Abstract
Background: The rising prevalence of metabolic-dysfunction-associated steatotic liver disease (MASLD) is a significant health challenge, and the consumption of ultra-processed foods (UPFs) could play a key role. Aim: The aim is assess the impact of UPF consumption changes on the development and progression of MASLD in adults. Design: This is a longitudinal study to assess how changes in UPF consumption affect liver fat and MASLD parameters over 6 months in 70 participants. Methods: Dietary intake was assessed using a validated food frequency questionnaire, and foods were classified according to the NOVA system. Participants were divided into three groups based on UPF consumption changes: maximum (T1), medium (T2), and minimum reduction (T3). Fatty liver parameters were assessed with magnetic resonance imaging and ultrasonography. Mediterranean diet (Med-diet) adherence and sociodemographic parameters were also recorded. The General Linear Model was used to determine relationships between UPF consumption, fatty liver disease parameters, and diet. Results: Participants in T1 experienced a 7.7% reduction in intrahepatic fat content (IFC) compared to 2.6% in T3. T1 showed increased Med-diet adherence and decreased meat and sweets consumption. The energy intake decreased by 605.3 kcal/day in T1, while T3 showed an increase of 209.5 kcal/day. Conclusions: Reducing UPF consumption leads to a decrease in IFC, associated with high Med-diet adherence and low calorie intake. Adopting these dietary patterns aligns with global sustainability goals and could further benefit MASLD patients by addressing environmental challenges alongside improving liver health.
1. Introduction
The rising prevalence of metabolic-dysfunction-associated steatotic liver disease (MASLD) has prompted significant concern among healthcare professionals and researchers [1]. A growing body of evidence suggests that one of the key contributors to this condition is the widespread consumption of ultra-processed foods (UPFs), which are linked to MASLD through a variety of metabolic and epidemiological mechanisms [2].
MASLD, previously known as non-alcoholic fatty liver disease (NAFLD) [3], is characterized by the accumulation of fat in the liver in individuals who do not consume excessive amounts of alcohol [4]. This condition can progress to steatohepatitis, fibrosis, and eventually cirrhosis of the liver [3,5]. According to data from the Institute for Health Metrics and Evaluation, NAFLD, currently MASLD, resulted in approximately 3.67 million global disability-adjusted life years (DALYs) in 2021, with an increase in DALYs within the age range of 30 to 85 years, for both sexes.
High-income countries exhibit the highest DALYs. DALYs are a measure of overall disease burden, expressed as the number of years lost due to ill health, disability, or early death. Specifically, cirrhosis and liver cancer caused by MASLD were responsible for 72.5% and 27.5% of the DALYs, respectively [6]. As shown by data from 2018 to 2021, the trend in MASLD-related deaths has been increasing, with the 2021 data showing 0.138 million deaths globally, affecting both women and men similarly [6,7]. These figures emphasize the substantial global health burden of MASLD, highlighting the need to address preventable risk factors like the consumption of UPFs.
UPFs are industrially manufactured food products that are high in added sugars, saturated fats, salt, and additives while being low in essential nutrients and fiber [8]. Excessive UPF consumption contributes to obesity, insulin resistance, and hepatic inflammation, key risk factors for MASLD [8,9].
Due to their high caloric density and low nutrient content, UPFs contribute to increased total caloric intake, leading to weight gain and obesity [9]. Obesity is a well-established risk factor for fat accumulation in the liver and the development of insulin resistance, which are critical initial steps in the pathogenesis of MASLD [3].
Research has shown that the high consumption of UPFs among children and adolescents is linked to an increased incidence of MASLD and other metabolic conditions [10]. Similarly, it has been observed that in both adult and elderly populations, a diet high in UPFs increases the risk of developing fatty liver and its long-term complications [11,12].
The biological mechanisms linking UPF consumption with MASLD involve several processes. First, UPFs often contain high levels of fructose, which is metabolized in the liver and can promote hepatic lipogenesis, thereby increasing fat accumulation in the liver [13]. The negative effects of fructose in UPFs are amplified by other components, such as saturated fats, food additives, and the absence of fiber and essential nutrients, which stimulate de novo lipogenesis and exacerbate metabolic dysfunction [14]. Second, UPFs can induce oxidative stress and inflammation, which are key factors in the progression of MASLD to more advanced states like steatohepatitis [15]. Third, the poor nutritional quality of UPFs, being deficient in fiber and antioxidants and high in additives, can disrupt the balance of the gut microbiome, leading to harmful health effects [16].
Currently, there are no medical treatments that directly cure MASLD, making lifestyle factors, particularly diet, essential for its management [17,18].
This study underscores the importance of reducing UPF consumption as a preventive dietary strategy to slow MASLD progression. Specifically, we aim to explore the relationship between UPF intake and metabolic indicators, such as liver fat accumulation and weight gain, in adult populations.
2. Methods
2.1. Design
The present study is a longitudinal analysis within the frame of the FLIPAN study, registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT04442620; accessed 22 February 2022) with the identifier NCT04442620 [19]. The FLIPAN study is a prospective randomized trial conducted across multiple centers in Spain, featuring personalized nutritional counseling based on a customized Mediterranean diet (Med-diet), combined with physical activity promotion, aimed at preventing and reversing metabolic-dysfunction-associated fatty liver disease (MASLD) among patients with metabolic syndrome (MetS). A schema explaining the design of the present study can be seen in Figure 1.
Figure 1.
Design of the present study.
2.2. Participants, Recruitment, and Ethics
The recruitment of the FLIPAN study participants was conducted between June 2018 and January 2020. In total, 143 people were contacted. Participants were required to be between 40 and 60 years old, be characterized as overweight or obese (with a body mass index of between 27 and 40 kg/m2), be diagnosed with MASLD using magnetic resonance imaging (MRI), and meet at least three MetS criteria according to the International Diabetes Federation guidelines [20] to be included.
Participants were excluded if they had a history of cardiovascular disease, liver disease (excluding MASLD), cancer or malignancy within the past five years, haemochromatosis, prior bariatric surgery, untreated depression, alcohol or drug abuse, pregnancy, primary endocrinological disorders (except untreated hypothyroidism), severe psychiatric disorders (including schizophrenia, bipolar disorder, eating disorders, or depression requiring hospitalization within the past six months), a Beck Depression Inventory score above 30, or concurrent steroid therapy or if they were unable to provide informed consent.
After excluding participants who did not meet the criteria or chose not to participate, 74 participants took part in the clinical trial. Four additional participants were excluded because they did not complete the food frequency questionnaire (FFQ) in one of the two visits. This questionnaire is used in the present study to assess UPF consumption. A total of 70 participants (n = 70) were included in the definitive analysis. A flow-chart of the eligibility process can be seen in Figure 2.
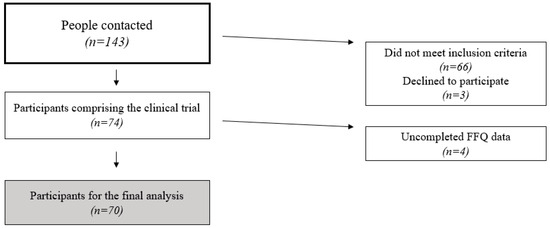
Figure 2.
Participant eligibility process flow-chart.
Participants were recruited in primary healthcare centers through general practitioners, who assessed whether the inclusion criteria were met (except for the MRI criteria, which were determined in a private clinic). This recruitment strategy, conducted through primary healthcare settings and standardized screening processes, strengthens the representativeness of the sample by ensuring a broad and diverse participant pool from the target population.
This study adhered to the ethical standards outlined in the Declaration of Helsinki and was approved by the Research Ethics Committee of the Balearic Islands (ref. IB 2251/14 PI; 26 February 2014). Participants were thoroughly informed about the study protocol and provided written consent.
2.3. Sociodemographic Characteristics
Sociodemographic data were collected via questionnaires, focusing on participants’ sex, age, educational level (primary school, secondary school, or university), and employment status (non-working, working, or retired). Information on physical activity levels was collected using the Minnesota Leisure Time Physical Activity Questionnaire, with energy expenditure expressed as the metabolic equivalents of tasks (min/week) [21,22].
2.4. Fatty Liver Disease Parameters
Abdominal MRI, with a 12-channel phased-array coil (Signa Explorer 1.5T, General Electric Healthcare, Chicago, IL, USA), was employed to quantify liver fat as the mean percentage, determined as intrahepatic fat content (IFC) (23). A more detailed explanation of this procedure, as well as its reliability and significance, is provided elsewhere [23,24,25].
Additionally, ultrasonography methods were used to evaluate the hepatic steatosis level. The process included a visual assessment of liver echogenicity quality, a visual comparison of the echo amplitude difference between the kidneys and the liver, and an evaluation of the clarity of the liver’s blood vessel structures. The clinical classification used a 4-point scale, less than 5% (grade 0), 5–33% (grade 1), 33–66% (grade 2), and greater than 66% (grade 3), as described elsewhere [26]. The hepatic fibrosis level and tissue stiffness (measured in kilopascals, kPa) were evaluated using transient elastography (FibroScan®, Echosens, París, France), with the participant lying down in a resting respiratory position, with the right arm elevated above the head for optimal intercostal access. Anthropometry was determined by bioimpedance with a segmental body composition analyzer (Tanita MC780P-MA, Tanita, Tokyo, Japan) and was utilized to measure weight (kg) and visceral fat quantity (13 being the cut-off point between low and high values).
2.5. Dietary Parameters
A validated 143-item semi-quantitative FFQ [27,28,29], administered by trained dietitians, assessed the participants’ usual dietary intake at baseline and after 6 months, with these visits also used to provide personalized follow-up and promote healthy lifestyle habits aligned with the Med-diet. Each food item in the FFQ had a standardized portion size, and consumption frequencies were recorded on a scale ranging from never or almost never to more than six times per day. The reported frequencies were converted into daily intake in grams by multiplying them by the portion size weight. The quantity of grams consumed per food group was calculated, including the following food groups: vegetables, fruits, legumes, cereals, dairy, meat, olive oil, fish, nuts, and sweets and pastries. The energy intake per person per day (kcal/day) was calculated using a computer program with data from Spanish food composition tables [30,31], multiplying the grams of each food item by its respective energy content in 100 g and finally adding all food items consumed. The Med-diet adherence was also reported. A previously validated 17-item energy-reduced Med-diet questionnaire was administered at baseline and after six months [32,33]. Each item of the Med-diet questionnaire corresponds to a characteristic of the Med-diet, with participants scoring 1 for adherence and 0 for non-adherence. Scores range up to 17 points, reflecting higher adherence and diet quality over time.
2.6. Ultra-Processed Food Consumption Assessment
The dietary information extracted from the FFQ was used to determine UPF consumption according to the NOVA system. This system was developed in 2010 by the NUPENS research group at the School of Public Health of the University of Sao Paulo, under the leadership of Dr. Carlos Monteiro. It classifies foods and beverages based on their level of processing and consists of four main groups: NOVA 1: unprocessed or minimally processed foods; NOVA 2: processed culinary ingredients; NOVA 3: processed foods; and NOVA 4: UPFs [8,34]. Each food item included in the FFQ was categorized according to these four NOVA groups. Following previous methodologies [35,36], the percentage of UPF consumption was calculated at both baseline and after 6 months. This was achieved by dividing the total grams of UPF consumed by the total grams of all food items consumed daily and multiplying by 100.
2.7. Statistics
Analyses were conducted using SPSS statistical software version 27.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean and standard deviation (SD), except for prevalence data, which are reported as the sample size and percentage. The Chi-squared test was employed for categorical variables, while one-way ANOVA was applied to continuous variables. To create our tertiles, we first calculated the UPF consumption and its percentage at each time point, as described in the previous section, and then assessed the changes in these percentages from baseline to 6 months. These changes were categorized into tertiles to reflect different levels of reduction: Tertile 1 (T1) represents the group with the highest reduction in UPF consumption, defined as a decrease of 7.27% or more (≤−7.27). Tertile 2 (T2) includes participants with a moderate reduction, with values ranging from −7.26% to −0.63% (from −7.26 to −0.63). Finally, Tertile 3 (T3) represents the smallest reduction in UPF consumption, with changes of 0.62% or less (≥−0.62). This approach allows us to differentiate participants based on the extent of their reduction in UPF consumption. The General Linear Model (GLM) was used to assess the relationships between changes in UPF consumption percentages, fatty liver disease parameters, and diet parameters over the 6-month period, with adjustments for the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and physical activity represented by metabolic equivalent tasks. Given that the study participants had MetS, including insulin resistance, obesity, and glucose intolerance, adjusting for the HOMA-IR helps to isolate the effects of UPF consumption on MASLD, reducing biases related to glucose levels and obesity. Bonferroni’s post hoc test was applied to identify statistically significant differences (p < 0.05) between groups across time (a, b, c), across time within groups (*), and between the time* group interactions.
3. Results
A total of 70 participants were included in the final analysis. These participants were separated into three groups according to UPF consumption. Table 1 shows the three groups mentioned: T1: participants with a maximum reduction in UPF% consumption (n = 23); T2: participants with a medium reduction in %UPF consumption (n = 24); and T3: participants with a minimum reduction in %UPF consumption (n = 23).

Table 1.
Descriptive characteristics of the sample according to changes in UPF consumption (%) in 6 months.
Table 1 also shows the descriptive characteristics of the sample according to changes in UPF% consumption within a period of 6 months. The studied sociodemographic variables were sex, educational level, job situation, age, and the metabolic syndrome parameters. A higher number of men were seen in all of the three tertiles, the educational level was similar between groups, and most participants were of an employed status, with the youngest participants showing the greatest reduction in percentage UPF consumption over the 6-month period with a mean (and standard deviation) of 50.8 (6.9) years old. None of the descriptive variables showed significant differences between groups (p < 0.05).
Table 2 shows the fatty liver parameters according to 6-month changes in UPF percentage consumption.

Table 2.
Fatty liver parameters according to 6-month changes in percentage (%) of UPF consumption.
Weight and visceral fat were also assessed and are shown in Table 2. IFC showed statistical significance when related to changes in UPF% consumption over 6 months, with a reduction of 7.7% in IFC in the group with the maximum reduction in %UPF consumption (T1) compared to a 2.6% reduction in the group with the minimum reduction (T3). The size effect of IFC was η2p = 0.33. The other fatty liver parameters showed minor changes between groups but were not statistically significant.
Table 3 shows the Med-diet adherence, the Med-diet food groups, and NOVA food groups according to 6-month changes in UPF consumption.

Table 3.
Diet parameters and NOVA food groups according to 6-month changes in percentage (%) of UPF consumption.
Med-Diet adherence was associated with a reduction in UPF% consumption. Hence, higher adherence to the Med-diet aligns with a more substantial reduction in UPF%. T1 increased its Med-diet adherence by a mean of 5.2 points. Significant changes were observed in the Med-diet food groups for meat, nuts, and sweets and pastries. A notable reduction in meat (−68.8 g/day) and sweets and pastries consumption (−23.7 g/day) was seen in T1 compared to T3. Nut consumption increased in all three groups. All NOVA food groups showed significant changes, except for non-processed foods. The greatest reduction was in UPFs, with a reduction of −399.8 g/day in T1 compared to an increase of 41.1 g/day in T3. All three groups reduced their high-processed food consumption, with a small reduction in low-processed foods in T1 and T2 (−19.8 g/day and −4.4 g/day, respectively) compared to an increase of 5.2 g/day in T3. The total energy intake was significantly related to the reduction in UPF% consumption, with a reduction of 605.3 kcal/day in T1, compared to a reduction of 89.2 kcal/day in T2 and an increase of 209.5 kcal/day in T3. The size effects calculated with η2p were 0.85, 0.63, 0.32, 0.27, 0.62, 0.54, 0.46, and 0.86 for Med-diet adherence, meat, nuts, sweets and pastries, low-processed foods, high-processed foods, UPFs, and total energy intake, respectively.
4. Discussion
A reduction in UPF consumption is a beneficial factor in improving MASLD parameters, including a significant decrease in IFC. Dietary changes, including a reduction in meat, sweets, and pastries and an overall improvement in Med-diet adherence, have been identified as factors contributing to reductions in UPF consumption and IFC. These results emphasize the importance of adopting dietary patterns that focus on minimally processed foods. Beyond health benefits, shifting to minimally processed foods has substantial implications for environmental sustainability. UPF production involves the extensive use of natural resources and often generates considerable amounts of waste, contributing significantly to global environmental degradation [37]. This is important to mention because a reduced consumption of UPF could help both planetary health and population health at the same time.
A remarkable reduction in total energy intake was seen when reducing UPF consumption, potentially being one of the factors contributing to the IFC reduction. This finding is consistent with previous research [38,39,40]. Specifically, one of the studies highlighted that energy intake is a key factor in decreasing fat accumulation in the liver [40]. An increase in calorie intake can be harmful, resulting in an imbalance between energy intake and energy expenditure, meaning that the calories consumed exceed the individual’s needs, leading primarily to overweight and obesity, among other health risks frequently linked to MASLD [17,41]. These results reinforce the idea that reducing UPF consumption can directly lower energy intake, facilitating weight loss and improvements in liver parameters.
A recent report from the Global Food Research Program, called “A global threat to public health”, determined that UPF consumption currently accounts for over half of the estimated total caloric intake in the United States, the United Kingdom, and Canada and approximately 20–40% of the caloric intake in other high- and middle-income countries, with sales increasing rapidly each year [42]. Decreasing the UPF intake can directly lead to a decrease in energy consumption and, consequently, a reduction in body weight and obesity [43].
UPFs are typically energy-dense and nutrient-poor, promoting caloric excess while disrupting metabolic pathways and increasing systemic inflammation and oxidative stress, all of which exacerbate MASLD [35,44]. UPFs can influence the homeostatic mechanisms of body weight regulation and create an intestinal environment conducive to the proliferation of microorganisms that promote inflammatory diseases [45,46]. The artificial additives and preservatives present in UPFs can disrupt the gut microbiome, leading to dysbiosis and the disruption of the intestinal mucus barrier [16,47]. Additionally, UPFs are typically low in dietary fiber, which is an essential nutrient for the growth and activity of beneficial gut microorganisms. By reducing the intake of UPFs, the consumption of fiber-rich foods such as fruits, vegetables, and whole grains can be increased, thereby providing nourishment for beneficial bacteria, which in turn can support liver health by reducing intestinal permeability, preventing the systemic inflammatory response and restoring microbial balance [48].
A reduction in UPF consumption has been associated with improvements in liver health, potentially through other mechanisms. First, reducing UPF intake can lower systemic inflammation, a key factor contributing to MASLD. UPFs, due to their high content of synthetic and pro-inflammatory compounds, can exacerbate liver inflammation, and their reduction may alleviate this [15,44,49]. Second, dietary patterns that focus on minimally processed foods and are rich in anti-inflammatory and antioxidant compounds, such as polyphenols and omega-3 fatty acids, can help improve liver fat metabolism and reduce hepatic fat accumulation [50]. Among them, the Med-diet has proven to be especially beneficial, being recognized as useful for the prevention and management of MASLD [51,52]. In the current study, the Med-diet appears to be related to a reduction in UPF consumption, reflecting how following the Med-diet could be a strategy to decrease UPFs and therefore reduce IFC in patients with MASLD. The Med-diet, rich in whole, minimally processed foods such as fruits, vegetables, whole grains, legumes, nuts, and fish, naturally limits the intake of UPFs and supports liver health through its anti-inflammatory and antioxidant properties [53,54].
By improving diet quality, the Med-diet helps reduce liver fat, making it a beneficial dietary approach for individuals with MASLD. Within the products of the Med-diet, the reduced consumption of meat products, sweets, and pastries was associated with a higher reduction in UPF consumption. This reduction in red and processed meats, sweets, and pre-cooked products was also observed in a previous study, which demonstrated that changes in UPF consumption were linked to increased adherence to the Med-diet, as well as reductions in weight, BMI, and energy intake. Additionally, the study highlighted how these dietary changes led to decreases in environmental strain, such as CO2 emissions and energy use [36].
UPFs contribute significantly to environmental damage, mainly due to the greenhouse gas emissions from their production [36,55]. As their consumption increases, it creates a dually negative impact on both consumer and environmental health [56]. Adopting a Med-diet pattern, which emphasizes local, seasonal, and plant-based foods that generally require fewer resources than UPFs, red meat products, and sweets, could provide dual benefits for the environment and public health [57]. Research has demonstrated that lower-CO2-emission diets, which emphasize minimally processed foods and the reduced consumption of UPFs, contribute to improved outcomes for MetS and glycemic control [58]. The shift to these dietary patterns would also benefit MASLD patients, given the shared pathophysiological mechanisms [59].
When proposing a diet for MASLD patients, the dietary components must address associated comorbidities such as cardiovascular diseases and type 2 diabetes mellitus, as well as contributing risk factors, since these are highly interconnected [2,60]. Therefore, the current analysis has been adjusted for the HOMA-IR, to avoid bias in the results, since patients with MASLD tend to have impaired glucose levels and insulin resistance. MASLD is closely associated with insulin resistance, which is a key metabolic dysfunction linked to the development and progression of fatty liver disease. By adjusting for the HOMA-IR, we account for the impact of insulin resistance on liver health, allowing for a more accurate assessment of how UPF consumption directly affects MASLD outcomes.
This is important because individuals with higher levels of insulin resistance are more likely to develop MASLD, and insulin resistance itself can influence the way the body processes fats and sugars, contributing to liver fat accumulation [61]. Including the HOMA-IR in the analysis ensures that the relationship between UPF consumption and MASLD parameters is not confounded by the metabolic status of the participants. It helps isolate the specific impact of UPF consumption on liver health, independent of insulin resistance, providing a clearer understanding of the dietary factors contributing to MASLD. This adjustment strengthens the validity and interpretability of the findings.
Following a Med-diet pattern requires a reduction in the intake of red and processed meats as well as processed sweets and sugary beverages. These are food groups that are normally classified as ultra-processed, and their minimization is a key factor in reducing MASLD [60,62,63]. Participants who had a higher reduction in UPFs showed significant improvements across several food categories, particularly in the reduction in meat and sweets consumption, alongside increased nut intake. This result further demonstrates the important benefits of the increased consumption of minimally processed products such as fruits, vegetables, whole grains, legumes, nuts, fish, and white meats [63]. These foods contribute to achieving a healthy body weight and reducing inflammation and oxidative stress, which are crucial factors for managing MASLD [64,65,66,67].
The existing literature predominantly consists of observational studies, highlighting the need for more randomized intervention studies and longitudinal research [2]. Our longitudinal study shows how reducing UPF consumption in the diet implied a notable decrease in IFC. Similar to our findings, previously published research reported that adults with a high UPF intake exhibited a significantly elevated fatty liver index [68]. A prospective analysis, included within an RCT among Spanish adults with MASLD, demonstrated that in older adults with chronic health conditions, the consumption of UPF was directly and strongly associated with fatty liver and hepatic steatosis scores [35]. Additionally, a UK cohort study discovered that high UPF intake was linked to an increased risk of NAFLD, liver fibrosis, cirrhosis, severe liver disease, and adverse levels of multiple clinical biomarkers, showing the importance of reducing UPF intake to improve liver health [69]. This study did not, however, examine other parameters such as IFC changes over time, such as in this current study. Another study found that high UPF consumption was associated with increased odds of NAFLD in both adolescents and adults, mainly due to increased body fat. If these results are validated, lowering UPF intake could potentially prevent NAFLD, currently MASLD, in both age groups [70].
Lifestyle interventions, especially dietary changes, play a critical role in managing MASLD. These findings highlight the critical need to prioritize dietary interventions targeting UPF consumption as an effective strategy for improving liver health and preventing related conditions, simultaneously mitigating environmental impacts such as global warming and resource depletion.
Strengths and Limitations
The current study’s strengths lie in its longitudinal design, which offers valuable insights into how changes in UPF consumption affect MASLD over a 6-month period. By employing both abdominal MRI and ultrasonography, this study ensures a comprehensive and accurate assessment of liver fat. The use of a validated 143-item semi-quantitative FFQ and the validated 17-item energy-reduced Med-diet questionnaire enhanced the precision of dietary evaluations. Moreover, the use of the NOVA system for classifying UPF consumption is a good choice because it is the most reliable and widely used system in the scientific literature. This choice allows for the effective comparison of results with other studies and ensures the accuracy and relevance of the dietary assessments. Adjusting for factors like the HOMA-IR in the analyses helps to isolate the specific impact of UPF consumption on liver health, mitigating potential biases related to glucose levels and obesity.
This study also has some limitations. The sample size of 70 participants may restrict the generalizability of the findings, as the results may not extend beyond the specific cohort studied or apply to other populations. The age of the participants (40–60 years old) could limit the extrapolation of the results to younger or older populations. The reliance on self-reported dietary intake data introduces the possibility of inaccuracies, potentially affecting the reliability of UPF consumption assessments. While the FFQ offers a general overview of dietary intake, it does not accurately capture the specific details of UPF consumption. There is a need for a validated questionnaire specifically designed to measure UPF consumption.
5. Conclusions
Reducing UPF consumption is beneficial for managing MASLD, as evidenced by the notable decrease in IFC among participants. This dietary shift, combined with increased adherence to the Med-diet and reduced calorie intake, can provide a dietary path to reducing adverse liver health outcomes. Moreover, adopting these dietary patterns aligns with global sustainability goals and could further benefit MASLD patients by addressing environmental challenges alongside improving liver health. Given the significant role of diet in MASLD management, the current lack of medical treatments, and the impact of UPF consumption on environmental health, our findings underscore the need for dietary interventions that specifically target UPF reduction, therefore improving liver health, mitigating the associated risks, and simultaneously enhancing planet sustainability.
Author Contributions
S.G., J.A.T. and C.B. designed this study and wrote the protocol; S.G., L.U. and E.A.-M. recruited participants; S.G. and D.M. collected samples; D.M. and C.G. conducted clinical measurements; M.C. conducted MRI measurements; M.M.-M. conducted biochemical tasks; S.G. and C.B. conducted statistical analysis; S.G., C.B. and J.A.T. wrote the first draft of this manuscript; S.G., M.M.-M., L.U., M.C., C.G., D.M., E.A.-M., J.A.T. and C.B. read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Instituto de Salud Carlos III through the Fondo de Investigación para la Salud (CIBEROBN CB12/03/30038), which are cofunded by the European Regional Development Fund. Fundació La Marató TV3 (Spain) project ref. 201630.10. IDISBA Grants (FOLIUM, PRIMUS, SYNERGIA, and LIBERI). Red EXERNET-Red de Ejercicio Físico y Salud (RED2022-134800-T) Agencia Estatal de Investigación (Ministerio de Ciencias e Innovación, Spain). The funding sponsors had no role in the design of this study, in the collection, analyses, or interpretation of the data; in the writing of this manuscript, or in the decision to publish the results.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Balearic Islands (ref. IB 2251/14 PI; 26 February 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. The results and writing of this manuscript followed the Committee on Publication Ethics (COPE) guidelines on how to deal with potential acts of misconduct, maintaining the integrity of this research and its presentation following the rules of good scientific practice, the trust in the journal, the professionalism of scientific authorship, and the entire scientific endeavor. Written informed consent has been obtained from the patient(s) to publish this paper if applicable.
Data Availability Statement
There are restrictions on the availability of data for this trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Requestors wishing to access the trial data used in this study can make a request to pep.tur@uib.es.
Acknowledgments
The authors especially thank the participants for their enthusiastic collaboration and the personnel for outstanding support and exceptional effort. The authors thank Octavio Barbero from Red Asistencial Juaneda, Palma de Mallorca, Spain, for technical assistance. CIBEROBN is an initiative of Instituto de Salud Carlos III, Spain.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Clinical Trials Registration
This trial was registered at ClinicalTrials.gov with the registry number NCT04442620 (https://clinicaltrials.gov/ct2/show/NCT04442620, accessed on 22 February 2022).
References
- Lee, E.C.Z.; Anand, V.V.; Razavi, A.C.; Alebna, P.L.; Muthiah, M.D.; Siddiqui, M.S.; Chew, N.W.S.; Mehta, A. The Global Epidemic of Metabolic Fatty Liver Disease. Curr. Cardiol. Rep. 2024, 26, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Grinshpan, L.S.; Eilat-Adar, S.; Ivancovsky-Wajcman, D.; Kariv, R.; Gillon-Keren, M.; Zelber-Sagi, S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. JHEP Rep. 2023, 6, 100964. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Musio, A.; Marchesini, G. Moderate Alcohol Intake in Non-Alcoholic Fatty Liver Disease: To Drink or Not to Drink? Nutrients 2019, 11, 3048. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; LaVine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). Global Health Metrics. Total Burden Related to NAFLD Cause. 2021. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-total-burden-related-nafld-level-0 (accessed on 7 November 2024).
- Institute for Health Metrics and Evaluation GBD Results. GBD 2021—(IHME)—GBD Compare. 2021. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 7 November 2024).
- Talens, P.; Cámara, M.; Daschner, A.; López, E.; Marín, S.; Martínez, J.A.; Morales-Navas, F.J. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) sobre el impacto del consumo de alimentos “ultra-procesados” en la salud de los consumidores. Rev. Com. Cient. AESAN 2020, 31, 49–76. [Google Scholar]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. [Google Scholar] [CrossRef]
- Konieczna, J.; Morey, M.; Abete, I.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Vioque, J.; Gonzalez-Palacios, S.; Daimiel, L.; Salas-Salvadó, J.; Fiol, M.; et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: Prospective analysis nested in the PREDIMED-Plus trial. Clin. Nutr. 2021, 40, 4290–4300. [Google Scholar] [CrossRef]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2266. [Google Scholar] [CrossRef]
- Lodge, M.; Dykes, R.; Kennedy, A. Regulation of Fructose Metabolism in Nonalcoholic Fatty Liver Disease. Biomolecules 2024, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu. Rev. Med. 2012, 63, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Casares, M.; Gómez, C.; Ugarriza, L.; Tur, J.A.; Sureda, A. Effects of a Two-Year Lifestyle Intervention on Intrahepatic Fat Reduction and Renal Health: Mitigation of Inflammation and Oxidative Stress, a Randomized Trial. Antioxidants 2024, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Brichacek, A.L.; Florkowski, M.; Abiona, E.; Frank, K.M. Ultra-Processed Foods: A Narrative Review of the Impact on the Human Gut Microbiome and Variations in Classification Methods. Nutrients 2024, 16, 1738. [Google Scholar] [CrossRef] [PubMed]
- Aerts, M.; Rosseel, Z.; De Waele, E. The Evolution in Non-Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective. Nutrients 2024, 16, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A.; Mustafa, M.R.; Ibrahim, M.; Othman, R. Lifestyle interventions for non-alcoholic fatty liver disease. Saudi J. Biol. Sci. 2019, 26, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- NCT04442620—Prevention and Reversion of NAFLD in Obese Patients with Metabolic Syndrome by Mediterranean Diet and Physical Activity (FLIPAN). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04442620 (accessed on 20 January 2022).
- The International Diabetic Federation (IDF). The IDF Consensus Worldwide Definition of Definition of the Metabolic Syndrome. 2022. Available online: http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf (accessed on 22 January 2022).
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J.; Investigators of the MARATDON Group. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E.; The MARATHOM Investigators. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reason. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef]
- Eskreis-Winkler, S.; Corrias, G.; Monti, S.; Zheng, J.; Capanu, M.; Krebs, S.; Fung, M.; Reeder, S.; Mannelli, L. IDEAL-IQ in an oncologic population: Meeting the challenge of concomitant liver fat and liver iron. Cancer Imaging 2018, 18, 51. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- De la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.Á. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos, Guía de Prácticas (Spanish Food Composition Tables), 17th ed.; Pirámide: Madrid, Spain, 2015. [Google Scholar]
- Mataix, J.; Mañas, M.; Llopis, J.; Martínez de Victoria, E.; Juan, J.; Borregón, A. Tablas de Composición de Alimentos (Spanish Food Composition Tables), 5th ed.; Universidad de Granada: Granada, Spain, 2013. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Schröder, H.; Zomeño, M.D.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Vioque, J.; Romaguera, D.; Alfredo Martínez, J.; Tinahones, F.J.; López Miranda, J.; et al. Validity of the energy-restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Konieczna, J.; Fiol, M.; Colom, A.; Martínez-Gonzáles, M.Á.; Salas-Salvadó, J.; Corella, D.; Soria-Florido, M.T.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Does Consumption of Ultra-Processed Foods Matter for Liver Health? Prospective Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 4142. [Google Scholar] [CrossRef]
- García, S.; Pastor, R.; Monserrat-Mesquida, M.; Álvarez-Álvarez, L.; Rubín-García, M.; Martínez-Gonzáles, M.Á.; Salas Salvadó, J.; Corella Piquer, D.; Fitó Colomer, M.; Martínez Hernández, J.A.; et al. Ultra-processed foods consumption as a promoting factor of greenhouse gas emissions, water, energy, and land use: A longitudinal assessment. Sci. Total Environ. 2023, 891, 164417. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Ultra-Processed Foods and Food System Sustainability: What Are the Links? Sustainability 2020, 12, 6280. [Google Scholar] [CrossRef]
- Ashiqueali, S.A.; Zhu, X.; Wiesenborn, D.S.; Gesing, A.; Schneider, A.; Noureddine, S.A.; Correa-Garcia, C.G.; Masternak, M.M.; Siddiqi, S.A. Calorie restriction and life-extending mutation downregulate miR-34a to facilitate lipid metabolism in the liver. Exp. Gerontol. 2024, 194, 112506. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Bouzas, C.; Mascaró, C.M.; Casares, M.; Llompart, I.; Abete, I.; Angullo-Martinez, E.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 2223. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Nutritional Modulation of Non-Alcoholic Fatty Liver Disease and Insulin Resistance. Nutrients 2015, 7, 9127–9138. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Global Food Research Program. Utlra-Processed Foods: A Global Threat to Public Health. 2023. Available online: https://www.globalfoodresearchprogram.org/wp-content/uploads/2023/11/GFRP_FactSheet_UltraProcessedFoods_2023_11.pdf (accessed on 29 July 2024).
- Aramburu, A.; Alvarado-Gamarra, G.; Cornejo, R.; Curi-Quinto, K.; Díaz-Parra, C.d.P.; Rojas-Limache, G.; Lanata, C.F. Ultra-processed foods consumption and health-related outcomes: A systematic review of randomized controlled trials. Front. Nutr. 2024, 11, 1421728. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Dicken, S.J.; Batterham, R.L. Ultra-processed Food and Obesity: What Is the Evidence? Curr. Nutr. Rep. 2024, 13, 23–38. [Google Scholar] [CrossRef]
- Lv, J.-L.; Wei, Y.-F.; Sun, J.-N.; Shi, Y.-C.; Liu, F.-H.; Sun, M.-H.; Chang, Q.; Wu, Q.-J.; Zhao, Y.-H. Ultra-processed food consumption and metabolic disease risk: An umbrella review of systematic reviews with meta-analyses of observational studies. Front. Nutr. 2024, 11, 1306310. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Fichtel-Epstein, C.; Huang, J.; Rich, B.J.; Taswell, C.S.; Isrow, D.; Jin, W. Ultra-Processed Food and Prostate Cancer Risk: A Systemic Review and Meta-Analysis. Cancers 2024, 16, 3953. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Argelich, E.; Casares, M.; Ugarriza, L.; Llompart, I.; Tur, J.A.; Sureda, A. Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants 2024, 13, 480. [Google Scholar] [CrossRef]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Hassani Zadeh, S.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Effect of Mediterranean diet on liver enzymes: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2022, 128, 1231–1239. [Google Scholar] [CrossRef]
- George, E.S.; Reddy, A.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; Tierney, A.C. Impact of a Mediterranean diet on hepatic and metabolic outcomes in non-alcoholic fatty liver disease: The MEDINA randomised controlled trial. Liver. Int. 2022, 42, 1308–1322. [Google Scholar] [CrossRef]
- Vellinga, R.E.; van Bakel, M.; Biesbroek, S.; Toxopeus, I.B.; de Valk, E.; Hollander, A.; van’t Veer, P.; Temme, E.H.M. Evaluation of foods, drinks and diets in the Netherlands according to the degree of processing for nutritional quality, environmental impact and food costs. BMC Public Health 2022, 22, 877. [Google Scholar] [CrossRef]
- Lorca-Camara, V.; Bosque-Prous, M.; Batlle-Bayer, L.; Bes-Rastrollo, M.; O’Callaghan-Gordo, C.; Bach-Faig, A. Environmental and Health Sustainability of the Mediterranean Diet: A Systematic Review. Adv. Nutr. Int. Rev. J. 2024, 15, 100322. [Google Scholar] [CrossRef]
- García, S.; Bouzas, C.; Mateos, D.; Pastor, R.; Álvarez, L.; Rubín, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Goday, A.; et al. Carbon dioxide (CO2) emissions and adherence to Mediterranean diet in an adult population: The Mediterranean diet index as a pollution level index. Environ. Health. 2023, 22, 1. [Google Scholar] [CrossRef]
- García, S.; Pastor, R.; Monserrat-Mesquida, M.; Álvarez-Álvarez, L.; Rubín-García, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Goday, A.; Martínez, J.A.; et al. Metabolic syndrome criteria and severity and carbon dioxide (CO2) emissions in an adult population. Global Health 2023, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Clark, J.M. The epidemiology of nonalcoholic fatty liver disease: A global perspective. Semin Liver Dis. 2008, 28, 339–350. [Google Scholar] [CrossRef]
- Beygi, M.; Ahi, S.; Zolghadri, S.; Stanek, A. Management of Metabolic-Associated Fatty Liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: From Medication Therapy to Nutritional Interventions. Nutrients 2024, 16, 2220. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Ramaiah, P.; Jamel Baljon, K.; Alsulami, S.A.; Lindsay, G.M.; Chinnasamy, L. Diet quality indices and odds of metabolic dysfunction-associated fatty liver disease: A case-control study. Front. Nutr. 2024, 10, 1251861. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. [Google Scholar] [CrossRef]
- Abdallah, J.; Assaf, S.; Das, A.; Hirani, V. Effects of anti-inflammatory dietary patterns on non-alcoholic fatty liver disease: A systematic literature review. Eur. J. Nutr. 2023, 62, 1563–1578. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program With Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, H.; Zeng, Y.; Chen, Y.; Xu, C. Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: A population-based analysis of NHANES 2011-2018. Br. J. Nutr. 2023, 130, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Clay-Gilmour, A.; Zhang, J.; Zhang, X.; Steck, S.E. Higher ultra-processed food intake is associated with adverse liver outcomes: A prospective cohort study of UK Biobank participants. Am. J. Clin. Nutr. 2024, 119, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Martinez Steele, E.; Lo, C.H.; Zhang, F.F.; Zhang, X. Higher ultra-processed food intake was positively associated with odds of NAFLD in both US adolescents and adults: A national survey. Hepatol. Commun. 2023, 7, e0240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).