Anti-Inflammatory Effect of a Polysaccharide Derived from Artocarpus heterophyllus Lam. Pulp on Lipopolysaccharide-Stimulated RAW264.7 Macrophages Through Inhibiting MAPK/ERK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Culture
2.3. Analysis of Cell Viability
2.4. Determination of Nitric Oxide (NO) Levels
2.5. Determination of Intracellular Reactive Oxygen Species (ROS)
2.6. Measurement of Apoptosis
2.7. Assay of Antioxidant Activities
2.8. Cytokine Assay
2.9. Real Time Quantitative PCR
- β-actin (5′-3′) CTGAGAGGGAAATCGTGCGTGAC, (3′-5′) AGGAAGAGGATGCGGCAGTGG
- IL-6 (5′-3′) CTTCTTGGGACTGATGCTGGTGAC, (3′-5′) TCTGTTGGGAGTGGTATCCTCTGTG
- TNF-α (5′-3′) GGACTAGCCAGGAGGGAGAACAG, (3′-5′) GCCAGTGAGTGAAAGGGACAGAAC
- IL-1β (5′-3′) AATCTCACAGCAGCATCTCGACAAG, (3′-5′) TCCACGGGCAAGACATAGGTAGC
2.10. Western Blotting
2.11. Analysis of UPLC-Q-TOF-MS/MS
2.12. Statistical Analysis
3. Results
3.1. Cell Viability, NO Production, and Intracellular ROS Levels
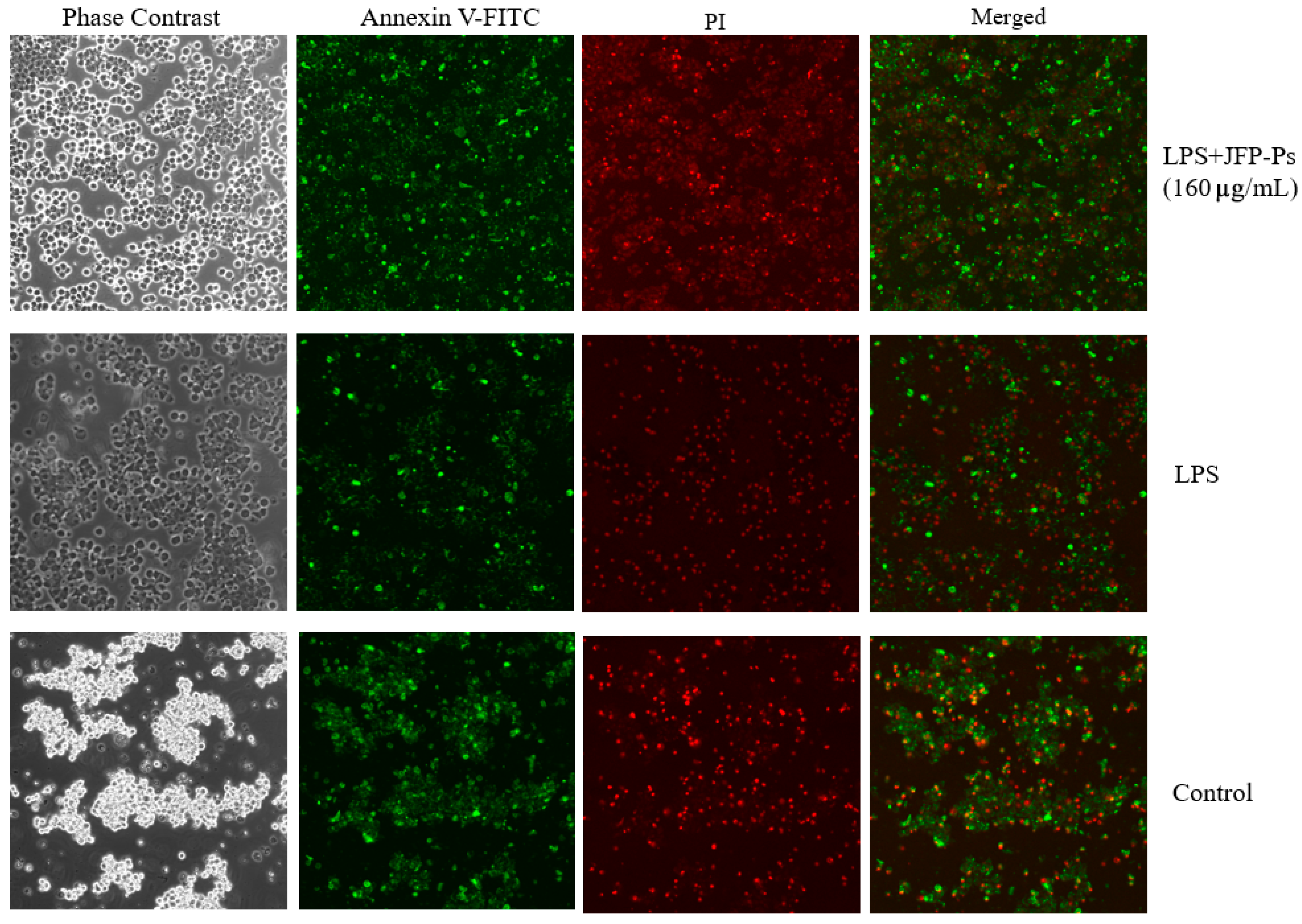
3.2. Impact of JFP-Ps on Apoptosis
3.3. Effect of JFP-Ps on the Activity of Antioxidant Enzymes
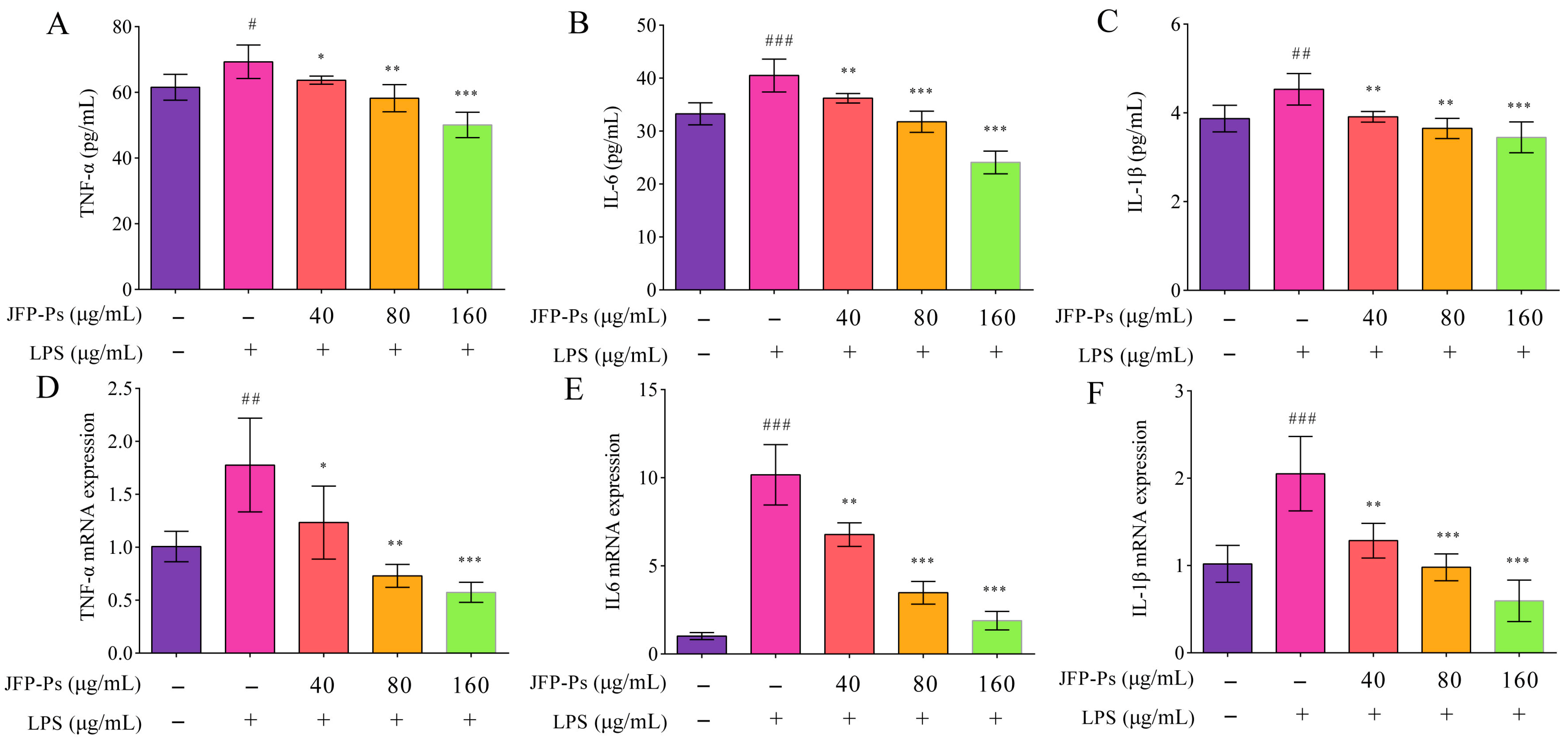
3.4. Inhibition of Pro-Inflammatory Cytokine Production via JFP-Ps
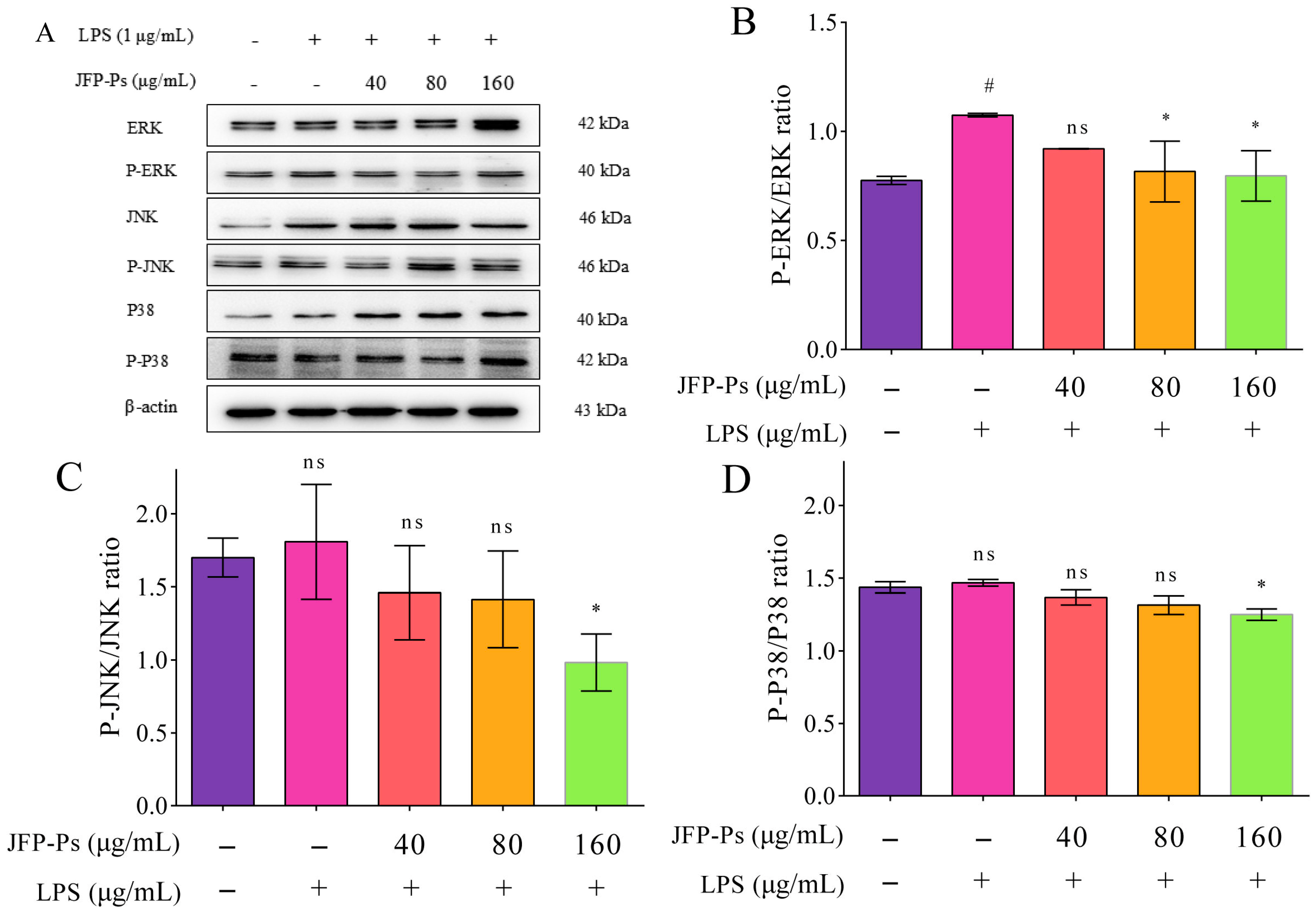
3.5. Impact of JFP-Ps on MAPK Signaling Pathway
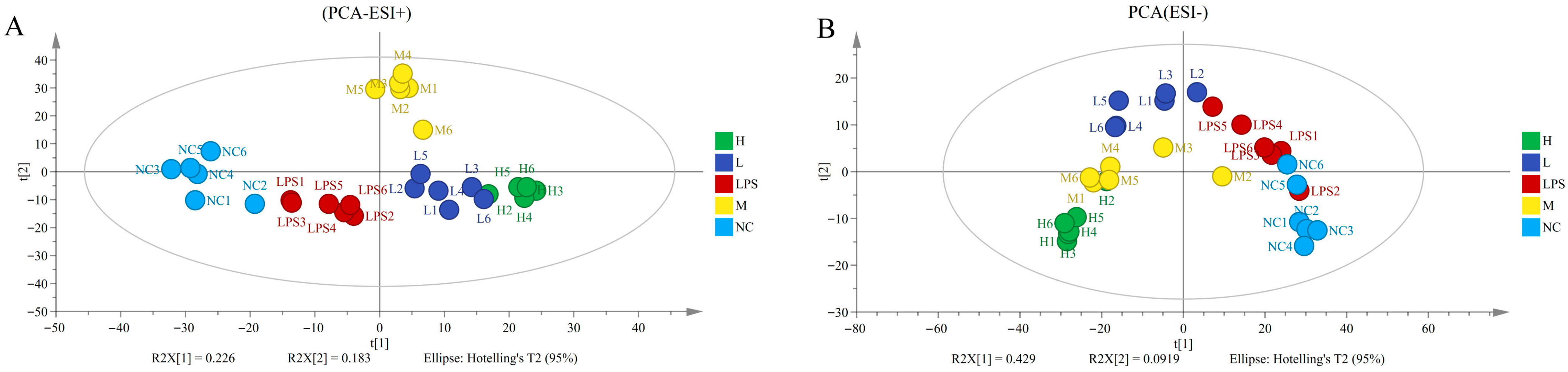
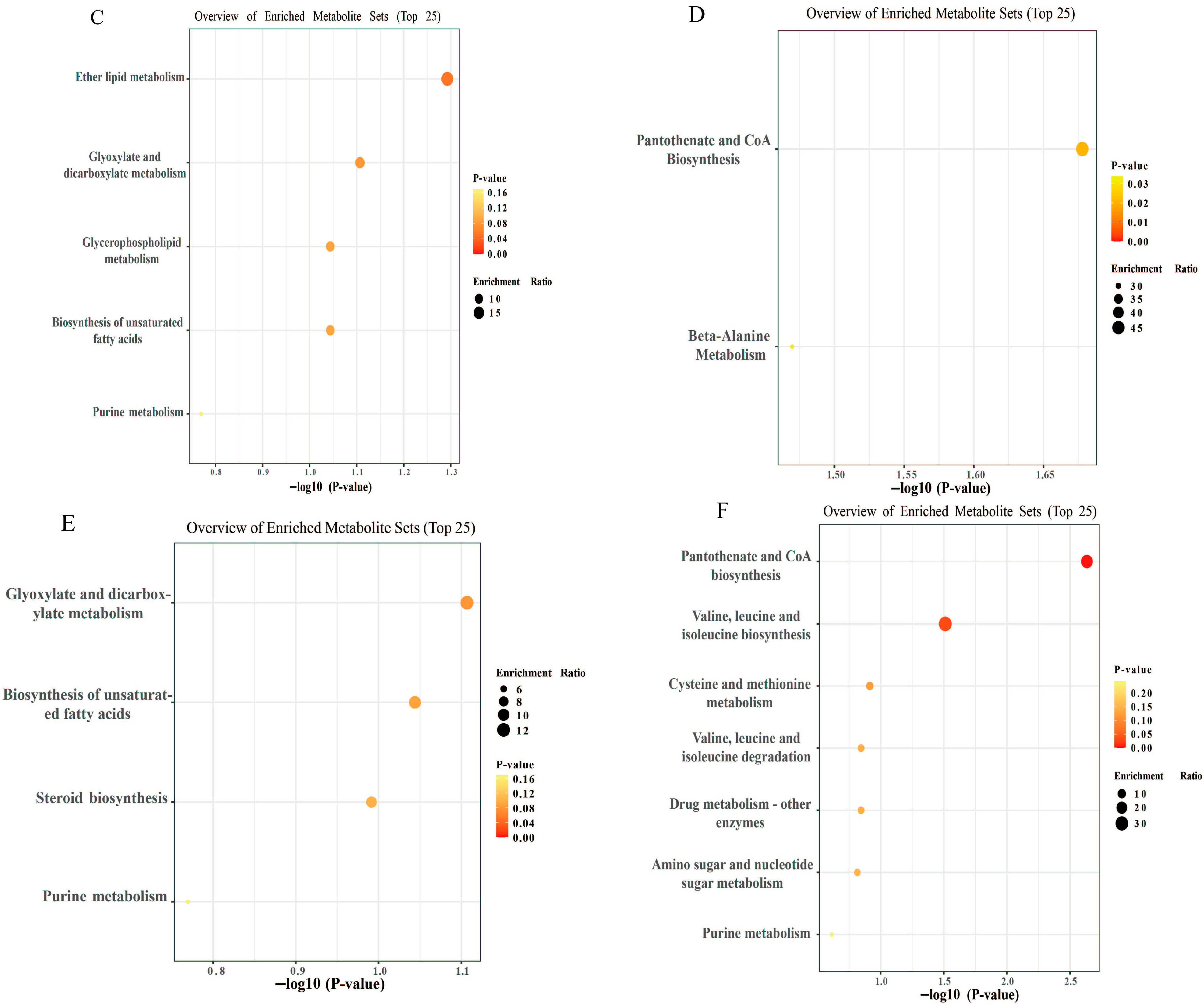
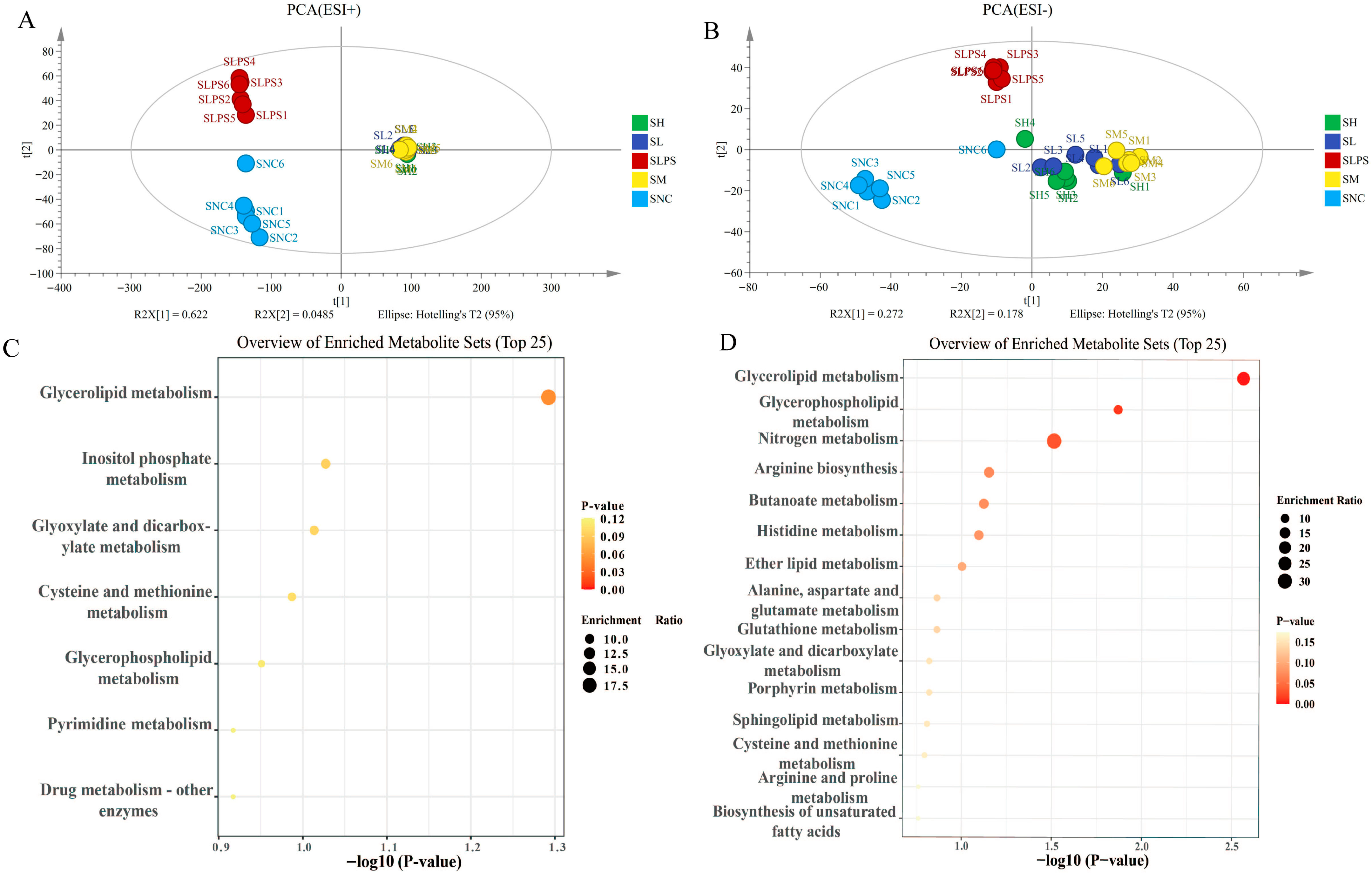
3.6. Non-Targeted Metabolomic Analysis of JFP-Ps in LPS-Induced RAW264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Kim, S.; Vetrivel, P.; Kim, H.; Ha, S.; Saralamma, V.V.G.; Kim, G. Artemisia iwayomogi (Dowijigi) inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppressing the NF-κB signaling pathway. Exp. Ther. Med. 2020, 3, 2161–2170. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorgan. Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Yu, W.W.; Zhou, H.C.; Lan, Z.C.; Wu, T.; Xiong, S.M.; Yan, L.; Liu, H.B. Lycium barbarum polysaccharides ameliorate LPS-induced inflammation of RAW264.7 cells and modify the behavioral score of peritonitis mice. J. Food Biochem. 2021, 45, e13889. [Google Scholar] [CrossRef]
- Yeom, M.; Kim, J.H.; Min, J.H.; Hwang, M.K.; Jung, H.S.; Sohn, Y. Xanthii fructus inhibits inflammatory responses in LPS-stimulated RAW 264.7 macrophages through suppressing NF-κB and JNK/p38 MAPK. J. Ethnopharmacol. 2015, 176, 394–401. [Google Scholar] [CrossRef]
- Han, J.M.; Lee, E.K.; Gong, S.Y.; Sohng, J.K.; Kang, Y.J.; Jung, H.J. Sparassis crispa exerts anti-inflammatory activity via suppression of TLR-mediated NF-κB and MAPK signaling pathways in LPS-induced RAW264.7 macrophage cells. J. Ethnopharmacol. 2019, 231, 10–18. [Google Scholar] [CrossRef]
- Feng, S.; Ding, H.; Liu, L.; Peng, C.; Huang, Y.; Zhong, F.; Li, W.; Meng, T.; Li, J.; Wang, X.; et al. Astragalus polysaccharide enhances the immune function of RAW264.7 macrophages via the NF-κB p65/MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 20. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Huang, J.; Qian, N.; Shen, G.; Chen, L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Silva, I.S.; Nicolau, L.A.D.; Sousa, F.B.M.; Araújo, S.D.; Oliveira, A.P.; Araújo, T.S.L.; Souza, L.K.M.; Martins, C.S.; Aquino, P.E.A.; Carvalho, L.L.; et al. Evaluation of anti-inflammatory potential of aqueous extract and polysaccharide fraction of Thuja occidentalis Linn. in mice. Int. J. Biol. Macromol. 2017, 105, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Rod-In, W.; You, S.; Park, W.J.; Surayot, U. Suaeda maritima polysaccharides attenuate LPS-induced inflammation of RAW264. 7 cells and antioxidative activity. Int. Immunopharmacol. 2024, 137, 112482. [Google Scholar] [CrossRef]
- Bemmo, U.L.K.; Bindzi, J.M.; Kamseu, P.R.T.; Ndomou, S.C.H.; Tambo, S.T.; Zambou, F.N. Physicochemical properties, nutritional value, and antioxidant potential of jackfruit (Artocarpus heterophyllus) pulp and seeds from Cameroon eastern forests. Food Sci. Nutr. 2023, 11, 4722–4734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhang, Y.; Nie, S.; Xu, F.; He, S.; Gong, D.; Wu, G.; Tan, L. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. pulp. Carbohyd. Polym. 2017, 155, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zheng, Y.; Wu, G.; Tan, L.; Xu, F.; Zhang, Y.; Chen, X.; Zhu, K. Protective effect of Artocarpus heterophyllus Lam. (jackfruit) polysaccharides on liver injury induced by cyclophosphamide in mice. Nutrients 2024, 16, 166. [Google Scholar] [CrossRef]
- Cheng, X.D.; Wu, Q.X.; Zhao, J.; Su, T.; Lu, Y.M.; Zhang, W.N.; Wang, Y.; Chen, Y. Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. Int. J. Biol. Macromol. 2019, 128, 732–739. [Google Scholar] [CrossRef]
- Yang, C.L.; Wang, S.B.; He, W.P.; Liu, J.J. Anti-oxidant and anti-inflammatory effects of ethanol extract from Polygala sibirica L. var megalopha Fr. on lipopolysaccharide-stimulated RAW264.7 cells. Chin. J. Integr. Med. 2023, 29, 905–913. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Guo, R.; Jing, W.; Lu, H. Cell metabolomics reveals berberine-inhibited pancreatic cancer cell viability and metastasis by regulating citrate metabolism. J. Proteome Res. 2020, 19, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Biworo, A.; Tanjung, E.; Iskandar; Khairina; Suhartono, E. Antidiabetic and antioxidant activity of jackfruit (Artocarpus heterophyllus) extract. J. Med. Bioeng. 2015, 4, 318–323. [Google Scholar] [CrossRef]
- Zeng, S.; Cao, J.; Wei, C.; Chen, Y.; Liu, Q.; Li, C.; Zhang, Y.; Zhu, K.; Wu, G.; Tan, L. Polysaccharides from Artocarpus heterophyllus Lam. (jackfruit) pulp alleviate obesity by modulating gut microbiota in high fat diet-induced rats. Food Hydrocolloid 2023, 139, 108521. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Liu, J.; Wang, Y.; Hu, J.H.; Wang, M.-H. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr. Res. Pract. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Oh, H.J.; Magar, T.B.T.; Pun, N.T.; Lee, Y.; Kim, E.H.; Lee, E.S.; Park, P.H. YJI-7 Suppresses ROS production and expression of inflammatory mediators via modulation of p38MAPK and JNK signaling in RAW 264.7 macrophages. Biomol. Ther. 2018, 26, 191–200. [Google Scholar] [CrossRef]
- Li, J.; Ruzhi, D.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.Q. Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef]
- Huang, Q.; He, W.; Khudoyberdiev, I.; Ye, C.L. Characterization of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg roots and their effects on antioxidant activity and H2O2-induced oxidative damage in RAW 264.7 cells. BMC Chem. 2021, 15, 9. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, M.; Luo, A. Extraction, characterization and antioxidant activities of an acidic polysaccharide from Dendrobium devonianum. J. Food Meas. Charact. 2021, 16, 867–879. [Google Scholar] [CrossRef]
- Fang, S.C.; Hsu, C.L.; Yen, G.C. Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus. J. Agr. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Roth, J.; Barczyk-Kahlert, K. More than suppression: Glucocorticoid action on monocytes and macrophages. Front. Immunol. 2019, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural characterization and anti-inflammatory activity of a polysaccharide from the lignified okra. Carbohydr. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef]
- Liao, J.; Li, C.; Huang, J.; Liu, W.; Chen, H.; Liao, S.; Chen, H.; Rui, W. Structure characterization of honey-processed Astragalus polysaccharides and its anti-inflammatory activity in vitro. Molecules 2018, 23, 168. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, K.; Jing, Y.; Du, R.; Zhu, Z.; Lu, L.; Zhang, R. The effects of low-dose nepsilon-(carboxymethyl)lysine (CML) and nepsilon-(carboxyethyl)lysine (CEL), two main glycation free adducts considered as potential uremic toxins, on endothelial progenitor cell function. Cardiovasc. Diabetol. 2012, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ye, C.; Huang, Y.; Zhang, N.; Zhang, X.; Xiao, M. Ginkgo biloba sarcotesta polysaccharide inhibits inflammatory responses through suppressing both NF-κB and MAPK signaling pathway. J. Sci. Food Agr. 2019, 99, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lin, Q.; Guo, T.; Yang, T.; Zhou, W.; Deng, X.; Yan, J.K.; Luo, Y.; Ju, M.; Luo, F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohyd. Polym. 2018, 200, 487–497. [Google Scholar] [CrossRef]
- Lee, S.G.; Rod-In, W.; Jung, J.J.; Jung, S.K.; Lee, S.M.; Park, W.J. Lipids extracted from Aptocyclus ventricosus eggs possess immunoregulatory effects on RAW264.7 cells by activating the MAPK and NF-kB signaling pathways. Mar. Drugs 2024, 22, 368. [Google Scholar] [CrossRef]
- Su, A.; Ma, G.; Ma, N.; Pei, F.; Yang, W.; Hu, Q. Effects of Flammulina velutipes polysaccharides on gut microbiota composition and metabolism in vitro fermentation. Food Sci. Biotechnol. 2022, 32, 361–369. [Google Scholar] [CrossRef]
- McMahon, A.N.; Lee, E.; Takita, C.; Reis, I.M.; Wright, J.L.; Hu, J.J. Metabolomics in radiotherapy-induced early adverse skin reactions of breast cancer patients. Breast Cancer Targets Ther. 2024, 16, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ryback, B.; Vorholt, J.A. Coenzyme biosynthesis in response to precursor availability reveals incorporation of beta-alanine from pantothenate in prototrophic bacteria. J. Biol. Chem. 2023, 299, 104919. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Song, X.; Zhao, M.; Chen, H.; Wang, Y.; Zhao, B.; Yu, S.; Ma, T.; Gao, L. Oral administration of live combined Bacillus subtilis and Enterococcus faecium alleviates colonic oxidative stress and inflammation in osteoarthritic rats by improving fecal microbiome metabolism and enhancing the colonic barrier. Front. Microbiol. 2022, 13, 1005842. [Google Scholar] [CrossRef]
- Li, S.; Zhuge, A.; Chen, H.; Han, S.; Shen, J.; Wang, K.; Xia, J.; Xia, H.; Jiang, S.; Wu, Y.; et al. Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice. J. Adv. Res. 2025, 69, 413–426. [Google Scholar] [CrossRef]
- Haj, A.K.; Hasan, H.; Raife, T.J. Heritability of protein and metabolite biomarkers associated with COVID-19 severity: A metabolomics and proteomics Analysis. Biomolecules 2022, 13, 46. [Google Scholar] [CrossRef]
- Li, K.; Cui, L.J.; Cao, Y.X.; Li, S.Y.; Shi, L.X.; Qin, X.M.; Du, Y.G. UHPLC Q-exactive MS-based serum metabolomics to explore the effect mechanisms of immunological activity of Astragalus polysaccharides with different molecular weights. Front. Pharmacol. 2020, 11, 595692. [Google Scholar] [CrossRef]

| Control Group | LPS | LPS+JFP-Ps Concentrations (μg/mL) | |||
|---|---|---|---|---|---|
| 40 | 80 | 160 | |||
| CAT (µmol/min/mg prot) | 59.91 ± 10.05 ab | 69.18 ± 13.46 a | 53.98 ± 3.09 bc | 46.39 ± 2.32 cd | 37.49 ± 8.03 d |
| GSH-Px (µmol/mg prot) | 253.51 ± 23.23 a | 168.37 ± 13.82 b | 191.11 ± 11.93 b | 224.52 ± 31.33 a | 257.32 ± 29.49 a |
| SOD (U/mg prot) | 0.54 ± 0.064 a | 0.29 ± 0.038 c | 0.42 ± 0.042 b | 0.46 ± 0.041 b | 0.52 ± 0.032 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, B.; Liu, M.; Xu, P.; Zhang, Y.; Xu, F.; Wu, G.; Zhou, Y.; Zhu, K. Anti-Inflammatory Effect of a Polysaccharide Derived from Artocarpus heterophyllus Lam. Pulp on Lipopolysaccharide-Stimulated RAW264.7 Macrophages Through Inhibiting MAPK/ERK Signaling Pathway. Nutrients 2025, 17, 3879. https://doi.org/10.3390/nu17243879
Bai B, Liu M, Xu P, Zhang Y, Xu F, Wu G, Zhou Y, Zhu K. Anti-Inflammatory Effect of a Polysaccharide Derived from Artocarpus heterophyllus Lam. Pulp on Lipopolysaccharide-Stimulated RAW264.7 Macrophages Through Inhibiting MAPK/ERK Signaling Pathway. Nutrients. 2025; 17(24):3879. https://doi.org/10.3390/nu17243879
Chicago/Turabian StyleBai, Benyan, Mengyang Liu, Panjie Xu, Yanjun Zhang, Fei Xu, Gang Wu, Yan Zhou, and Kexue Zhu. 2025. "Anti-Inflammatory Effect of a Polysaccharide Derived from Artocarpus heterophyllus Lam. Pulp on Lipopolysaccharide-Stimulated RAW264.7 Macrophages Through Inhibiting MAPK/ERK Signaling Pathway" Nutrients 17, no. 24: 3879. https://doi.org/10.3390/nu17243879
APA StyleBai, B., Liu, M., Xu, P., Zhang, Y., Xu, F., Wu, G., Zhou, Y., & Zhu, K. (2025). Anti-Inflammatory Effect of a Polysaccharide Derived from Artocarpus heterophyllus Lam. Pulp on Lipopolysaccharide-Stimulated RAW264.7 Macrophages Through Inhibiting MAPK/ERK Signaling Pathway. Nutrients, 17(24), 3879. https://doi.org/10.3390/nu17243879






