Efficacy and Safety of Phase 1 of Very Low Energy Ketogenic Therapy (VLEKT) in Subjects with Obesity and Mild Renal Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
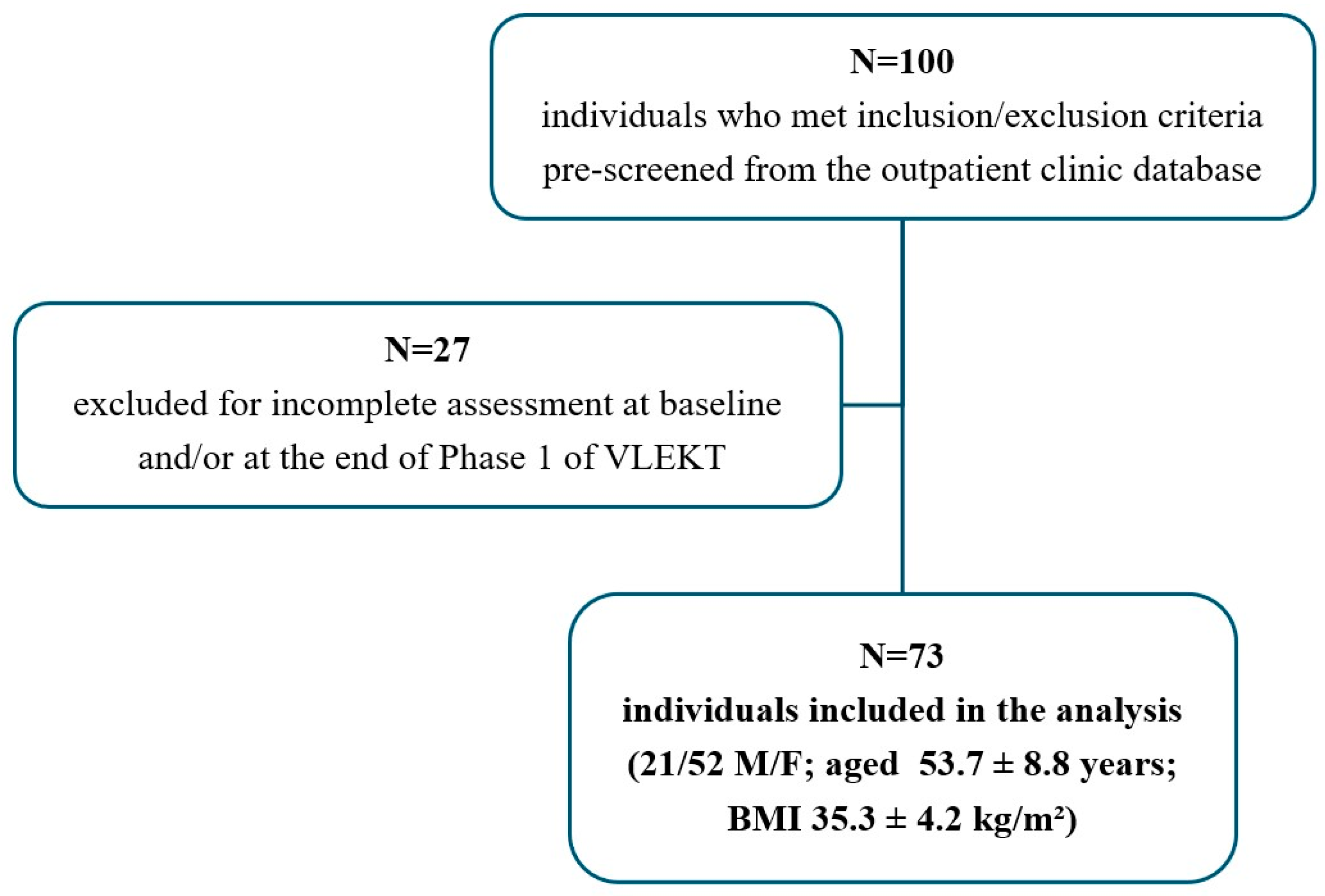
2.2. Study Population
2.3. Study Protocol
2.4. Anthropometric Measurements
2.5. Laboratory Assessments
2.6. Renal Function Assessment
2.7. Insulin Resistance Assessment
2.8. Nutritional Intervention
2.9. Adherence to the Nutritional Intervention
2.10. Power Analysis
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Mallamaci, F.; Tripepi, G. Risk Factors of Chronic Kidney Disease Progression: Between Old and New Concepts. J. Clin. Med. 2024, 13, 678. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carro, C.; Vergara, A.; Bermejo, S.; Azancot, M.A.; Sellares, J.; Soler, M.J. A Nephrologist Perspective on Obesity: From Kidney Injury to Clinical Management. Front. Med. 2021, 8, 655871. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, F.F.; Schytz, P.A.; Heerspink, H.J.L.; von Scholten, B.J.; Idorn, T. Obesity-Related Kidney Disease: Current Understanding and Future Perspectives. Biomedicines 2023, 11, 2498. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Chinnadurai, R.; Al-Chalabi, S.; Evans, P.; Kalra, P.A.; Syed, A.A.; Sinha, S. Obesity and chronic kidney disease: A current review. Obes. Sci. Pract. 2023, 9, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic Kidney Disease: Its Relationship With Obesity. Cureus 2022, 14, e30535. [Google Scholar] [CrossRef] [PubMed]
- Mascali, A.; Franzese, O.; Nistico, S.; Campia, U.; Lauro, D.; Cardillo, C.; Di Daniele, N.; Tesauro, M. Obesity and kidney disease: Beyond the hyperfiltration. Int. J. Immunopathol. Pharmacol. 2016, 29, 354–363. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. J. Nephrol. 2017, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Henegar, J.R.; Dwyer, T.M.; Liu, J.; Da Silva, A.A.; Kuo, J.J.; Tallam, L. Is obesity a major cause of chronic kidney disease? Adv. Ren. Replace. Ther. 2004, 11, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Grassi, D.; Cicero, A.F.G.; Bagnato, C.; Paolini, B.; Muscogiuri, G. A New Nomenclature for the Very Low-Calorie Ketogenic Diet (VLCKD): Very Low-Energy Ketogenic Therapy (VLEKT). Ketodiets and Nutraceuticals Expert Panels: “KetoNut”, Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). Curr. Nutr. Rep. 2024, 13, 552–556. [Google Scholar]
- Joshi, S.; Shi, R.; Patel, J. Risks of the ketogenic diet in CKD—The con part. Clin. Kidney J. 2024, 17, sfad274. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Camajani, E.; Verde, L.; Perrini, S.; Cignarelli, A.; Prodam, F.; Gambineri, A.; Isidori, A.M.; Colao, A.; et al. Ketogenic nutritional therapy (KeNuT)-a multi-step dietary model with meal replacements for the management of obesity and its related metabolic disorders: A consensus statement from the working group of the Club of the Italian Society of Endocrinology (SIE)-diet therapies in endocrinology and metabolism. J. Endocrinol. Investig. 2024, 47, 487–500. [Google Scholar]
- Annunziata, G.; Caprio, M.; Verde, L.; Carella, A.M.; Camajani, E.; Benvenuto, A.; Paolini, B.; De Nicola, L.; Aucella, F.; Bellizzi, V.; et al. Nutritional assessment and medical dietary therapy for management of obesity in patients with non-dialysis chronic kidney disease: A practical guide for endocrinologist, nutritionists and nephrologists. A consensus statement from the Italian society of endocrinology (SIE), working group of the club nutrition-hormones and metabolism; the Italian society of nutraceuticals (SINut), club ketodiets and nutraceuticals “KetoNut-SINut”; and the Italian society of nephrology (SIN). J. Endocrinol. Investig. 2024, 47, 2889–2913. [Google Scholar]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients With Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.F.; Benjamin, O.; Lappin, S.L. End-Stage Renal Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Janiszewski, P.M.; Ross, R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010, 33, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Gaspa, G.; Naciu, A.M.; Rosa, C.D.; Lattanzi, G.; Beato, I.; Micheli, V.; Turriziani, C.; Khazrai, Y.M.; Cesareo, R. Short- and long-term effects of very low- and low-calorie ketogenic diets on metabolism and cardiometabolic risk factors: A narrative review. Minerva Endocrinol. 2023, 48, 318–333. [Google Scholar] [CrossRef]
- Dilliraj, L.N.; Schiuma, G.; Lara, D.; Strazzabosco, G.; Clement, J.; Giovannini, P.; Trapella, C.; Narducci, M.; Rizzo, R. The Evolution of Ketosis: Potential Impact on Clinical Conditions. Nutrients 2022, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, B.; Gong, A.Y.; Malhotra, D.K.; Gupta, R.; Dworkin, L.D.; Gong, R. The ketone body beta-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021, 100, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.; Yasuda-Yamahara, M.; Kuwagata, S.; Chin-Kanasaki, M.; Kume, S. Ketone Body Metabolism in Diabetic Kidney Disease. Kidney360 2024, 5, 320–326. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Roberts, C.G.P.; Vangala, C.; Shetty, G.K.; McKenzie, A.L.; Weimbs, T.; Volek, J.S. The case for a ketogenic diet in the management of kidney disease. BMJ Open Diabetes Res. Care 2024, 12, e004101. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
| Parameters | Baseline (n = 73) | After 45 Days (±2 Days) of Phase 1 (n = 73) | p-Value | Δ% |
|---|---|---|---|---|
| Anthropometrical parameters | ||||
| Weight (kg) | 97.7 ± 13.3 | 89.0 ± 12.5 | <0.001 | −8.9 ± 2.9 |
| BMI (kg/m2) | 35.3 ± 4.2 | 32.2 ± 4.2 | <0.001 | −8.9 ± 3.0 |
| Normal weight (n,%) | 0 | 1 (1.4) | χ2= 0.01, p = 1.000 | |
| Overweight (n,%) | 6 (8.2) | 23 (31.5) | χ2= 11.02, p < 0.001 | |
| Obesity I class (n,%) | 30 (41.1) | 30 (41.1) | χ2= 0.03, p = 0.866 | |
| Obesity II class (n,%) | 26 (35.6) | 16 (21.9) | χ2= 2.71, p = 0.099 | |
| Obesity III class (n,%) | 11 (15.1) | 3 (4.1) | χ2= 3.87, p = 0.048 | |
| WC (cm) | 105.5 ± 10.0 | 98.5 ±10.1 | <0.001 | −6.6 ± 3.1 |
| Biochemical parameters | ||||
| Fasting plasma glucose (mg/dL) | 102.1 ± 15.7 | 91.9 ± 12.4 | <0.001 | −8.9 ± 12.1 |
| Insulin (µIU/mL) | 18.0 ± 10.2 | 8.3 ± 3.8 | <0.001 | −47.3 ± 26.7 |
| HOMA-IR | 4.7 ± 3.3 | 1.9 ± 1.0 | <0.001 | −50.7 ± 29.2 |
| Total cholesterol (mg/dL) | 214 ± 39 | 174 ± 35 | <0.001 | −19.3 ± 15.2 |
| HDL cholesterol (mg/dL) | 53 ± 16 | 52 ± 14 | 0.253 | −0.0 ± 21.2 |
| LDL cholesterol (mg/dL) | 135 ± 33 | 104 ± 28 | <0.001 | −22.2 ± 15.7 |
| LDL/HDL | 2.7 ± 1.1 | 2.1 ± 0.7 | <0.001 | −18.7 ± 23.7 |
| Triglycerides (mg/dL) | 132 ± 73 | 93 ± 33 | <0.001 | −22.9 ± 25.7 |
| AST (U/L) | 24 ± 11 | 20 ± 9 | 0.001 | −10.1 ± 27.3 |
| ALT (U/L) | 30 ± 16 | 24 ± 14 | <0.001 | −12.1 ± 28.7 |
| eGFR (mL/min/1.73 m2) | 90.2 ± 14.6 | 92.5 ± 13.7 | 0.062 | 3.6 ± 12.7 |
| <90 mL/min/1.73 m2 (n,%) | 40 (54.8) | 29 (39.7) | χ2 = 2.75, p = 0.097 | |
| ≥90 mL/min/1.73 m2 (n,%) | 33 (45.2) | 44 (60.3) | ||
| Urea (mg/dL) | 37 ± 10 | 37 ± 12 | 0.987 | 1.3 ± 27.3 |
| Parameters | <90 mL/min/1.73 m2 | ≥90 mL/min/1.73 m2 | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 40) | After 45 Days (±2 Days) of Phase 1 (n = 40) | p-Value | Baseline (n = 33) | After 45 Days (±2 Days) of Phase 1 (n = 33) | p-Value | |
| Anthropometrical parameters | ||||||
| Weight (kg) | 95.9 ± 12.0 | 86.9 ± 10.9 | <0.001 | 99.9 ± 14.6 | 91.4 ± 14.0 | <0.001 |
| BMI (kg/m2) | 34.2 ± 3.9 | 31.0 ± 3.8 | <0.001 | 36.6 ± 4.3 | 33.5 ± 4.4 | <0.001 |
| Normal weight (n,%) | 0 | 1 (2.5%) | 0 | 0 | 0 | |
| Overweight (n,%) | 5 (12.5%) | 15 (37.5%) | χ2 = 5.40, p = 0.020 | 1 (3.0%) | 8 (24.2%) | χ2 = 4.63, p = 0.031 |
| Obesity I class (n,%) | 18 (45.5%) | 20 (50.0%) | χ2 = 0.05, p = 0.823 | 12 (36.4%) | 10 (30.3%) | χ2 = 0.07, p = 0.794 |
| Obesity II class (n,%) | 14 (35.0%) | 3 (7.5%) | χ2 = 7.47, p = 0.006 | 12 (36.4%) | 13 (39.4%) | χ2 = 0.00, p = 1.000 |
| Obesity III class (n,%) | 3 (7.5%) | 1 (2.5%) | χ2 = 0.26, p = 0.608 | 8 (24.2%) | 2 (6.1%) | χ2 = 2.95, p = 0.086 |
| WC (cm) | 104.1 ± 10.2 | 97.1 ± 10.6 | <0.001 | 107.1 ± 9.7 | 100.3 ± 9.4 | <0.001 |
| Biochemical parameters | ||||||
| Fasting plasma glucose (mg/dL) | 102.7 ± 16.5 | 90.4 ± 10.3 | <0.001 | 101.3 ± 15.0 | 93.7 ± 14.5 | 0.004 |
| Insulin (µIU/mL) | 17.0 ± 9.4 | 8.4 ± 3.3 | <0.001 | 19.1 ± 11.2 | 8.1 ± 4.4 | <0.001 |
| HOMA-IR | 4.5 ± 3.5 | 1.9 ± 0.9 | <0.001 | 4.9 ± 3.0 | 1.9 ± 1.1 | <0.001 |
| Total cholesterol (mg/dL) | 214 ± 34 | 173 ± 33 | <0.001 | 213 ± 45 | 175 ± 37 | <0.001 |
| HDL cholesterol (mg/dL) | 54 ± 18 | 54 ± 15 | 0.503 | 51 ± 14 | 50 ± 13 | 0.316 |
| LDL cholesterol (mg/dL) | 133 ± 28 | 102 ± 26 | <0.001 | 137 ± 38 | 107 ± 30 | <0.001 |
| LDL/HDL | 2.7 ± 1.0 | 2.0 ± 0.7 | <0.001 | 2.8 ± 1.2 | 2.2 ± 0.8 | 0.002 |
| Triglycerides (mg/dL) | 122 ± 35 | 88 ± 25 | <0.001 | 144 ± 101 | 99 ± 30 | 0.009 |
| AST (U/L) | 24 ± 10 | 21 ± 9 | 0.034 | 24 ± 11 | 20 ± 11 | 0.016 |
| ALT (U/L) | 27 ± 11 | 23 ± 9 | 0.009 | 32 ± 20 | 25 ± 18 | 0.004 |
| eGFR (mL/min/1.73 m2) | 79.1 ± 8.3 | 85.6 ± 12.0 | <0.001 | 103.8 ± 7.0 | 101.0 ± 10.5 | 0.072 |
| Urea (mg/dL) | 40 ±10 | 38 ± 11 | 0.393 | 34 ± 8 | 36 ± 13 | 0.523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verde, L.; Barrea, L.; Galasso, M.; Lucà, S.; Camajani, E.; Pisani, A.; Colao, A.; Caprio, M.; Muscogiuri, G. Efficacy and Safety of Phase 1 of Very Low Energy Ketogenic Therapy (VLEKT) in Subjects with Obesity and Mild Renal Impairment. Nutrients 2025, 17, 721. https://doi.org/10.3390/nu17040721
Verde L, Barrea L, Galasso M, Lucà S, Camajani E, Pisani A, Colao A, Caprio M, Muscogiuri G. Efficacy and Safety of Phase 1 of Very Low Energy Ketogenic Therapy (VLEKT) in Subjects with Obesity and Mild Renal Impairment. Nutrients. 2025; 17(4):721. https://doi.org/10.3390/nu17040721
Chicago/Turabian StyleVerde, Ludovica, Luigi Barrea, Martina Galasso, Stefania Lucà, Elisabetta Camajani, Antonio Pisani, Annamaria Colao, Massimiliano Caprio, and Giovanna Muscogiuri. 2025. "Efficacy and Safety of Phase 1 of Very Low Energy Ketogenic Therapy (VLEKT) in Subjects with Obesity and Mild Renal Impairment" Nutrients 17, no. 4: 721. https://doi.org/10.3390/nu17040721
APA StyleVerde, L., Barrea, L., Galasso, M., Lucà, S., Camajani, E., Pisani, A., Colao, A., Caprio, M., & Muscogiuri, G. (2025). Efficacy and Safety of Phase 1 of Very Low Energy Ketogenic Therapy (VLEKT) in Subjects with Obesity and Mild Renal Impairment. Nutrients, 17(4), 721. https://doi.org/10.3390/nu17040721









