The Gut Nexus: Unraveling Microbiota-Mediated Links Between Type 2 Diabetes and Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Results
3.1. Microbiota Composition Across T2DM, CRC, and T2DM+CRC
| Taxon/Metabolite | Qualitative Direction Across T2DM/CRC/DCRC | Representative Studies |
|---|---|---|
| Faecalibacterium spp. | Generally ↓ in CRC; often reduced in metabolic disorders | [22] |
| Roseburia spp. | ↓ in CRC and obesogenic states; SCFA-producing | [22] |
| Butyricicoccus spp. | ↓ in DCRC vs. controls | [18] |
| Lactobacillus spp. | Decreased in some DCRC cohorts; context-dependent | [18] |
| Paraprevotella spp. | ↓ in DCRC | [18] |
| Eggerthella spp. | ↑ in DCRC; enriched in dysbiosis | [18] |
| Hungatella spp. | ↑ in DCRC | [18] |
| Peptostreptococcus spp. | ↑ in DCRC; associated with CRC and inflammation | [18] |
| Parvimonas spp. | ↑ in DCRC and CRC | [18] |
| Veillonella spp. | ↑ in DCRC; associated with chemoresistance in CRC | [18] |
| Akkermansia muciniphila | ↓ in obesity/T2DM; often ↑ after beneficial interventions | [27,28,31] |
| SCFAs (total) | Often ↓ in CRC and dysmetabolic states; ↑ with fiber interventions | [20,24,30] |
| Butyrate | ↓ in CRC/dysbiosis; ↑ with high-fiber/prebiotic intake | [20,21,22] |
| Acetate | Altered in DCRC; direction varies by study | [18,20] |
| Propionate | Altered SCFA profile; direction varies | [29,30] |
| Total bile acids | Dysregulated in DCRC and T2DM–CRC | [18,26] |
| Secondary bile acids (e.g., DCA) | ↑ in DCRC/CRC; tumor-promoting | [18,26,30] |
| TMAO | ↑ or altered in DCRC; pro-atherogenic and potentially pro-tumorigenic | [18] |
| ROS and oxidative stress markers | ↑ in dysbiosis and SCFA/BA imbalance contexts | [20,46] |
| Inflammatory cytokines (TNF-α, IL-6, IL-1β, etc.) | ↑ in obesity, T2DM, and dysbiotic CRC | [27,30] |
| Intervention/Strategy | Type | Context | Microbiota Effects | CRC-Related Outcomes | Metabolic/T2DM-Related Outcomes | Therapeutic Implications | Reference |
|---|---|---|---|---|---|---|---|
| Garlic intake | Dietary | Human; observational | Alters blood/gut microbiome signatures; supports beneficial taxa and anti-inflammatory profile | Medium/high garlic intake linked to reduced CRC risk | Associated with better inflammatory and metabolic parameters | Supports garlic-rich diets as microbiota- and immune-modulating chemopreventive strategy | [19] |
| Prebiotic fiber supplementation | Dietary | Human; prospective cohort | Expected enrichment of Bifidobacterium and SCFA-producing taxa | Associated with modestly lower CRC risk and improved post-CRC survival | Linked to better cardiometabolic profiles | Supports high-fiber/prebiotic intake as part of CRC and metabolic risk reduction | [21] |
| High-fiber, plant-rich diet in obese patients with prior adenomatous polyps | Dietary | Human; interventional | Increases SCFA producers; reduces pro-inflammatory/pathogenic taxa | Reduces markers and intermediate endpoints related to colon cancer risk | Improves weight, insulin sensitivity, and lipid profile | Fiber-rich diets provide dual protection for colon and metabolic health in high-risk populations | [24] |
| High-fat Western-style diet | Dietary | Mouse; HFD models | Reduces diversity, enriches pro-inflammatory taxa, disrupts barrier | Favors a pro-tumorigenic colonic environment and may increase CRC susceptibility | Promotes insulin resistance, obesity, and T2DM-like metabolic dysfunction | Reducing HFD and replacing with fiber-rich patterns may lower both CRC and T2DM risk | [25,46] |
| SCFA-enhancing strategies (high fiber, prebiotics) | Dietary/microbiota-directed | In vitro, human, and animal data | Increase butyrate and other SCFAs; enrich butyrate-producing taxa | SCFAs inhibit CRC cell proliferation and induce apoptosis; improve barrier | SCFAs improve insulin sensitivity and energy homeostasis | Reinforces dietary/prebiotic strategies to increase SCFA production as a shared T2DM–CRC target | [20,30] |
| Akkermansia muciniphila supplementation/EVs | Microbiota-directed | Mouse and preclinical | Increases A. muciniphila, improves mucus layer and tight junctions; modulates TLR signaling | Not yet directly tested in CRC models, but reduces inflammatory milieu that favors carcinogenesis | Reduces obesity-related inflammation and improves insulin sensitivity | Promising candidate for next-generation probiotics aimed at obesity, T2DM, and CRC prevention | [27,28,31] |
| Metformin therapy | Pharmacologic | Mouse tumor models; human epidemiology (cited in text) | Modulates gut microbiota, increasing some beneficial taxa and altering SCFA/BA profiles | Reduces tumor growth in preclinical models; observational data suggest lower CRC risk in metformin users | Improves glycemic control and insulin resistance in T2DM | Supports potential dual benefit of metformin on metabolic and oncologic outcomes, though causal links remain to be confirmed | [29] |
| General high-fiber/Mediterranean-style diets | Dietary | Human/review | Enrich SCFA-producing taxa, increase microbial diversity, reduce bile acid dysregulation | Associated with lower CRC risk in epidemiologic studies | Linked to lower risk of obesity, insulin resistance, and T2DM | Support guidelines promoting plant-rich, high-fiber patterns to jointly address CRC and T2DM burdens | [30] |
3.2. Metabolic Pathways & Microbial Metabolites
3.3. Immune and Inflammatory Modulation
3.4. Metabolic Dysfunction Amplified by Dysbiosis
3.5. Interventions Targeting Microbiota
3.6. Diagnostic and Research Applications
4. Discussion
4.1. Microbiota as a Convergent Axis in T2DM and CRC
4.2. Metabolomic Shifts and Carcinogenic Potential
4.3. Immune Dysregulation and Barrier Breakdown
4.4. Obesity and Hyperinsulinemia: A Feed-Forward Loop
4.5. Therapeutic Modulation and Translational Insights
4.6. Clinical and Surgical Implications
4.7. Limitations and Knowledge Gaps
4.8. Future Research Directions
4.9. Conceptual Framework and Conclusions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| T2DM | Type 2 Diabetes Mellitus |
| SCFA | Short Chain Fatty Acids |
| DCA | Deoxycholic Acid |
| TMAO | Trimethylamine-N-oxide |
| FMT | Fecal Microbiota Transplantation |
| EOCRC | Early-Onset Colorectal Cancer |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear Factor Kappa B |
| IL | Interleukine |
| TNF-ɑ | Tumor Necrosis Factor-alpha |
| GLP-1 | Glucagon-Like Peptide 1 |
| IGF-1 | Insulin-Like Growth Factor 1 |
References
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.-X.; Yin, X.-F.; Zhang, M.-X.; Qiao, J.; Xin, X.-H.; Chang, M.-J.; Gao, C.; Li, Y.-F.; Li, X.-F. The Gut Microbiota and Its Relevance to Peripheral Lymphocyte Subpopulations and Cytokines in Patients with Rheumatoid Arthritis. J. Immunol. Res. 2021, 2021, 6665563. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef]
- Chong, S.; Lin, M.; Chong, D.; Jensen, S.; Lau, N.S. A systematic review on gut microbiota in type 2 diabetes mellitus. Front. Endocrinol. 2024, 15, 1486793. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas. IDF Diabetes Atlas, 10th ed.; IDF Diabetes Atlas: Brussels, Belgium, 2021; Available online: www.diabetesatlas.org (accessed on 21 July 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Peeters, P.J.H.L.; Bazelier, M.T.; Leufkens, H.G.M.; De Vries, F.; De Bruin, M.L. The risk of colorectal cancer in patients with type 2 Diabetes: Associations with treatment stage and obesity. Diabetes Care 2015, 38, 495–502. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; O’SUllivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of Vitamin D pathway genetic variation and circulating 25-hydroxyVitamin D on cancer outcome: Systematic review and meta-Analysis. Br. J. Cancer 2017, 116, 1095–1110. [Google Scholar] [CrossRef]
- Burcelin, R.; Serino, M.; Chabo, C.; Blasco-Baque, V.; Amar, J. Gut microbiota and diabetes: From pathogenesis to therapeutic perspective. Acta Diabetol. 2011, 48, 257–273. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Liu, G.; Tang, J.; Zhou, J.; Dong, M. Short-chain fatty acids play a positive role in colorectal cancer. Discov. Oncol. 2024, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’COnnell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef]
- Huang, Q.-Y.; Yao, F.; Zhou, C.-R.; Huang, X.-Y.; Wang, Q.; Long, H.; Wu, Q.-M. Role of gut microbiome in regulating the effectiveness of metformin in reducing colorectal cancer in type 2 diabetes. World J. Clin. Cases 2020, 8, 6213–6228. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Z.; Gao, Z.; Chen, J.; Song, C.; Xu, J.; Wang, H.; Huang, A.; Shi, J.; Gu, J. Metagenomic and targeted metabolomic analyses reveal distinct phenotypes of the gut microbiota in patients with colorectal cancer and type 2 diabetes mellitus. Chin. Med. J. 2023, 136, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Speciani, M.C.; Gargari, G.; Penagini, R.; Mutignani, M.; Ferraroni, M.; Natale, A.; Katsoulis, M.; Cintolo, M.; Leone, P.; Airoldi, A.; et al. Garlic consumption in relation to colorectal cancer risk and to alterations of blood bacterial DNA. Eur. J. Nutr. 2023, 62, 2279–2292. [Google Scholar] [CrossRef]
- Huang, C.; Deng, W.; Xu, H.-Z.; Zhou, C.; Zhang, F.; Chen, J.; Bao, Q.; Zhou, X.; Liu, M.; Li, J.; et al. Short-chain fatty acids reprogram metabolic profiles with the induction of reactive oxygen species production in human colorectal adenocarcinoma cells. Comput. Struct. Biotechnol. J. 2023, 21, 1606–1620. [Google Scholar] [CrossRef]
- Skiba, M.B.; Kohler, L.N.; Crane, T.E.; Jacobs, E.T.; Shadyab, A.H.; Kato, I.; Snetselaar, L.; Qi, L.; Thomson, C.A. The Association between Prebiotic Fiber Supplement Use and Colorectal Cancer Risk and Mortality in the Women’s Health Initiative. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1884–1890. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, Y.; Zheng, Q.; Zhang, R.; Hu, C.; Jia, W. Altered intestinal microbiota associated with colorectal cancer. Front. Med. 2019, 13, 461–470. [Google Scholar] [CrossRef]
- Iwama, N.; Matsuda, M.; Tsuruta, M.; Okabayashi, K.; Shigeta, K.; Kanai, T.; Kitagawa, Y. Relationship between obesity-related colorectal tumors and the intestinal microbiome: An animal-based trial. J. Cancer Res. Clin. Oncol. 2023, 149, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Christie, J.; Wilson, A.; Ziegler, T.R.; Methe, B.; Flanders, W.D.; Rolls, B.J.; Eberhart, B.L.; Li, J.V.; Huneault, H.; et al. Fibre-rich Foods to Treat Obesity and Prevent Colon Cancer trial study protocol: A randomised clinical trial of fibre-rich legumes targeting the gut microbiome, metabolome and gut transit time of overweight and obese patients with a history of noncancerous adenomatous polyps. BMJ Open 2024, 14, e081379. [Google Scholar] [PubMed]
- Xie, Y.; Ding, F.; Di, W.; Lv, Y.; Xia, F.; Sheng, Y.; Yu, J.; Ding, G. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 2020, 21, 1133–1144. [Google Scholar] [CrossRef]
- Lv, Y.; Lin, S.; Liu, M.; Wang, L.; Wang, X.; Cui, L.; Xu, J. Impacts of pre-existing diabetes mellitus on colorectal cancer in a mice model. Cancer Med. 2023, 12, 11641–11650. [Google Scholar] [CrossRef]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat. Commun. 2024, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, J.; Meng, Y.; Yang, J.; Cui, Q.; Zhou, Y. Metformin Alters Gut Microbiota of Healthy Mice: Implication for Its Potential Role in Gut Microbiota Homeostasis. Front. Microbiol. 2018, 9, 1336. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Saigal, A.; Szamosi, J.C.; Hammill, J.A.; Bezverbnaya, K.; Wang, D.; Gautam, J.; Tsakiridis, E.E.; Di Pastena, F.; McNicol, J.; et al. Metformin-induced reductions in tumor growth involves modulation of the gut microbiome. Mol. Metab. 2022, 61, 101498. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Tilg, H. Dietary Factors: Major Regulators of the Gut’s Microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]
- Ashrafian, F.; Behrouzi, A.; Shahriary, A.; Badi, S.A.; Davari, M.; Khatami, S.; Jamnani, F.R.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Chu, C.; Gao, X.; Li, X.; Zhang, X.; Ma, R.; Jia, Y.; Li, D.; Wang, D.; Xu, F. Involvement of estrogen receptor-α in the activation of Nrf2-antioxidative signaling pathways by silibinin in pancreatic β-cells. Biomol. Ther. 2020, 28, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Q.; Zhu, S.; Liu, B.; Liu, F.; Xu, Y. Mulberry leaf (Morus alba L.): A review of its potential influences in mechanisms of action on metabolic diseases. Pharmacol. Res. 2022, 175, 106029. [Google Scholar] [CrossRef]
- Zhang, W.; An, Y.; Qin, X.; Wu, X.; Wang, X.; Hou, H.; Song, X.; Liu, T.; Wang, B.; Huang, X.; et al. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front. Oncol. 2021, 11, 739648. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front. Cell. Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.-X.; Xie, C.-Y.; Fan, J.; Lv, J.; Xu, X.-J.; Lv, J.; Kuai, W.-T.; Jia, Y.-T. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J. Gastroenterol. 2020, 26, 7633–7651. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxidative Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Cui, Z.; Yan, Y.Q.; Ning, L.J.; Wang, Z.H.; Hong, J. Microbe-based management for colorectal cancer. Chin. Med. J. 2021, 134, 2922–2930. [Google Scholar] [CrossRef]
- Luan, S.; Cheng, W.; Wang, C.; Gong, J.; Zhou, J. Impact of glucagon-like peptide 1 analogs on cognitive function among patients with type 2 diabetes mellitus: A systematic review and meta−analysis. Front. Endocrinol. 2022, 13, 1047883. [Google Scholar] [CrossRef]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Wu, J.; Xia, C.; Liu, C.; Zhang, Q.; Xia, C. The role of gut microbiota and drug interactions in the development of colorectal cancer. Front. Pharmacol. 2023, 14, 1265136. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, W.; Shen, Q.; Miao, C.; Chen, L.; Li, Y.; Gu, X.; Fan, M.; Ma, Y.; Wang, H.; et al. Bile acid metabolism dysregulation associates with cancer cachexia: Roles of liver and gut microbiome. J. Cachex Sarcopenia Muscle 2021, 12, 1553–1569. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, G.; Liu, Y.; Zhang, J.; Chen, Y.; Liu, L.; Zhang, G. HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances. Biomolecules 2023, 13, 601. [Google Scholar] [CrossRef]
- Chen, G.; Ren, Q.; Zhong, Z.; Li, Q.; Huang, Z.; Zhang, C.; Yuan, H.; Feng, Z.; Chen, B.; Wang, N.; et al. Exploring the gut microbiome’s role in colorectal cancer: Diagnostic and prognostic implications. Front. Immunol. 2024, 15, 1431747. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.A.; Rosa-Neto, P. World Alzheimer Report 2022—Life After Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Grnøtved, A.; Rimm, E.B.; Willett, W.C.; Andersen, L.B.; Hu, F.B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 2012, 172, 1306–1312. [Google Scholar] [CrossRef]
- Zhao, S.; Lu, Z.; Zhao, F.; Tang, S.; Zhang, L.; Feng, C. Assessing the impact of probiotics on immunotherapy effectiveness and antibiotic-mediated resistance in cancer: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1538969. [Google Scholar] [CrossRef] [PubMed]
- Fuchsberger, C.; Flannick, J.; Teslovich, T.M.; Mahajan, A.; Agarwala, V.; Gaulton, K.J.; Ma, C.; Fontanillas, P.; Moutsianas, L.; McCarthy, D.J.; et al. The genetic architecture of type 2 diabetes. Nature 2016, 536, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lau, H.C.H.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef] [PubMed]
- Stendell-Hollis, N.R.; Thomson, C.A.; Thompson, P.A.; Bea, J.W.; Cussler, E.C.; Hakim, I.A. Green tea improves metabolic biomarkers, not weight or body composition: A pilot study in overweight breast cancer survivors. J. Hum. Nutr. Diet. 2010, 23, 590–600. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Fang, A.; Zhang, Q.; Fan, H.; Zhou, Y.; Yao, Y.; Zhang, Y.; Huang, X. Discovery of human lactate dehydrogenase A (LDHA) inhibitors as anticancer agents to inhibit the proliferation of MG-63 osteosarcoma cells. MedChemComm 2017, 8, 1720–1726. [Google Scholar] [CrossRef]
- Liu, B.; Yang, P.; Ye, Y.; Zhou, Y.; Li, L.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Role of ROS in the protective effect of silibinin on sodium nitroprusside-induced apoptosis in rat pheochromocytoma PC12 cells. Free Radic. Res. 2011, 45, 835–847. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–958. [Google Scholar] [CrossRef]
- Kent, B.A.; Holman, C.; Amoako, E.; Antonietti, A.; Azam, J.M.; Ballhausen, H.; Bediako, Y.; Belasen, A.M.; Carneiro, C.F.D.; Chen, Y.-C.; et al. Recommendations for empowering early career researchers to improve research culture and practice. PLoS Biol. 2022, 20, e3001680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lin, H.; Yu, X.; Wang, T.; Xu, Z. Prediction of postoperative complications following transanal total mesorectal excision in middle and low rectal cancer: Development and internal validation of a clinical prediction model. Int. J. Color. Dis. 2024, 39, 133. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Clay, S.L.; Fonseca-Pereira, D.; Garrett, W.S. Colorectal cancer: The facts in the case of the microbiota. J. Clin. Investig. 2022, 132, e155101. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26. [Google Scholar] [CrossRef]
- Wu, H.; Ma, W.; Wang, Y.; Wang, Y.; Sun, X.; Zheng, Q. Gut microbiome-metabolites axis: A friend or foe to colorectal cancer progression. Biomed. Pharmacother. 2024, 173, 116410. [Google Scholar] [CrossRef]
- Vipperla, K.; O’Keefe, S.J. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr. Clin. Pract. 2012, 27, 624–635. [Google Scholar] [CrossRef]
- Pournourmohammadi, S.; Grimaldi, M.; Stridh, M.H.; Lavallard, V.; Waagepetersen, H.S.; Wollheim, C.B.; Maechler, P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ß-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017, 88, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Cheng, L.; Cao, X.; Liu, C. Gut microbiota in colorectal cancer: A review of its influence on tumor immune surveillance and therapeutic response. Front. Oncol. 2025, 15, 1557959. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack Europe PMC Funders Group. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Nochi, H. Role and cytotoxicity of amylin and protection of pancreatic islet β-cells from amylin cytotoxicity. Cells 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef]
- Hansen, T.H.; Gøbel, R.J.; Hansen, T.; Pedersen, O. The gut microbiome in cardio-metabolic health. Genome Med. 2015, 7, 33. [Google Scholar] [CrossRef]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active Smoking and the Risk of Type 2 Diabetes A Systematic Review and Meta-Analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Kesh, K.; Mendez, R.; Abdelrahman, L.; Banerjee, S.; Banerjee, S. Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb. Cell Factories 2020, 19, 75. [Google Scholar] [CrossRef]
- Guttmacher, A.E.; Collins, F.S.; Nussbaum, R.L.; Ellis, C.E. Genomic Medicine. N. Engl. J. Med. 2003, 347, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valbuena, I.; Valenti-Azcarate, R.; Amat-Villegas, I.; Riverol, M.; Marcilla, I.; de Andrea, C.E.; Sánchez-Arias, J.A.; Carmona-Abellan, M.d.M.; Marti, G.; Erro, M.; et al. Amylin as a potential link between type 2 diabetes and alzheimer disease. Ann. Neurol. 2019, 86, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Haowen, J.; Vimalesvaran, S.; Myint Kyaw, B.; Tudor Car, L. Virtual reality in medical students’ education: A scoping review protocol. BMJ Open 2021, 11, e046986. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Kyte, D.G.; Aiyegbusi, O.L.; Keeley, T.J.; Calvert, M.J. Assessing the impact of healthcare research: A systematic review of methodological frameworks. PLoS Med. 2017, 14, e1002370. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Masgrau, A.; Boirie, Y. Is protein metabolism changed with obesity? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 89–92. [Google Scholar] [CrossRef]
- Ma, L.; Yang, C.; Huang, L.; Chen, Y.; Li, Y.; Cheng, C.; Cheng, B.; Zheng, L.; Huang, K. Glycated Insulin Exacerbates the Cytotoxicity of Human Islet Amyloid Polypeptides: A Vicious Cycle in Type 2 Diabetes. ACS Chem. Biol. 2019, 14, 486–496. [Google Scholar] [CrossRef]



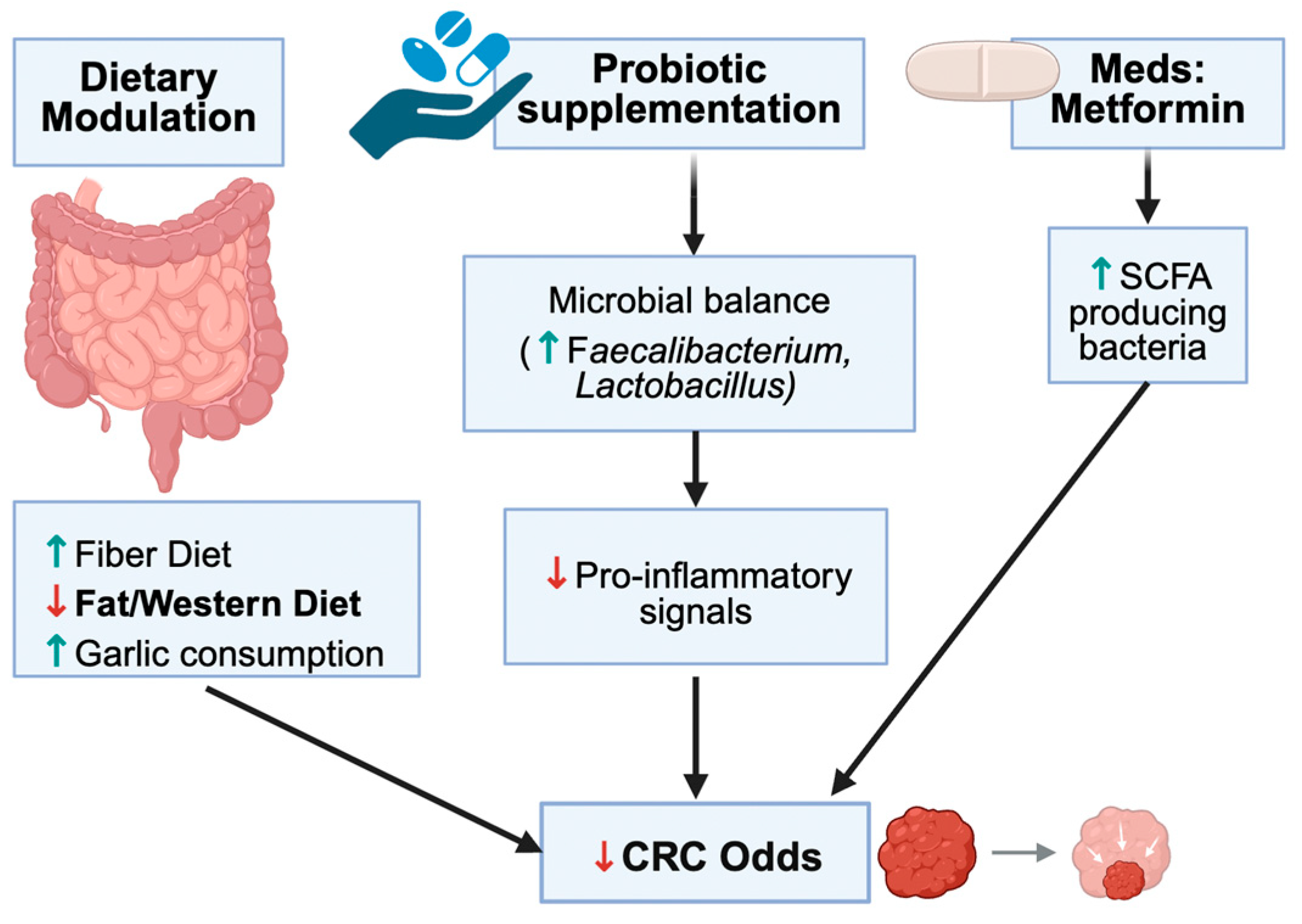

| Study | Reference | Model/Context | Key Microbiota Findings | CRC-Related Findings | T2DM/Metabolic Findings | Preventive/Therapeutic Insights |
|---|---|---|---|---|---|---|
| Metagenomic and targeted metabolomic analyses reveal distinct patterns in patients with colorectal cancer and type 2 diabetes mellitus | [18] | Human patients; DCRC vs. CRC vs. controls | DCRC group shows enrichment of Peptostreptococcus, Porphyromonas, Parvimonas, Eggerthella, Hungatella, Veillonella; depletion of Butyricicoccus, Lactobacillus spp., Paraprevotella and other butyrate producers | Compounded dysbiosis and altered bile acid/SCFA profiles may favor colorectal carcinogenesis | DCRC patients show diabetes-related metabolic dysregulation superimposed on CRC, with altered BA–SCFA interactions | Highlights targeting BA–SCFA pathways and dysbiotic taxa in DCRC as a potential preventive/therapeutic strategy |
| Garlic consumption in relation to colorectal cancer risk and to alterations of blood bacterial DNA | [19] | Human; observational | Higher garlic intake associated with distinct blood bacterial DNA profiles suggestive of systemic microbiome modulation | Medium/high garlic intake significantly associated with lower CRC risk, particularly rectal cancer | Garlic intake associated with more favorable inflammatory/metabolic profile | Supports garlic-rich diets as chemopreventive via microbiota and immune modulation |
| Short-chain fatty acids reprogram metabolic profiles with the suppression of colorectal cancer cell species production in human colorectal adenocarcinoma cells | [20] | In vitro; CRC cell lines | SCFAs (especially butyrate) act as key microbial metabolites influencing cancer cell metabolism | Butyrate and other SCFAs suppress CRC progression by reprogramming glycolysis and mitochondrial metabolism, increasing ROS and inducing apoptosis | SCFAs are central to energy homeostasis and may improve insulin sensitivity (relevant to T2DM) | Supports strategies to enhance SCFA production via diet/microbiota modulation as CRC-preventive |
| The Association Between Prebiotic Fiber Supplement Use and Colorectal Cancer Risk and Mortality in the Women’s Health Initiative | [21] | Human; prospective cohort | Prebiotic fiber expected to enrich Bifidobacterium and other SCFA producers (microbiota not directly sequenced in all) | Prebiotic fiber supplement use associated with modestly lower CRC risk and improved survival after CRC | Fiber intake linked to better metabolic profile, indirectly relevant to T2DM | Encourages high-fiber/prebiotic intake as adjunct in CRC prevention and metabolic health |
| Altered intestinal microbiota associated with colorectal cancer | [22] | Human; CRC vs. controls | CRC patients have decreased SCFA-producing taxa (Faecalibacterium, Roseburia) and increased potentially pathogenic bacteria | Defines characteristic CRC-associated dysbiosis | Overlaps with patterns described in metabolic disorders but diabetes not primary focus | Supports microbiota-targeted prevention (diet, probiotics) aimed at restoring beneficial taxa |
| Relationship between obesity-related colorectal tumors and the intestinal microbiome: an animal-based trial | [23] | Mouse; obesity-related CRC model | Obesity-related CRC tumors show distinct microbial signatures vs. lean CRC, with enrichment of obesogenic and pro-inflammatory taxa | Obesity-associated dysbiosis correlates with greater tumor burden and more aggressive CRC | Obesity/metabolic dysfunction (T2DM-like) interact with dysbiosis to promote CRC | Suggests weight loss and microbiota modulation to reduce obesity-related CRC risk |
| Fibre-rich Foods to Treat Obesity and Prevent Colon Cancer trial in obese patients with a history of noncancerous adenomatous polyps | [24] | Human; interventional, high-fiber diet | Fiber-rich diet increases SCFA-producing taxa and reduces pro-inflammatory/pathogenic bacteria | Reduces markers associated with colon cancer risk (e.g., polyp recurrence, inflammatory markers) | Improves weight, insulin sensitivity, and lipids in obese participants | High-fiber diets offer dual benefit: colon cancer prevention and metabolic improvement |
| Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice | [25] | Mouse; high-fat diet (HFD) | HFD reduces microbial diversity, enriches pro-inflammatory taxa, and impairs gut barrier integrity | Barrier dysfunction and inflammation under HFD create a milieu favorable for CRC | HFD promotes insulin resistance and T2DM-like metabolic dysfunction | Suggests limiting HFD and increasing fiber to preserve barrier function and reduce CRC/T2DM risk |
| Impacts of pre-existing diabetes mellitus on colorectal cancer in a mice model | [26] | Mouse; T2DM + CRC | Diabetic CRC mice exhibit distinct dysbiosis vs. non-diabetic CRC, with shifts in SCFA producers and pathobionts | Pre-existing diabetes exacerbates tumor growth, aggressiveness, and inflammatory signaling | Shows that diabetes-induced metabolic changes and dysbiosis synergistically worsen CRC outcomes | Highlights need for aggressive metabolic control and possible microbiota modulation in diabetic CRC |
| Impacts of pre-existing diabetes mellitus on colorectal cancer in a mice model | [26] | Human; T2DM + CRC | Enrichment of bile acid-transforming bacteria and depletion of beneficial taxa | BA dysregulation (↑ secondary bile acids like DCA) associated with higher CRC risk and aggressiveness | T2DM-related metabolic alterations intersect with BA–microbiota axis | Suggests BA-targeted therapies and microbiota modulation as preventive/adjuvant approaches in T2DM-associated CRC |
| Impacts of pre-existing diabetes mellitus on colorectal cancer in a mice model | [26] | Mouse; HFD + tumor/cachexia | HFD and tumor burden reshape microbiota; specific taxa correlate with cachexia and systemic inflammation | Microbiota-mediated effects aggravate tumor progression and cachexia | Links diet-induced dysbiosis, liver dysfunction, and metabolic imbalance | Indicates diet/microbiota interventions could mitigate cachexia and improve cancer outcomes |
| Adipose tissue inflammation by recruitment of distinct neutrophils and its resolution by Akkermansia muciniphila and its extracellular vesicles | [27] | Mouse; microbiota-directed | Obesity reduces Akkermansia muciniphila; supplementation with A. muciniphila or its EVs reshapes microbiota and improves barrier | Not directly CRC-focused, but reduces inflammatory milieu relevant to carcinogenesis | Ameliorates obesity-related inflammation and metabolic impairment | Provides rationale for A. muciniphila/EV-based therapies to restore gut barrier, reduce inflammation, and indirectly lower CRC risk |
| Akkermansia muciniphila: A new hope in obesity prevention and treatment… | [28] | Human/animal; review | Summarizes evidence linking A. muciniphila to healthy mucus layer, gut barrier, and microbial balance | Suggests possible protective role against carcinogenesis via barrier and immune modulation | Strongly linked to improved metabolic outcomes (obesity, insulin sensitivity) | Proposes A. muciniphila as a candidate probiotic/next-gen therapy for obesity, T2DM, and possibly CRC risk reduction |
| Metformin-induced reductions in tumor growth involves modulation of the gut microbiome | [29] | Mouse; metformin + tumor model | Metformin reshapes the gut microbiota, increasing beneficial taxa and altering SCFA/BA profiles | Metformin reduces tumor growth, partly through microbiota-dependent mechanisms | Confirms metformin’s glucose-lowering and insulin-sensitizing effects with added microbiota-related actions | Supports repurposing metformin as adjuvant therapy in metabolically dysregulated cancer patients |
| Dietary Factors: Major Regulators of the Gut’s Microbiota | [30] | Human/animal; review | Reviews how macronutrients, fiber, and specific foods regulate gut microbiota | Summarizes diet–microbiota–CRC links | Links diet-induced dysbiosis to obesity, insulin resistance, and T2DM | Advocates high-fiber, plant-rich diets and limited processed meat to promote eubiosis and reduce CRC/metabolic risk |
| Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on Toll-like receptors and tight junction | [31] | In vitro/preclinical; microbiota-directed | A. muciniphila and its EVs improve microbial balance and increase tight junction protein expression | Not directly CRC-focused, but improved barrier and reduced inflammation are protective against carcinogenesis | Relevant to T2DM via reduced endotoxemia and inflammatory burden | Suggests A. muciniphila/EV-based approaches to restore gut barrier and immune–microbiota homeostasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboob, A.; Shin, C.; Almughanni, S.; Hornakova, L.; Kubatka, P.; Büsselberg, D. The Gut Nexus: Unraveling Microbiota-Mediated Links Between Type 2 Diabetes and Colorectal Cancer. Nutrients 2025, 17, 3803. https://doi.org/10.3390/nu17233803
Mahboob A, Shin C, Almughanni S, Hornakova L, Kubatka P, Büsselberg D. The Gut Nexus: Unraveling Microbiota-Mediated Links Between Type 2 Diabetes and Colorectal Cancer. Nutrients. 2025; 17(23):3803. https://doi.org/10.3390/nu17233803
Chicago/Turabian StyleMahboob, Anns, Chehbin Shin, Shahd Almughanni, Lubica Hornakova, Peter Kubatka, and Dietrich Büsselberg. 2025. "The Gut Nexus: Unraveling Microbiota-Mediated Links Between Type 2 Diabetes and Colorectal Cancer" Nutrients 17, no. 23: 3803. https://doi.org/10.3390/nu17233803
APA StyleMahboob, A., Shin, C., Almughanni, S., Hornakova, L., Kubatka, P., & Büsselberg, D. (2025). The Gut Nexus: Unraveling Microbiota-Mediated Links Between Type 2 Diabetes and Colorectal Cancer. Nutrients, 17(23), 3803. https://doi.org/10.3390/nu17233803






