Bioconversion-Based Postbiotics Enhance Muscle Strength and Modulate Gut Microbiota in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
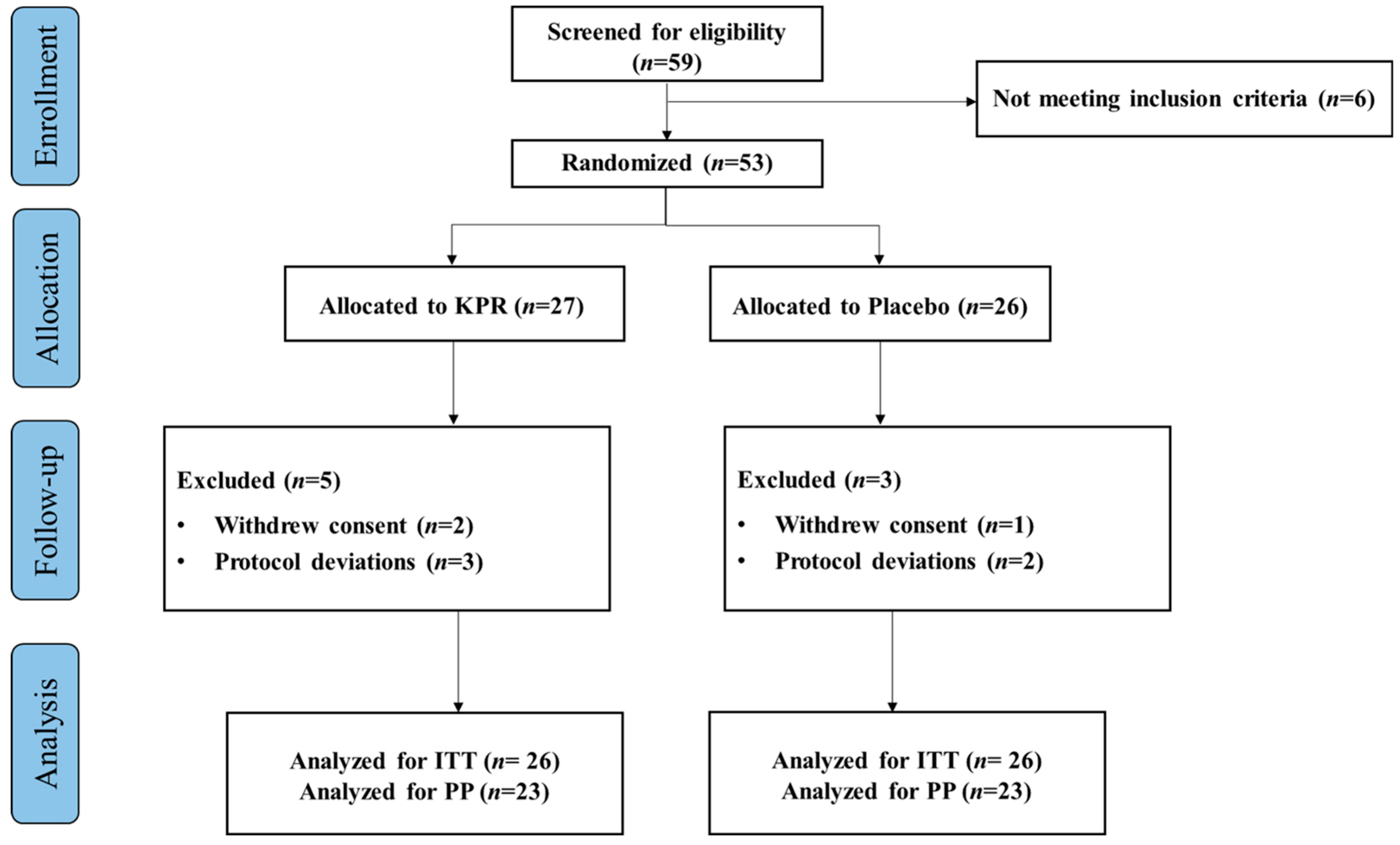
2. Methods
2.1. Study Design and Participants
2.2. Outcomes
2.2.1. Blood/Stool Sample Collection
2.2.2. Primary Outcome
2.2.3. Secondary Outcome
2.3. Statistical Analysis
3. Results
3.1. Primary Outcome: Dominant Hand Grip Strength Significantly Increased in the KP Group After 12 Weeks of Intervention in Both ITT and PP Analysis
3.2. Secondary Outcome: PP Analysis
3.2.1. Inflammatory and Muscle Biomarkers
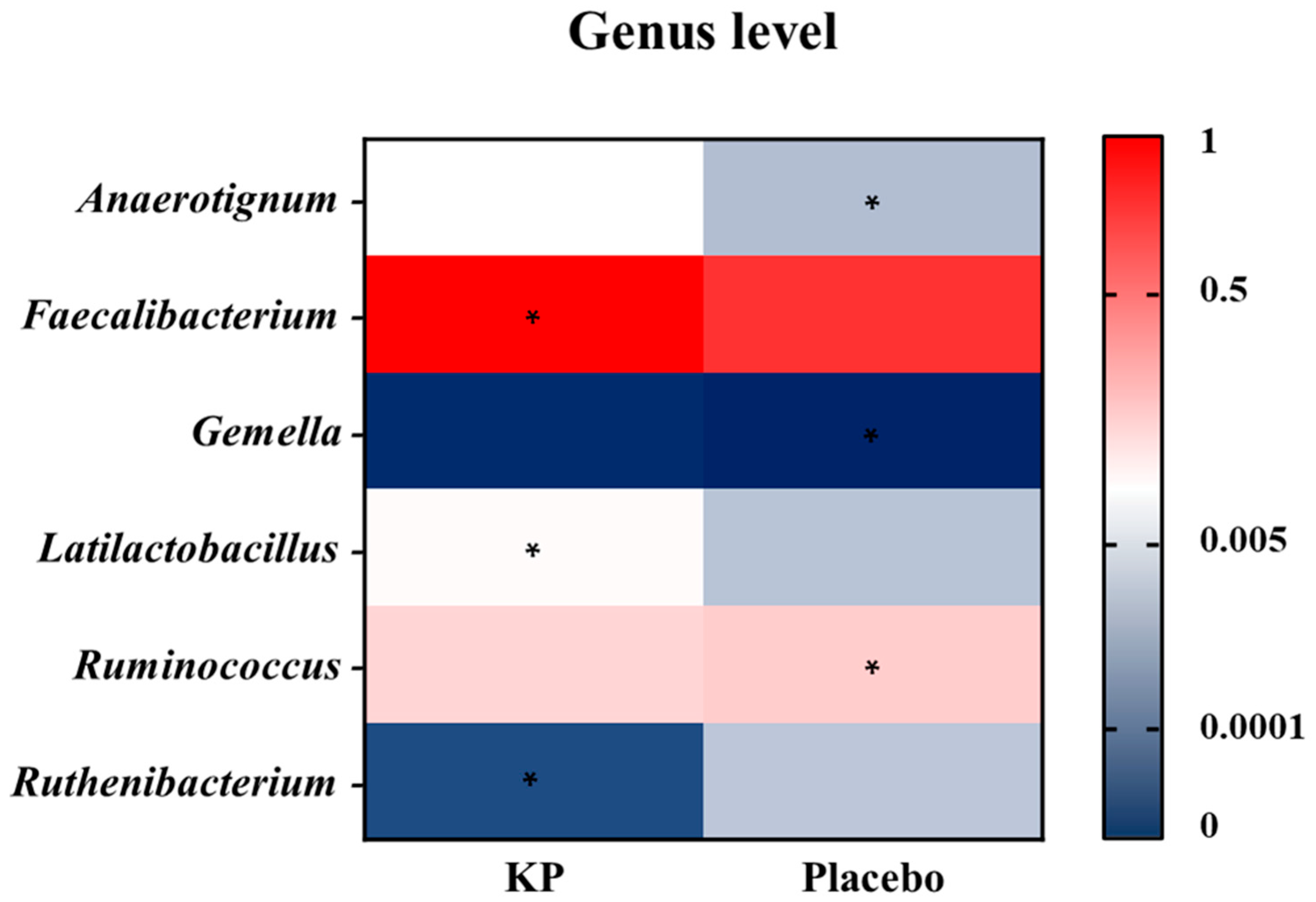
3.2.2. Microbial Profiling
3.2.3. Correlation Between Muscle Function Profile and Microbial Profile
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madra-Gackowska, K.; Szewczyk-Golec, K.; Gackowski, M.; Wozniak, A.; Kedziora-Kornatowska, K. Evaluation of Selected Parameters of Oxidative Stress and Adipokine Levels in Hospitalized Older Patients with Diverse Nutritional Status. Antioxidants 2023, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Madra-Gackowska, K.; Szewczyk-Golec, K.; Gackowski, M.; Holynska-Iwan, I.; Parzych, D.; Czuczejko, J.; Graczyk, M.; Husejko, J.; Jablonski, T.; Kedziora-Kornatowska, K. Selected Biochemical, Hematological, and Immunological Blood Parameters for the Identification of Malnutrition in Polish Senile Inpatients: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 1494. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bo, L.; Zhou, E.; Chen, Y.; Naranmandakh, S.; Xie, W.; Ru, Q.; Chen, L.; Zhu, Z.; et al. Progress of linking gut microbiota and musculoskeletal health: Casualty, mechanisms, and translational values. Gut Microbes 2023, 15, 2263207. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.D.; Wang, Y.Y.; Liao, Z.Z.; Xiao, X.H. Skeletal muscles and gut microbiota-derived metabolites: Novel modulators of adipocyte thermogenesis. Front. Endocrinol. 2023, 14, 1265175. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Si, S.C.; Wang, W.H.; Li, J.; Ma, Y.X.; Zhao, H.; Liu, J. Gut dysbiosis in primary sarcopenia: Potential mechanisms and implications for novel microbiome-based therapeutic strategies. Front. Microbiol. 2025, 16, 1526764. [Google Scholar] [CrossRef]
- Roy, S.; Alizadeh Bahmani, A.H.; Davids, M.; Herrema, H.; Nieuwdorp, M. Modulating the Gut–Muscle Axis: Increasing SCFA-Producing Gut Microbiota Commensals and Decreasing Endotoxin Production to Mitigate Cancer Cachexia. Microorganisms 2025, 13, 1356. [Google Scholar] [CrossRef]
- Chew, W.; Lim, Y.P.; Lim, W.S.; Chambers, E.S.; Frost, G.; Wong, S.H.; Ali, Y. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front. Med. 2022, 9, 1065365. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yin, D.; Shi, R. Gut-muscle axis mechanism of exercise prevention of sarcopenia. Front. Nutr. 2024, 11, 1418778. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the gut microbiota and sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, F.; Yang, H.; Luo, J.; Zhang, L.; Chen, X.; Liao, H.; Lei, H.; Liu, S.; Yang, T.; et al. Probiotics and muscle health: The impact of Lactobacillus on sarcopenia through the gut-muscle axis. Front. Microbiol. 2025, 16, 1559119. [Google Scholar] [CrossRef]
- Kang, C.H.; Jung, E.S.; Jung, S.J.; Han, Y.H.; Chae, S.W.; Jeong, D.Y.; Kim, B.C.; Lee, S.O.; Yoon, S.J. Pasteurized Akkermansia muciniphila HB05 (HB05P) Improves Muscle Strength and Function: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 4037. [Google Scholar] [CrossRef]
- Motei, D.E.; Beteri, B.; Hepsomali, P.; Tzortzis, G.; Vulevic, J.; Costabile, A. Supplementation with postbiotic from Bifidobacterium Breve BB091109 improves inflammatory status and endocrine function in healthy females: A randomized, double-blind, placebo-controlled, parallel-groups study. Front. Microbiol. 2023, 14, 1273861. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Arai, S.; Sato, S.; Iwabuchi, N.; Takara, T.; Tanaka, M. Effects of Heat-Killed Lacticaseibacillus paracasei MCC1849 on Immune Parameters in Healthy Adults—A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2024, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008291. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.-E.-H.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway: A review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Hwang, S.; Seo, K.H.; Kim, H. Probiotic-Derived Extracellular Vesicles Attenuate Sarcopenia via Muscle Regeneration. J. Food Sci. 2025, 90, e70586. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. The metabolic and temporal basis of muscle hypertrophy in response to resistance exercise. Eur. J. Sport Sci. 2016, 16, 633–644. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Yang, D.; Li, J.; Li, Z.; Zhao, M.; Wang, D.; Gou, F.; Wang, J.; Dai, Y.; et al. Irisin regulates oxidative stress and mitochondrial dysfunction through the UCP2-AMPK pathway in prion diseases. Cell Death Dis. 2025, 16, 66. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Rodrigues, V.D.; Laurindo, L.F.; Cherain, L.M.A.; de Lima, E.P.; Boaro, B.L.; da Silva Camarinha Oliveira, J.; Chagas, E.F.B.; Catharin, V.C.S.; Dos Santos Haber, J.F.; et al. Targeting AMPK with Irisin: Implications for metabolic disorders, cardiovascular health, and inflammatory conditions—A systematic review. Life Sci. 2025, 360, 123230. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin reduces inflammatory signaling pathways in inflammation-mediated metabolic syndrome. Mol. Cell Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Li, W.; Moylan, J.S.; Chambers, M.A.; Smith, J.; Reid, M.B. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 2009, 297, C706–C714. [Google Scholar] [CrossRef]
- Parker, E.; Khayrullin, A.; Kent, A.; Mendhe, B.; Youssef El Baradie, K.B.; Yu, K.; Pihkala, J.; Liu, Y.; McGee-Lawrence, M.; Johnson, M.; et al. Hindlimb Immobilization Increases IL-1β and Cdkn2a Expression in Skeletal Muscle Fibro-Adipogenic Progenitor Cells: A Link Between Senescence and Muscle Disuse Atrophy. Front. Cell Dev. Biol. 2021, 9, 790437. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Choi, H.I.; Hong, S.W.; Kang, S.; Jegal, H.Y.; Choi, E.W.; Park, B.S.; Kim, J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines 2020, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Carasi, P.; Racedo, S.M.; Jacquot, C.; Romanin, D.E.; Serradell, M.A.; Urdaci, M.C. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J. Immunol. Res. 2015, 2015, 361604. [Google Scholar] [CrossRef] [PubMed]
- Filannino, F.M.; Ruggiero, M.; Panaro, M.A.; Lofrumento, D.D.; Trotta, T.; Benameur, T.; Cianciulli, A.; Calvello, R.; Zoila, F.; Porro, C. Irisin Attenuates Neuroinflammation Targeting the NLRP3 Inflammasome. Molecules 2024, 29, 5623. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Guo, Q.; Wang, W.; Zhang, L. High-throughput sequencing analysis of the characteristics of the gut microbiota in aged patients with sarcopenia. Exp. Gerontol. 2023, 182, 112287. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, Y.; Liu, X.; Liu, H.; Zhao, X.; Xue, L.; Yang, S.; Wang, Y.; Liu, X. Changes in the gut microbiota of patients with sarcopenia based on 16S rRNA gene sequencing: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1429242. [Google Scholar] [CrossRef]
- Sugimura, Y.; Yang, Y.; Kanda, A.; Mawatari, A.; Tamada, Y.; Mikami, T.; Nakaji, S.; Ihara, K. Association between Gut Microbiota and Muscle Strength in Japanese General Population of the Iwaki Health Promotion Project. Microorganisms 2024, 12, 622. [Google Scholar] [CrossRef]
- Du, C.; Zhou, X.; Zhang, K.; Huang, S.; Wang, X.; Zhou, S.; Chen, Y. Inactivation of the MSTN gene expression changes the composition and function of the gut microbiome in sheep. BMC Microbiol. 2022, 22, 273. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, B.; Han, P.; Wang, Z.; Cao, R.; Chen, S.; Cheng, C.; Lian, H.; Zha, Y.; Li, M. Gut microbiota-metabolome remodeling associated with low bone mass: An integrated multi-omics study in fracture patients. Front. Mol. Biosci. 2025, 12, 1646361. [Google Scholar] [CrossRef]
- Solvang, M.; Farquharson, F.M.; Horgan, G.; Pisano, S.; Holck, J.; Zeuner, B.; Russell, W.R.; Louis, P. Roles of human colonic bacteria in pectin utilization and associated cross-feeding networks revealed using synthetic co-cultures. Microbiology 2025, 171, 001559. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Chassard, C.; Clerget, M.; Potrykus, J.; Campbell, G.; Mayer, C.D.; Young, P.; Rucklidge, G.; Ramsay, A.G.; et al. Substrate-driven gene expression in Roseburia inulinivorans: Importance of inducible enzymes in the utilization of inulin and starch. Proc. Natl. Acad. Sci. USA 2011, 108, 4672–4679. [Google Scholar] [CrossRef]
- Marzouk, N.H.; Rashwan, H.H.; El-Hadidi, M.; Ramadan, R.; Mysara, M. Proinflammatory and GABA eating bacteria in Parkinson’s disease gut microbiome from a meta-analysis prospective. NPJ Park. Dis. 2025, 11, 145. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Horst, R.T.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1897. [Google Scholar] [CrossRef]
- Han, S.; Seo, K.H.; Gyu Lee, H.; Kim, H. Effect of Cucumis melo L. peel extract supplemented postbiotics on reprograming gut microbiota and sarcopenia in hindlimb-immobilized mice. Food Res. Int. 2023, 173, 113476. [Google Scholar] [CrossRef]
- Rastelli, M.; Knauf, C.; Cani, P.D. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity 2018, 26, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Chen, W.; Tan, H.; Ye, L.; Chen, J.; Xu, X. Gut Microbiota in Primary Sarcopenia: Mechanisms and Potential Therapeutic Targets. Front. Biosci. 2025, 30, 36204. [Google Scholar] [CrossRef] [PubMed]
- Dungan, C.M.; Peck, B.D.; Walton, R.G.; Huang, Z.; Bamman, M.M.; Kern, P.A.; Peterson, C.A. In vivo analysis of gammaH2AX+ cells in skeletal muscle from aged and obese humans. FASEB J. 2020, 34, 7018–7035. [Google Scholar] [CrossRef]
- Philippou, A.; Maridaki, M.; Halapas, A.; Koutsilieris, M. The role of the insulin-like growth factor 1 (IGF-1) in skeletal muscle physiology. In Vivo 2007, 21, 45–54. [Google Scholar]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Jang, I.Y.; Jung, H.W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.J. Serum irisin level is independent of sarcopenia and related muscle parameters in older adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; Chai, J.; Maley, K.; Cozens, G.; Grounds, G.; Winn, N.; Rosenthal, N.; Grounds, M.D. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J. Cell Sci. 2010, 123, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Alharbi, A.; Gibson, R.; Rodriguez-Mateos, A. (Poly)phenol-gut microbiota interactions and their impact on human health. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 316–322. [Google Scholar] [CrossRef]



| KP | Placebo | ||
|---|---|---|---|
| Intention to treat | (n = 27) | (n = 26) | p-value |
| Age (yrs) | 53.70 ± 7.45 | 55.04 ± 8.83 | 0.465 |
| Female (%) | 25 (93) | 24 (92) | |
| Height (cm) | 158.50 ± 5.66 | 159.54 ± 6.20 | 0.525 |
| Weight (kg) | 59.76 ± 9.91 | 59.93 ± 8.18 | 0.946 |
| BMI (kg/m2) | 23.70 ± 3.12 | 23.52 ± 2.68 | 0.822 |
| Per protocol | (n = 22) | (n = 23) | |
| Age (yrs) | 52.55 ± 6.46 | 55.74 ± 8.96 | 0.179 |
| Female (%) | 20 (91) | 21 (91) | |
| Height (cm) | 159.59 ± 5.47 | 159.65 ± 6.53 | 0.973 |
| Weight (kg) | 60.70 ± 9.35 | 60.52 ± 8.27 | 0.946 |
| BMI (kg/m2) | 23.77 ± 2.97 | 23.72 ± 2.70 | 0.948 |
| KP | p-Value (1) | Placebo | p-Value (1) | p-Value (2) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | ||||
| Intention to treat | (n = 27) | (n = 26) | |||||
| Grip strength | |||||||
| Dominant hand (kg) | 19.80 ± 7.09 | 21.42 ± 6.77 | 0.012 * | 19.77 ± 5.81 | 20.84 ± 5.22 | 0.128 | 0.498 |
| Non-dominant hand (kg) | 19.03 ± 8.12 | 18.65 ± 7.47 | 0.475 | 19.39 ± 5.61 | 20.57 ± 5.46 | 0.252 | 0.128 |
| BMC (kg) | 1.92 ± 0.31 | 1.90 ± 0.27 | 0.379 | 1.91 ± 0.31 | 1.91 ± 0.30 | 0.644 | 0.541 |
| BMD (g/cm2) | 1.04 ± 0.09 | 1.06 ± 0.14 | 0.582 | 1.04 ± 0.09 | 1.04 ± 0.09 | 0.414 | 0.343 |
| Fat (kg) | 20.88 ± 6.03 | 21.08 ± 5.72 | 0.638 | 20.12 ± 6.78 | 19.89 ± 6.41 | 0.861 | 0.749 |
| Lean (kg) | 36.32 ± 5.84 | 35.86 ± 5.99 | 0.276 | 34.98 ± 8.15 | 36.34 ± 6.21 | 0.757 | 0.403 |
| T-score | −0.90 ± 1.11 | −0.09 ± 1.14 | 0.888 | −0.94 ± 1.08 | −0.98 ± 1.04 | 0.390 | 0.626 |
| Z-score | −0.59 ± 0.88 | −0.58 ± 0.89 | 0.787 | −0.51 ± 0.80 | −0.49 ± 0.79 | 0.697 | 0.867 |
| Per protocol | (n = 22) | (n = 23) | |||||
| Grip strength | |||||||
| Dominant hand (kg) | 19.55 ± 7.56 | 21.24 ± 7.32 | 0.025 * | 19.83 ± 5.87 | 20.90 ± 5.01 | 0.204 | 0.363 |
| Non-dominant hand (kg) | 19.08 ± 8.94 | 18.67 ± 8.13 | 0.531 | 19.14 ± 5.77 | 20.22 ± 5.70 | 0.286 | 0.251 |
| BMC (kg) | 1.95 ± 0.32 | 1.93 ± 0.27 | 0.327 | 1.92 ± 0.33 | 1.91± 0.32 | 0.312 | 0.569 |
| BMD (g/cm2) | 1.05 ± 0.09 | 1.08 ± 0.15 | 0.614 | 1.05 ± 0.09 | 1.08 ± 0.15 | 0.224 | 0.198 |
| Fat (kg) | 20.76 ± 6.31 | 21.07 ± 5.71 | 0.570 | 20.76 ± 6.31 | 20.51 ± 7.12 | 0.815 | 0.577 |
| Lean (kg) | 36.95 ± 5.97 | 36.47 ± 6.25 | 0.306 | 36.95 ± 5.97 | 36.47 ± 6.25 | 0.738 | 0.454 |
| T-score | −0.80 ± 1.17 | −0.82 ± 1.17 | 0.761 | −0.80 ± 1.17 | −0.82 ± 1.17 | 0.227 | 0.555 |
| Z-score | −0.52 ± 0.92 | −0.51 ± 0.92 | 0.844 | −0.52 ± 0.92 | −0.51 ± 0.92 | 0.895 | 0.996 |
| KP (n = 22) | p-Value (1) | Placebo (n = 23) | p-Value (1) | p-Value (2) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | ||||
| Per protocol | |||||||
| IL-1β (pg/mL) | 0.02 ± 0.02 | 0.01 ± 0.00 | 0.011 * | 0.02 ± 0.01 | 0.02 ± 0.03 | 0.869 | 0.054 |
| IL-10 (pg/mL) | 1.42 ± 0.70 | 1.79 ± 0.87 | 0.108 | 1.10 ± 0.65 | 2.27 ± 2.20 | 0.001 * | 0.302 |
| IL-2 (pg/mL) | 25.18 ± 22.00 | 19.95 ± 20.87 | 0.398 | 25.73 ± 36.69 | 20.54 ± 20.12 | 0.573 | 0.909 |
| IGF-1 (ng/mL) | 78.00 ± 32.25 | 71.85 ± 30.16 | 0.783 | 78.23 ± 22.94 | 75.56 ± 30.43 | 0.670 | 0.937 |
| Irisin (ng/mL) | 21.20 ± 9.86 | 28.11 ± 14.29 | 0.027 * | 27.39 ± 17.31 | 35.38 ± 22.34 | 0.119 | 0.854 |
| Myostatin (ng/mL) | 4.07 ± 3.57 | 2.51 ± 2.39 | 0.001 * | 4.69 ± 3.74 | 2.85 ± 2.54 | 0.001 * | 0.340 |
| At Phylum–Genus–Species | KP (n =22) | p-Value (1) | Placebo (n =23) | p-Value (1) | p-Value (2) | ||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | ||||
| Per protocol | |||||||
| Actinomycetota | |||||||
| Bifidobacterium adolescentis | 0.046 ± 0.076 | 0.070 ± 0.102 | 0.041 * | 0.021 ± 0.039 | 0.040 ± 0.072 | 0.182 | 0.393 |
| Bacillota | |||||||
| Anaerobutyricum soehngenii | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.317 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 | 0.496 |
| Blautia faecicola | 0.002 ± 0.004 | 0.003 ± 0.005 | 0.505 | 0.001 ± 0.002 | 0.002 ± 0.004 | 0.038 * | 0.845 |
| Coprococcus catus | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.355 | 0.002 ± 0.001 | 0.002 ± 0.002 | 0.037 * | 0.040 † |
| Coprococcus comes | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.792 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.317 | 0.792 |
| Dorea phocaeensis | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.062 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.593 | 0.062 |
| Evtepia gabavorous | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.458 | 0.002 ± 0.010 | 0.000 ± 0.000 | 0.461 | 0.458 |
| Faecalibacillus intestinalis | 0.001 ± 0.001 | 0.001 ± 0.002 | 0.350 | 0.001 ± 0.002 | 0.001 ± 0.001 | 0.158 | 0.350 |
| Hominisplanchenecus faecis | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.250 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.336 | 0.250 |
| Lachnospira eligens | 0.004 ± 0.006 | 0.003 ± 0.010 | 0.039 * | 0.004 ± 0.008 | 0.002 ± 0.002 | 0.528 | 0.073 |
| Latilactobacillus sakei | 0.000 ± 0.001 | 0.002 ± 0.005 | 0.008 * | 0.002 ± 0.005 | 0.000 ± 0.001 | 0.347 | 0.122 |
| Roseburia inulinivorans | 0.005 ± 0.006 | 0.002 ± 0.002 | 0.035 * | 0.002 ± 0.003 | 0.002 ± 0.003 | 0.570 | 0.711 |
| Ruminococcoides bili | 0.002 ± 0.006 | 0.003 ± 0.011 | 0.500 | 0.006 ± 0.013 | 0.010 ± 0.023 | 0.139 | 0.041 † |
| Ruminococcus bromii | 0.006 ± 0.008 | 0.010 ± 0.014 | 0.099 | 0.009 ± 0.013 | 0.016 ± 0.024 | 0.013 * | 1.000 |
| Turicibacter bilis | 0.000 ± 0.000 | 0.003 ± 0.007 | 0.805 | 0.000 ± 0.000 | 0.003 ± 0.005 | 0.717 | 0.805 |
| Vescimonas fastidiosa | 0.002 ± 0.003 | 0.001 ± 0.001 | 0.021 * | 0.002 ± 0.003 | 0.002 ± 0.006 | 0.575 | 0.288 |
| Bacteroidota | |||||||
| Alistipes onderdonkii | 0.002 ± 0.009 | 0.001 ± 0.002 | 0.851 | 0.004 ± 0.007 | 0.002 ± 0.003 | 0.041 * | 0.165 |
| Bacteroides fragilis | 0.000 ± 0.001 | 0.002 ± 0.008 | 0.878 | 0.002 ± 0.003 | 0.001 ± 0.001 | 0.051 | 0.038 † |
| Bacteroides stercoris | 0.003 ± 0.006 | 0.008 ± 0.016 | 0.182 | 0.002 ± 0.004 | 0.001 ± 0.002 | 0.013 * | 0.039 † |
| Phocaeicola plebeius | 0.005 ± 0.008 | 0.015 ± 0.020 | 0.005 * | 0.010 ± 0.019 | 0.015 ± 0.030 | 0.422 | 0.354 |
| Pseudomonadota | |||||||
| Enterobacter cloacae | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.176 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.465 | 0.176 |
| Parameter | Species Level | |
|---|---|---|
| Dominant grip strength | Anaerobutyricum soehngenii | Hominisplanchenecus faecis |
| 0.69 (0.024) | −0.68 (0.028) | |
| IL-1β | Coprococcus catus | Evtepia gabavorous |
| 0.72 (0.013) | 0.67 (0.039) | |
| IL-2 | Coprococcus comes | |
| −0.70 (0.022) | ||
| Myostatin | Dorea phocaeensis | |
| 0.72 (0.014) | ||
| Irisin | Faecalibacillus intestinalis | Turicibacter bilis |
| 0.66 (0.045) | −0.68 (0.032) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.H.; Hwang, S.; Seo, K.-H.; Park, Y.; Kim, M.J.; Kim, H. Bioconversion-Based Postbiotics Enhance Muscle Strength and Modulate Gut Microbiota in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 3937. https://doi.org/10.3390/nu17243937
Jung SH, Hwang S, Seo K-H, Park Y, Kim MJ, Kim H. Bioconversion-Based Postbiotics Enhance Muscle Strength and Modulate Gut Microbiota in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(24):3937. https://doi.org/10.3390/nu17243937
Chicago/Turabian StyleJung, Seung Hyeon, Subin Hwang, Kun-Ho Seo, Yongsoon Park, Mi Jung Kim, and Hyunsook Kim. 2025. "Bioconversion-Based Postbiotics Enhance Muscle Strength and Modulate Gut Microbiota in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 24: 3937. https://doi.org/10.3390/nu17243937
APA StyleJung, S. H., Hwang, S., Seo, K.-H., Park, Y., Kim, M. J., & Kim, H. (2025). Bioconversion-Based Postbiotics Enhance Muscle Strength and Modulate Gut Microbiota in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 17(24), 3937. https://doi.org/10.3390/nu17243937







