disease and promising modulatory effects of herbal medicine. Exp. Gerontol. 2019, 119, 100–110. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.R.; Qu, Y.J.; Hu, B.; An, H.M. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacol. 2022, 152, 113208. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Hu, T.; Wei, J.; Wang, X.; Cai, C.; Gu, Y.; Hu, Y.; Wang, W.; Wu, Q.; Fang, J. Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine. Comput. Struct. Biotechnol. J. 2024, 23, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Silva, V.; Pereira, G.C.; Ribeiro, A.M. Therapeutic approaches using natural substances on the streptozotocin-induced animal model of sporadic Alzheimer’s disease: A systematic review. Adv. Tradit. Med. 2024, 24, 145–169. [Google Scholar] [CrossRef]
- Kalia, M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism 2003, 52, 36–38. [Google Scholar] [CrossRef]
- Pandey, S.N.; Rangra, N.K.; Singh, S.; Arora, S.; Gupta, V. Evolving role of natural products from traditional medicinal herbs in the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2021, 12, 2718–2728. [Google Scholar] [CrossRef]
- Dubey, T.; Chinnathambi, S. Brahmi (Bacopa monnieri): An ayurvedic herb against the Alzheimer’s disease. Arch. Biochem. Biophys. 2019, 676, 108153. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D. Alzheimer’s disease: Review of current nanotechnological therapeutic strategies. Expert Rev. Neurother. 2020, 20, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Datta, S.; Patil, S. Evaluation of Traditional Herb Extract Salvia officinalis in Treatment of Alzheimers Disease. Pharmacogn. J. 2020, 12, 1735–1747. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Tredici, K.D.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Woo, J.H.; Lee, J.H.; Kim, H.; Park, S.J.; Joe, E.H.; Jou, I. Control of inflammatory responses: A new paradigm for the treatment of chronic neuronal diseases. Exp. Neurobiol. 2015, 24, 95. [Google Scholar] [CrossRef]
- Peric, A.; Annaert, W. Early etiology of Alzheimer’s disease: Tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol. 2015, 129, 363–381. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Hodson, R. Alzheimer’s disease. Nature 2018, 559, S1. [Google Scholar] [CrossRef]
- Fink, H.A.; Linskens, E.J.; MacDonald, R.; Silverman, P.C.; McCarten, J.R.; Talley, K.M.C.; Forte, M.L.; Desai, P.J.; Nelson, V.A.; Miller, M.A.; et al. Benefits and harms of prescription drugs and supplements for treatment of clinical Alzheimer-type dementia: A systematic review and meta-analysis. Ann. Intern. Med. 2020, 172, 656–668. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, C.; Chen, W. A network pharmacology-based study on Alzheimer disease prevention and treatment of Qiong Yu Gao. BioData Min. 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Soheili, M.; Karimian, M.; Hamidi, G.; Salami, M. Alzheimer’s disease treatment: The share of herbal medicines. Iran. J. Basic Med. Sci. 2021, 24, 123. [Google Scholar]
- Ma, Y.; Liu, S.; Zhou, Q.; Li, Z.; Zhang, Z.; Yu, B. Approved drugs and natural products at clinical stages for treating Alzheimer’s disease. Chin. J. Nat. Med. 2024, 22, 699–710. [Google Scholar] [CrossRef]
- Huang, L.K.; Kuan, Y.C.; Lin, H.W.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease: A 2020-2023 update. J. Biomed. Sci. 2023, 30, 83. [Google Scholar] [CrossRef] [PubMed]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; Silva, B.S.d.; Passamani Ambrosio, R.; Castro, F.C.d.A.Q.; Teixeira, S.F.; et al. Advances in Alzheimer’s disease’s pharmacological treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Zhang, Z.; Yu, B. Herbal medicine in the treatment of Alzheimer’s disease. Chin. J. Integr. Med. 2014, 21, 102–107. [Google Scholar] [CrossRef]
- Soheili, M.; Khalaji, F.; Mirhashemi, M.; Salami, M. The effect of essential oil of Lavandula angustifolia on amyloid beta polymerization: An in vitro study. Iran. J. Chem. Chem Eng. 2018, 7, 103–107. [Google Scholar]
- Oseni, O.A.; Okoh, O.S.; Kayode, A.A. Acetylcholinesterase inhibition and antioxidant potentials of some Nigerian medicinal plants for the treatment of alzheimer disease and other related complications. J. Nat. Prod. Res. 2020, 4, 417–434. [Google Scholar]
- Yadav, A.; Jangra, M.; Kr, P. Herbal and synthetic approaches for the treatment of epilepsy. Int. J. Nutri. 2014, 4, 43. [Google Scholar] [CrossRef]
- Lalotra, S.; Vaghela, J.S. Scientific reports of medicinal plants used for the prevention and treatment of neurodegenerative diseases. Pharm. Biosci. J. 2019, 7, 15–25. [Google Scholar] [CrossRef]
- Sharma, R.; Kuca, K.; Nepovimova, E.; Kabra, A.; Rao, M.M.; Prajapati, P.K. Traditional Ayurvedic and herbal remedies for Alzheimer’s disease: From bench to bedside. Expert Rev. Neurother. 2019, 19, 359–374. [Google Scholar] [CrossRef]
- Zhao, Z.; Yuan, Y.; Li, S.; Wang, X.; Yang, X. Natural compounds from herbs and nutraceuticals as glycogen synthase kinase-3β inhibitors in Alzheimer’s disease treatment. CNS Neurosci. Ther. 2024, 30, e14885. [Google Scholar] [CrossRef]
- Ratheesh, G.; Tian, L.; Venugopal, J.R.; Ezhilarasu, H.; Sadiq, A.; Fan, T.P.; Ramakrishna, S. Role of medicinal plants in neurodegenerative diseases. Biomanuf. Rev. 2017, 2, 2. [Google Scholar] [CrossRef]
- Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sci. 2020, 10, 964. [Google Scholar] [CrossRef]
- Rajamanickam, G.; Manju, S.L. A review on phyto-nanotechnology for therapy of alzheimer’s disease. Indian J. Biochem. Biophys. 2021, 59, 867–872. [Google Scholar]
- Sivaraman, D.; Anbu, N.; Kabilan, N. Review on current treatment strategy in Alzheimer’s disease and role of herbs in treating neurological disorders. Int. J. Trans. Res. Ind. Med. 2019, 1, 33–43. [Google Scholar]
- Singh, A.; Agarwal, S.; Singh, S. Age related neurodegenerative Alzheimer’s disease: Usage of traditional herbs in therapeutics. Neurosci. Lett. 2019, 717, 134679. [Google Scholar] [CrossRef]
- Patwardhan, B.A. The designer medicine, review of ethnopharmacology and Bioprospecting Research. Indian Drugs 2020, 37, 213–227. [Google Scholar]
- Hügel, H.M. Brain food for Alzheimer-free ageing: Focus on herbal medicines. Adv. Exp. Med. Biol. 2015, 863, 95–116. [Google Scholar]
- Habtemariam, S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid.-Based Complement. Alternat. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Indi, S.S.; Rao, K.S.J. Garlic extract exhibits antiamyloidogenic activity on amyloid-beta fibrillogenesis: Relevance to Alzheimer’s disease. Phytother. Res. 2009, 23, 111–115. [Google Scholar] [CrossRef]
- Bordoloi, S.; Pathak, K.; Devi, M.; Saikia, R.; Das, J.; Kashyap, V.H.; Das, D.; Ahmad, M.Z.; Abdel-Wahab, B.A. Some promising medicinal plants used in Alzheimer’s disease: An ethnopharmacological perspective. Discov. Appl. Sci. 2024, 6, 215. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malaria J. 2011, 10, S4. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective herbs for the management of Alzheimer’s disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.H.; Li, M. Synergic effects of berberine and curcumin on improving cognitive function in an Alzheimer’s disease mouse model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rai, S.N.; Maurya, A.; Mishra, G.; Awasthi, R.; Shakya, A.; Chellappan, D.K.; Dua, K.; Vamanu, E.; Chaudhary, S.K.; et al. Therapeutic potential of phytoconstituents in management of Alzheimer’s disease. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5578574. [Google Scholar] [CrossRef]
- Kushwah, S.; Maurya, N.S.; Kushwaha, S.; Scotti, L.; Chawade, A.; Mani, A. Herbal therapeutics for alzheimer’s disease: Ancient Indian medicine system from the modern viewpoint. Curr. Neuropharmacol. 2023, 21, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, M.; Holcomb, L.A.; Hitt, A.R.; Tharakan, B.; Porter, J.W.; Young, K.A.; Manyam, B.V. Centella asiatica extract selectively decreases amyloid β levels in hippocampus of alzheimer’s disease animal model. Phytotherapy Res. 2009, 23, 14–19. [Google Scholar] [CrossRef]
- Lyle, N.; Gomes, A.; Sur, T.; Munshi, S.; Paul, S.; Chatterjee, S.; Bhattacharyya, D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav. Brain Res. 2009, 202, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Descamps, O.; John, V.; Bredesen, D.E. Ayurvedic medicinal plants for Alzheimer’s disease: A review. Alzheimers Res. Ther. 2012, 4, 22. [Google Scholar] [CrossRef]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Scient Rep. 2019, 9, 561. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Sahiner, N. A review on phyto-therapeutic approaches in alzheimer’s disease. J. Funct. Biomater. 2023, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Chandra, P. Effects of natural remedies on memory loss and Alzheimer’s disease. Afr. J. Biol. Sci. 2024, 6, 187–211. [Google Scholar]
- Kim, D.S.; Park, S.Y.; Kim, J.Y. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA (1–42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, R.; Dey, A.; Chakrabarty, A.K.; Banerjee, D.; Narwaria, A.; Sharma, S.; Rai, R.K.; Kati, C.K.; Dubay, S.K. Diabetes Mellitus and Alzheimer’s Disease: Understanding Disease Mechanisms, their Correlation, and Promising Dual Activity of Selected Herbs. J. Ethnopharmacol. 2024, 333, 118402. [Google Scholar] [CrossRef]
- Menghani, Y.R.; Bhattad, D.M.; Chandak, K.K.; Taksande, J.R. A Review: Pharmacological and herbal remedies in The Management of Neurodegenerative disorder (Alzheimer’s). Int J. Pharamcogn. 2021, 2, 18–27. [Google Scholar] [CrossRef]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.H.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Song, S.B.; Tung, N.H.; Quang, T.H.; Ngan, N.T.T.; Kim, K.E.; Kim, Y.H. Inhibition of TNF-α-mediated NF-κB Transcriptional Activity in HepG2 Cells by Dammarane-type Saponins from Panax ginseng Leaves. J. Ginseng. Res. 2012, 36, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Mathiyalagan, R.; Yang, D.C. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-κB activation in macrophages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M. Anti-inflammatory Effects of Ginsenosides Rg5, Rz1, and Rk1: Inhibition of TNF-α-induced NF-κB, COX-2, and iNOS transcriptional expression. Phytother. Res. 2014, 28, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Taranalli, A.D.; Cheeramkuzhy, T.C. Influence of Clitoria ternatea extracts on memory and central cholinergic activity in rats. Pharm. Biol. 2020, 38, 51–56. [Google Scholar] [CrossRef]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007, 73, 283–285. [Google Scholar] [CrossRef]
- Chheng, C.; Waiwut, P.; Plekratoke, K.; Chulikhit, Y.; Daodee, S.; Manthakantirat, O.; Pitiporn, S.; Musigavong, N.; Kwankhao, P.; Boonyarat, C. Multitarget activities of Kleeb Bua Daeng, a Thai traditional herbal formula, against Alzheimer’s disease. Pharm. 2020, 13, 79. [Google Scholar] [CrossRef]
- Jyothi, C.H.; Shashikala, G.; Vidya, H.K.; Shashikala, G.H. Evaluation of effect of alcoholic extract of Tinospora cordifolia on learning and memory in alprazolam induced amnesia in albino mice. Int. J. Basic Clin. Pharm. 2016, 5, 2159–2163. [Google Scholar]
- Rai, K.S.; Murthy, K.D.; Karanth, K.S.; Nalini, K.; Rao, M.S.; Srinivasan, K.K. Clitoria ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia. 2002, 73, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Alama, B.; Haque, E. Anti-alzheimer and antioxidant activity of Celastrus paniculatus seed. Iran. J. Pharm. Sci. 2011, 7, 49–56. [Google Scholar]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Damodaran, T.; Cheah, P.S.; Murugaiyah, V.; Hassan, Z. The nootropic and anticholinesterase activities of Clitoria ternatea Linn. root extract: Potential treatment for cognitive decline. Neurochem Int. 2020, 139, 104785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, C.; Zhang, L.; Cui, Y.; Gu, Y.; Guo, J.; Wu, D.; Li, Q.; Song, W. Cognitive improvement during treatment for mild Alzheimer’s disease with a Chinese herbal formula: A randomized controlled trial. PLoS ONE 2015, 10, e0130353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, C.; Wei, D.; Li, H.; Leung, E.L.H.; Deng, Q.; Liu, Z.; Fan, X.X.; Zhang, Z. Long-term efficacy of Chinese medicine Bushen Capsule on cognition and brain activity in patients with amnestic mild cognitive impairment. Pharmacol. Res. 2019, 146, 104319. [Google Scholar] [CrossRef]
- Park, H.; Kang, S.; Nam, E.; Suh, Y.H.; Chang, K.A. The protective effects of PSM-04 against beta amyloid-induced neurotoxicity in primary cortical neurons and an animal model of Alzheimer’s disease. Front. Pharmacol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Ning, F.; Chen, L.; Chen, L.; Liu, X.; Zhu, Y.; Hu, J.; Xie, G.; Xiu, J.; Shi, K.; Lan, Z.; et al. Combination of polygoni multiflori radix praeparata and acori tatarinowii rhizoma alleviates learning and memory impairment in scopolamine-treated mice by regulating synaptic-related proteins. Front. Pharmacol. 2021, 12, 679573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacol. 2020, 121, 109670. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, Y.J.; Jeong, S.J. Korean traditional herbal formula Soshiho-tang attenuates memory impairment and neuronal damage in mice with amyloid-beta-induced Alzheimer’s disease. Integr. Med. Res. 2021, 10, 100723. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP 3 inflammasomes and TLR 4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Ahmed, H.I.; Zaky, H.O.; Badr, A.M. Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 2021, 267, 113468. [Google Scholar] [CrossRef] [PubMed]
- Cuya, T.; Baptista, L.; Costa França, T.C. A molecular dynamics study of components of the ginger (Zingiber officinale) extract inside human acetylcholinesterase: Implications for Alzheimer disease. J. Biomol. Struct. Dyn. 2018, 36, 3843–3855. [Google Scholar] [CrossRef]
- Liu, J.; Yin, F.; Guo, L.; Gu, G.; Gao, R.; Peng, B.; Wang, Y.; Li, A.; Guo, J.; Xu, X.; et al. Molecular Mechanisms of Geniposide and Genipin Against Alzheimer’s Disease. Bioact. Nutraceuticals Diet. Suppl. Neurol. Brain Dis. 2015, 221–227. [Google Scholar]
- Cao, Z.; Wang, F.; Xiu, C.; Zhang, J.; Li, Y. Hypericum perforatum extract attenuates behavioral, biochemical, and neurochemical abnormalities in aluminum chloride-induced Alzheimer’s disease rats. Biomed. Parmacother. 2017, 91, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Thancharoen, O.; Limwattananon, C.; Waleekhachonloet, O.; Rattanachotphanit, T.; Limwattananon, P.; Limpawattana, P. Ginkgo biloba extract (EGb761), cholinesterase inhibitors, and memantine for the treatment of mild-to-moderate alzheimer’s disease: A network meta-analysis. Drugs Aging. 2019, 36, 435–452. [Google Scholar] [CrossRef]
- Müller, W.E.; Eckert, A.; Eckert, G.P.; Fink, H.; Friedland, K.; Gauthier, S.; Hoerr, R.; Möller, H.J.; Ihl, R.; Kasper, S.; et al. Therapeutic efficacy of the Ginkgo special extract EGb761® within the framework of the mitochondrial cascade hypothesis of Alzheimer’s disease. The World J. Biol. Psychiatry 2019, 20, 173–189. [Google Scholar] [CrossRef]
- Yancheva, S.; Ihl, R.; Nikolova, G.; Panayotov, P.; Schlaefke, S.; Hoerr, R. Ginkgo biloba extract EGb 761®, donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: A randomised, double-blind, exploratory trial. Aging Ment Health. 2009, 13, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.; Yoshitake, S.; Ijiri, S.; Koch, E.; Nöldner, M.; Yoshitake, T. Ginkgo biloba leaf extract (EGb 761®) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: Possible implications for the cognitive enhancing properties of EGb 761®. Int. Psychogeriatr. 2012, 24, S25–S34. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ramassamy, C.; Doré, S.; Christen, Y.; Poirier, J.; Quirion, R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef]
- Nikmahzar, E.; Jahanshahi, M.; Babakordi, F. Ginkgo biloba Extract Decreases Scopolamine-Induced Congophilic Amyloid Plaques Accumulation in Male Rat’s Brain. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e69143. [Google Scholar] [CrossRef]
- Soheili, M.; Tavirani, M.R.; Salami, M. Clearance of amyloid beta plaques from brain of Alzheimeric rats by Lavandula angustifolia. Neurosci Medic. 2012, 3, 362–367. [Google Scholar] [CrossRef][Green Version]
- Taiwo, A.E.; Leite, F.B.; Lucena, G.M.; Barros, M.; Silveira, D.; Silva, M.V.; Ferreira, V.M. Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: Influence of administration and gender. Indian J. Pharmacol. 2012, 44, 189. [Google Scholar]
- Bounihi, A.; Hajjaj, G.; Alnamer, R.; Cherrah, Y.; Zellou, A. In vivo potential anti-inflammatory activity of Melissa officinalis L. essential oil. Adv. Pharmcol. Sci. 2013, 2013, 101759. [Google Scholar]
- Leśniewicz, A.; Jaworska, K.; Żyrnicki, W. Macro-and micro-nutrients and their bioavailability in polish herbal medicament. Food Chem. 2006, 99, 670–679. [Google Scholar] [CrossRef]
- Hassanzadeh, G.; Pasbakhsh, P.; Akbar, M.; Shorki, S.; Ghahremani, M.; Amin, G.; Kashani, I.; Tameh, A.A. Neuroprotective properties of Melissa officinalis L. extract against ecstasy-induced neurotoxicity. Cell J. 2013, 13, 25–30. [Google Scholar]
- Hu, Y.R.; Xing, S.L.; Chen, C.; Shen, D.Z.; Chen, J.L. Tiaoxin Recipe, a Chinese herbal formula, inhibits microRNA-34a expression in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. J. Integr. Med. 2019, 17, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Sampson, J.; Theobald, A.E.; Hart, S.; Lis-Balchin, M.; Hoult, J.R.; Evans, P.; Jenner, P.; Milligan, S.; et al. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2001, 53, 1347–1356. [Google Scholar] [CrossRef]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Eskandari-Roozbahani, N.; Shomali, T.; Taherianfard, M. Neuroprotective Effect of Zataria Multiflora Essential Oil in Rats with Alzheimer’s Disease: A Mechanistic Study. Basic Clin. Neurosci. 2019, 10, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, M.V.; Gujarathi, P.P.; Mulla, T.; Bagban, I. Hypericum perforatum: A comprehensive review on pharmacognosy, preclinical studies, putative molecular mechanism, and clinical studies in neurodegenerative diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3803–3818. [Google Scholar] [CrossRef]

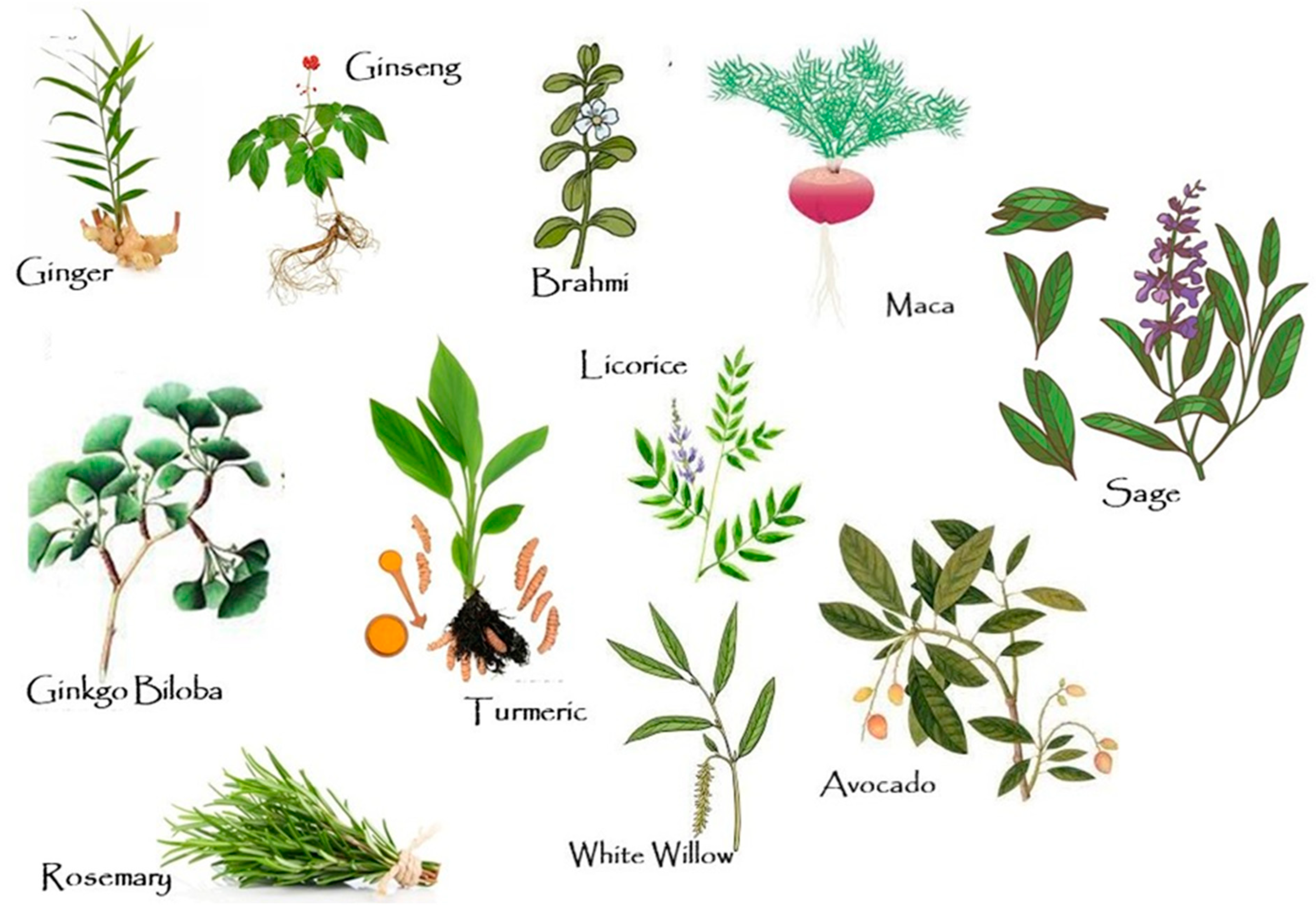
| Plant/Herbal Formula | Main Active Compounds | Dose/Exposure Time | Model | Neuroprotective Effects | Reference |
|---|---|---|---|---|---|
| Asiatic pennywort (Centella asiatica L.) | Phenols, Flavonoids | 2.5 or 5.0 g/kg/day, for 2 or 8 months | Mouse |
| [50,55] |
| Jatamansi (Nardostachys jatamansi) | Flavonoids, Polyphenols, Glycoside, Thankuniside, Triterpene | 200 and 500 mg/kg for 31 days | Rats |
| [51] |
| Curcuma longa | Curcumin | 7.5 mg/kg/day for 7 days | Mice |
| [70] |
| Ashwagandha (Withania somnifera) | Withanolides, Alkaloids | 200 and 500 mg/kg for 30 days | Mice |
| [44,59] |
| Rosemary (Rosmarinus officinalis) | Carnosic acid, Rosmarinic acid, Carnosol | 10, 20, 100, 250, 500, or 1000 μg/cm2 of RE or 2, 10 or 20 μg/cm2 of CA, 24 h | Mice |
| [44,64] |
| Kleeb Bua Daeng (KBD) | Phenols, Flavonoids | 100 and 500 mg/kg/day for 7 days | Mice |
| [66] |
| Tinospora cordifolia | Alkaloids (Choline), Phenolics | 140 mg/kg and 280 mg/kg for 14 days | Mice |
| [67] |
| Clitoria ternatea (C. ternatea) | Alkaloids, Sapoins, Flavonoids, Coumarins Lignans | 100 mg/kg for 30 days | Rats |
| [68] |
| 100, 200 and 300 mg/kg for 28 days | Rats |
| [71] |
| Plant/Herbal Formula | Main Active Compounds | Dose/Exposure Time | Model | Neuroprotective Effects | Reference |
|---|---|---|---|---|---|
| Soshiho-tang (SST) | Baicalin, Baicalein, Wogonin, Liquiritin, Glycyrrhizin | 500, 1000, or 2000 mg/kg/day for 20 days | Mice |
| [77] |
| Scutellaria baicalensis | Flavonoids (Baicalin) | 100 mg/kg for 33 days | Mice |
| [78] |
| Sesamum indicum L. | Sesame oil (sesamin) | 1 mL/kg or 2 mL/kg for 6 weeks | Mice |
| [79] |
| Hypericum perforatum | Catechin, Quercetin, Resveratrol, Curcumin, Isoflavones | 300 mg/kg for 90 days | Rats |
| [82,99] |
| Ginkgo biloba | Flavonoids (Quercetin, Kaempferol, Isorhamnetin), terpenoids | 240 mg/day for 22 weeks | Outpatients |
| [44,49,85] |
| Lavender (Lavandula angustifolia) | Linalool, Linalyl Acetate | 6.25, 12.5, 25, 50, and 100 μg/mL for 24 h | Human hepatoma G2 (HepG2) |
| [57] |
| Tiaoxin | Flavonoids Kaempferol Apigenin | 0.057 g/day for 12 weeks | Mice |
| [49,94] |
| Zataria multiflora | Carvacrol, Thymol, p-Cymene | 100 μL/kg/day for 20 days | Rats |
| [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Wójcik, E.; Gajowniczek-Ałasa, D.; Pawlikowska-Pawlęga, B.; Szwajgier, D. The Potential Role of Phytochemicals in Alzheimer’s Disease. Nutrients 2025, 17, 653. https://doi.org/10.3390/nu17040653
Baranowska-Wójcik E, Gajowniczek-Ałasa D, Pawlikowska-Pawlęga B, Szwajgier D. The Potential Role of Phytochemicals in Alzheimer’s Disease. Nutrients. 2025; 17(4):653. https://doi.org/10.3390/nu17040653
Chicago/Turabian StyleBaranowska-Wójcik, Ewa, Dorota Gajowniczek-Ałasa, Bożena Pawlikowska-Pawlęga, and Dominik Szwajgier. 2025. "The Potential Role of Phytochemicals in Alzheimer’s Disease" Nutrients 17, no. 4: 653. https://doi.org/10.3390/nu17040653
APA StyleBaranowska-Wójcik, E., Gajowniczek-Ałasa, D., Pawlikowska-Pawlęga, B., & Szwajgier, D. (2025). The Potential Role of Phytochemicals in Alzheimer’s Disease. Nutrients, 17(4), 653. https://doi.org/10.3390/nu17040653






