Plasma and Urinary TMAO and Methylamine Responses to Meat and Egg Ingestion: Links to Gut Microbiota Composition in Subjects With and Without Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Analysis of TMAO and Related Metabolites in Clinical and Food Samples
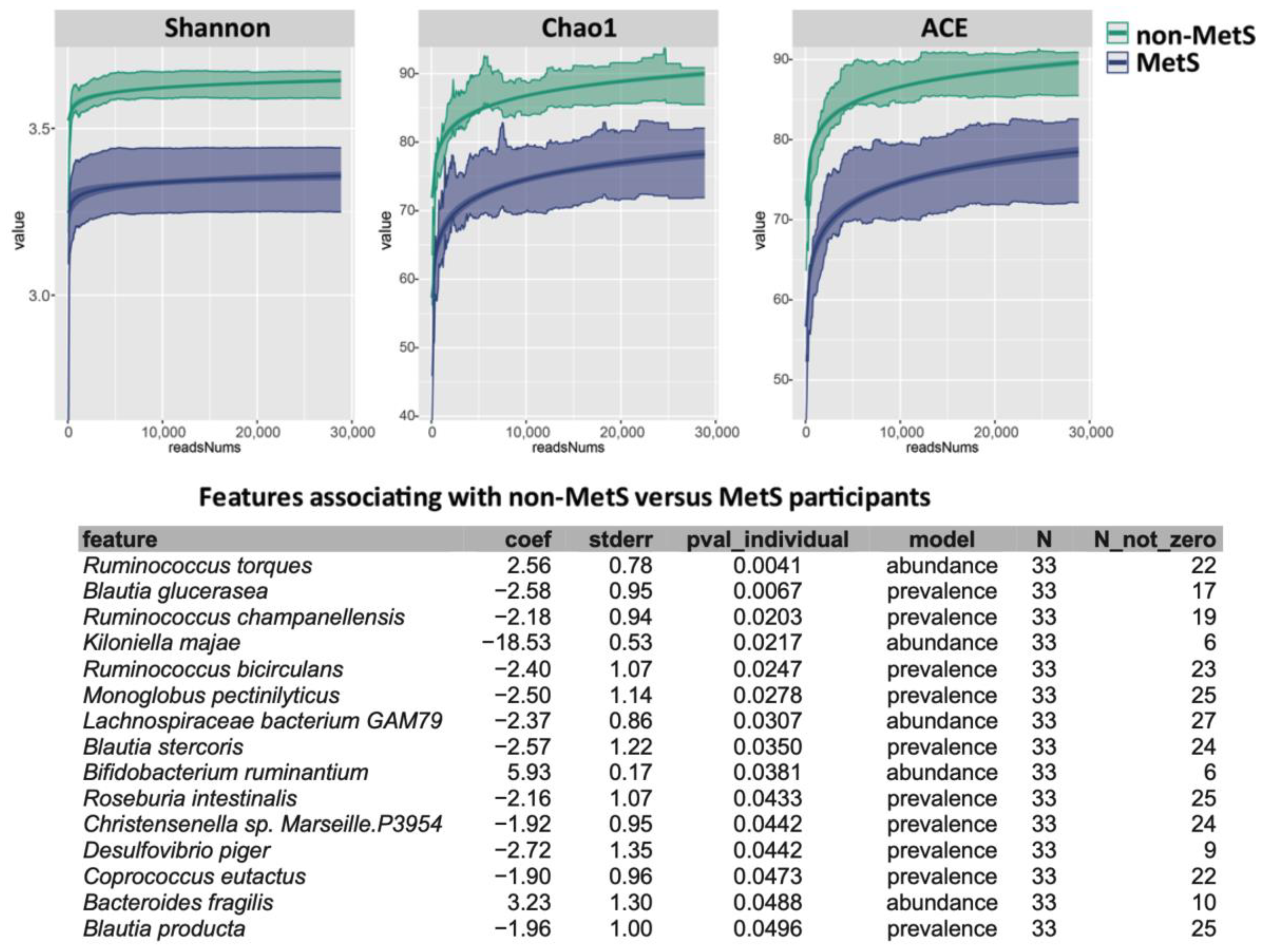
2.4. Microbiota Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP III | Adult Treatment Panel III |
| CntA/B | A two-component Rieske-type oxygenase/reductase system converts carnitine to TMA |
| CutC | Glycyl radical enzyme converts choline to TMA (choline TMA-lyase) |
| CutD | CutC-activating protein (the activator protein for choline TMA-lyase) |
| YeaW/X | A two-component Rieske-type oxygenase/reductase system converts betaine to TMA |
| CVD | Cardiovascular disease |
| FMO3 | Flavin-containing monooxygenase 3 |
| MetS | Metabolic syndrome |
| MUC2 | Mucin 2, oligomeric mucus/gel-forming |
| NCEP | National Cholesterol Education Program |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PLS | Partial Least Squares Discriminant Analysis |
| rho | Spearman correlation coefficients |
| SCFAs | Short-chain fatty acids |
| SD | Standard deviation |
| T2D | Type 2 diabetes |
| TMAO | Trimethylamine N-oxide |
| TMA | Trimethylamine |
| IACN | Iodoacetonitrile |
| ACN | Acetonitrile |
References
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Cleeman, J.I. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Di Leo, M.; Tartaglione, L.; De Leva, F.; Petruzziello, C.; Saviano, A.; Pontecorvi, A.; Ojetti, V. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1548–1562. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- El Hage, R.; Al-Arawe, N.; Hinterseher, I. The Role of the Gut Microbiome and Trimethylamine Oxide in Atherosclerosis and Age-Related Disease. Int. J. Mol. Sci. 2023, 24, 2399. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Evans, M.; Dai, L.; Avesani, C.M.; Kublickiene, K.; Stenvinkel, P. The dietary source of trimethylamine N-oxide and clinical outcomes: An unexpected liaison. Clin. Kidney J. 2023, 16, 1804–1812. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine Is a Proatherogenic Intermediate in Gut Microbial Metabolism of L-Carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; Silva, R.; McLaughlin, M.; Huet, B.; Jialal, I. Changes to trimethylamine-N-oxide and its precursors in nascent metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2018, 35, 20180015. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Hefni, M.E.; Witthöft, C.M. Development and Application of a UPLC–MRM–MS Method for Quantifying Trimethylamine, Trimethylamine-N-Oxide, and Related Metabolites in Individuals with and Without Metabolic Syndrome. Separations 2025, 12, 53. [Google Scholar] [CrossRef]
- Hefni, M.E.; Witthöft, C.M.; Hellström, P.; Johansson, I.; Esberg, A. Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study. Metabolites 2025, 15, 364. [Google Scholar] [CrossRef]
- Nickols, W.A.; Kuntz, T.; Shen, J.; Maharjan, S.; Mallick, H.; Franzosa, E.A.; Thompson, K.N.; Nearing, J.T.; Huttenhower, C. MaAsLin 3: Refining and extending generalized multivariable linear models for meta-omic association discovery. bioRxiv 2024. [Google Scholar] [CrossRef]
- RStudioTeam. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Ringel, C.; Dittrich, J.; Gaudl, A.; Schellong, P.; Beuchel, C.F.; Baber, R.; Beutner, F.; Teren, A.; Engel, C.; Wirkner, K.; et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis 2021, 335, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Liu, C.-H.; Wang, J.-H.; Hsu, B.-G. Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients. Int. J. Environ. Res. Public Health 2022, 19, 8710. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Corbin, K.D.; da Costa, K.A.; Zhang, S.C.; Zhao, X.Q.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef]
- DiMarco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983, 225, 320–324. [Google Scholar] [CrossRef]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G.; Tarsisano, M.G.; Di Renzo, L.; Federici, M.; Caprio, M.; De Lorenzo, A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021, 13, 1426. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Gordon, J.I. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef]
- Schaus, S.R.; Pereira, G.V.; Luis, A.S.; Madlambayan, E.; Terrapon, N.; Ostrowski, M.P.; Jin, C.; Henrissat, B.; Hansson, G.C.; Martens, E.C. Ruminococcus torques is a keystone degrader of intestinal mucin glycoprotein, releasing oligosaccharides used by Bacteroides thetaiotaomicron. mBio 2024, 15, e00039-24. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria with Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, B.; Srivastava, A.; Izzi, Z.; Abdalla, Y.; Shen, W.; Raj, D. Alterations of gut microbial pathways and virulence factors in hemodialysis patients. Front. Cell. Infect. Microbiol. 2022, 12, 904284. [Google Scholar] [CrossRef] [PubMed]
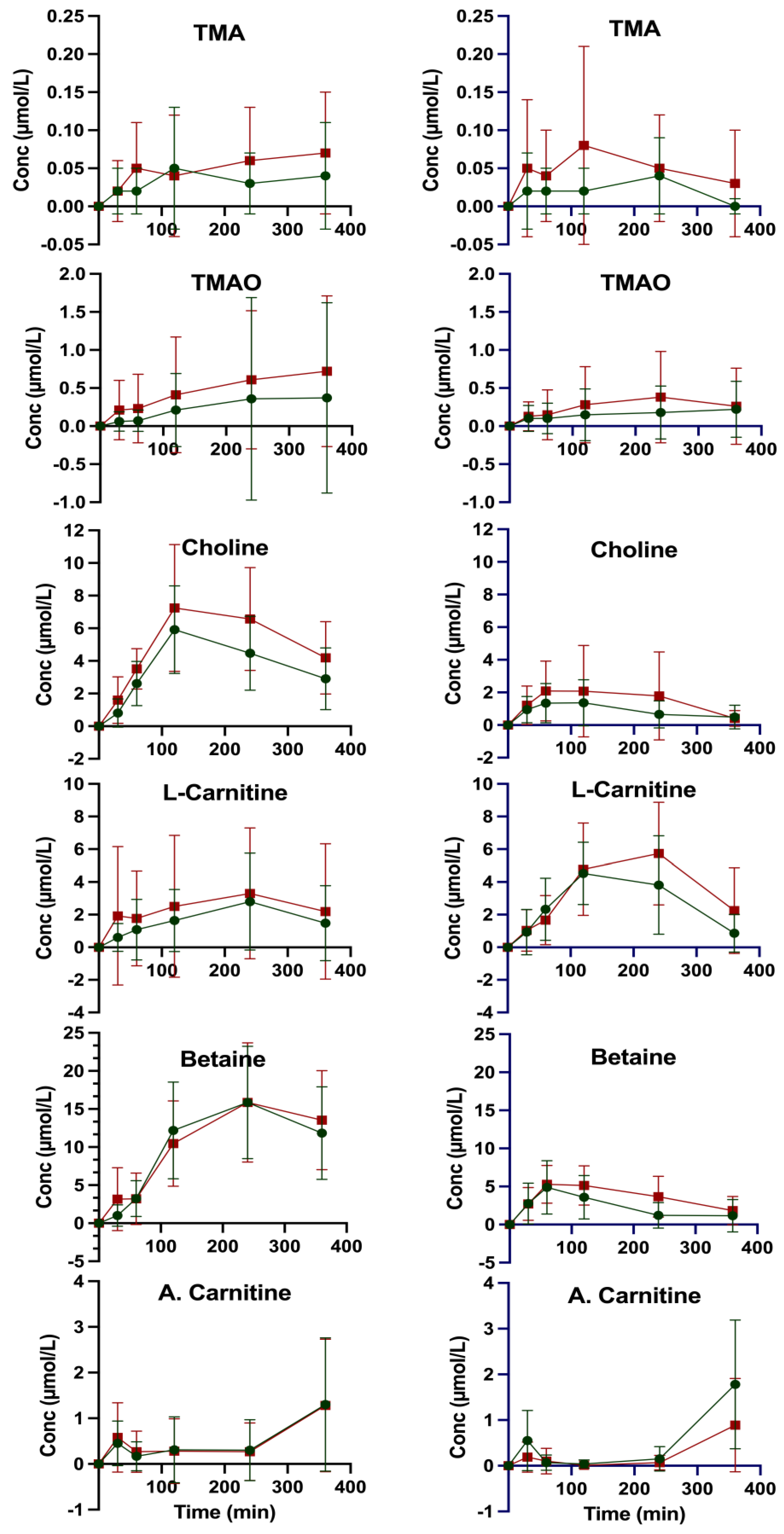
| Compound | TMA | TMAO | Carnitine | Choline | Betaine | A. Carnitine |
|---|---|---|---|---|---|---|
| Amount (mg) a in Ingested Egg | 357 ± 16.5 | 1.7 ± 0.1 | - | |||
| non-MetS | ||||||
| C0 (µM) b | 0.58 ± 0.27 | 3.32 ± 0.90 | 30.89 ± 4.87 | 9.04 ± 1.77 | 29.12 ± 6.47 | 6.44 ± 1.84 |
| Cmax (µM) c | 0.61 ± 0.27 | 3.32 ± 0.90 | 33.40 ± 4.87 | 15.28 ± 2.94 | 45.73 ± 10.21 | 7.31 ± 2.09 |
| ΔCmax (µM) d | 0.03 ± 0.38 | 0.00 | 2.51 ± 6.89 | 2.51 ± 6.89 | 6.24 ± 3.43 | 0.87 ± 2.79 |
| tmax (min) e | 120 | 0 | 240 | |||
| C360 f | 0.55 ± 0.24 | 2.87 ± 1.46 | 30.43 ± 5.35 | 12.04 ± 2.53 | 41.02 ± 8.07 | 7.31 ± 2.09 |
| IAUC0–360 (h × µM) g | 12.85 ± 15.44 | 93.39 ± 286.45 | 652.79 ± 583.61 | 1447.66 ± 503.44 | 4045.35 ± 1419.54 | 167.25 ± 175.02 |
| MetS | ||||||
| C0 (µM) b | 0.56 ± 0.22 | 8.02 ± 12.27 | 34.76 ± 7.17 | 9.24 ± 2.21 | 29.82 ± 9.11 | 6.32 ± 1.92 |
| Cmax (µM) c | 0.60 ± 0.23 | 8.02 ± 12.27 | 37.83 ± 5.40 | 16.48 ± 4.18 | 45.68 ± 12.00 | 7.36 ± 1.55 |
| ΔCmax (µM) d | 0.04 ± 0.32 | 0.00 | 3.07 ± 8.97 | 7.24 ± 4.73 | 15.86 ± 15.07 | 1.04 ± 2.47 |
| tmax (min) e | 240 | 0 | 240 | 120 | 240 | 360 |
| C360 f | 0.59 ± 0.19 | 5.69 ± 4.52 | 36.24 ± 6.03 | 13.43 ± 2.47 | 43.35 ± 10.98 | 7.36 ± 1.55 |
| IAUC0–360 (h × µM) g | 18.05 ± 19.56 | 170.34 ± 242.89 | 889.56 ± 1338.87 | 1898.78 ± 846.02 | 3896.75 ± 1928.66 | 161.06 ± 213.19 |
| Amount (mg) a in ingested meatballs | 54 ± 0.6 | 66 ± 0.7 | 16 ± 0.2 | |||
| non-MetS | ||||||
| C0 (µM) b | 0.60 ± 0.26 | 5.83 ± 8.72 | 29.85 ± 6.51 | 9.14 ± 2.15 | 29.18 ± 6.56 | 6.73 ± 2.52 |
| Cmax (µM) c | 0.60 ± 0.26 | 5.83 ± 8.72 | 34.30 ± 7.16 | 10.41 ± 2.58 | 34.06 ± 7.26 | 8.22 ± 2.51 |
| Δ C (µM) d | 0.00 | 0.00 | 4.45 ± 9.68 | 1.27 ± 3.36 | 4.88 ± 9.78 | 1.49 ± 3.56 |
| tmax (min) e | 0 | 0 | 120 | 120 | 60 | 360 |
| C360 f | 0.52 ± 0.21 | 3.62 ± 1.81 | 29.88 ± 5.64 | 9.03 ± 2.26 | 27.92 ± 6.25 | 8.22 ± 2.51 |
| IAUC0–360 (h × µM) g | 8.24 ± 8.30 | 56.34 ± 90.44 | 1047.83 ± 567.72 | 318.94 ± 241.12 | 840.67 ± 621.53 | 147.58 ± 85.30 |
| MetS | ||||||
| C0 (µM) b | 0.68 ± 0.38 | 11.09 ± 16.69 | 37.49 ± 6.25 | 9.51 ± 1.98 | 30.59 ± 8.78 | 7.21 ± 1.76 |
| Cmax (µM)c | 0.68 ± 0.38 | 11.09 ± 16.69 | 43.09 ± 7.51 | 11.60 ± 3.19 | 35.87 ± 9.93 | 7.82 ± 2.08 |
| Δ C (µM) d | 0.00 | 0.00 | 5.60 ± 9.77 | 2.09 ± 3.75 | 5.28 ± 13.26 | 0.61 ± 2.73 |
| tmax (min) e | 0 | 0 | 240 | 60 | 60 | 360 |
| C360 f | 0.56 ± 0.20 | 6.15 ± 5.99 | 38.64 ± 6.70 | 9.22 ± 1.43 | 31.58 ± 8.86 | 7.82 ± 2.08 |
| IAUC0–360 (h × µM) g | 18.10 ± 26.17 | 97.76 ± 154.06 | 1358.18 ± 735.17 | 555.60 ± 562.90 | 1331.37 ± 672.95 | 71.88 ± 68.33 |
| Variable | Non-MetS (n = 21) | MetS (n = 12) | Mann–Whitney U Test |
|---|---|---|---|
| After egg ingestion | |||
| TMA | 2.56 ± 0.97 | 2.95 ± 1.23 | ns |
| TMAO | 109.42 ± 45.86 | 204.54 ± 214.6 | 0.05 |
| L-Carnitine | 9.10 ± 7.58 | 11.61 ± 12.07 | ns |
| Choline | 20.77 ± 5.80 | 23.80 ± 5.92 | ns |
| Betaine | 33.42 ± 23.80 | 37.99 ± 8.53 | ns |
| Acetyl-L-carnitine | 5.48 ± 4.91 | 4.50 ± 4.48 | ns |
| After meat ingestion | |||
| TMA | 3.42 ± 1.85 | 4.37 ± 2.99 | ns |
| TMAO | 181.00 ± 169.71 | 222.30 ± 278.49 | ns |
| L-Carnitine | 31.07 ± 22.37 | 44.23 ± 43.02 | ns |
| Choline | 14.90 ± 5.96 | 13.73 ± 6.79 | ns |
| Betaine | 27.03 ± 21.96 | 35.09 ± 19.34 | ns |
| Acetyl-L-carnitine | 14.80 ± 11.06 | 16.59 ± 15.52 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefni, M.E.; Esberg, A.; Hellström, P.; Johansson, I.; Witthöft, C.M. Plasma and Urinary TMAO and Methylamine Responses to Meat and Egg Ingestion: Links to Gut Microbiota Composition in Subjects With and Without Metabolic Syndrome. Nutrients 2025, 17, 3719. https://doi.org/10.3390/nu17233719
Hefni ME, Esberg A, Hellström P, Johansson I, Witthöft CM. Plasma and Urinary TMAO and Methylamine Responses to Meat and Egg Ingestion: Links to Gut Microbiota Composition in Subjects With and Without Metabolic Syndrome. Nutrients. 2025; 17(23):3719. https://doi.org/10.3390/nu17233719
Chicago/Turabian StyleHefni, Mohammed E., Anders Esberg, Patrik Hellström, Ingegerd Johansson, and Cornelia M. Witthöft. 2025. "Plasma and Urinary TMAO and Methylamine Responses to Meat and Egg Ingestion: Links to Gut Microbiota Composition in Subjects With and Without Metabolic Syndrome" Nutrients 17, no. 23: 3719. https://doi.org/10.3390/nu17233719
APA StyleHefni, M. E., Esberg, A., Hellström, P., Johansson, I., & Witthöft, C. M. (2025). Plasma and Urinary TMAO and Methylamine Responses to Meat and Egg Ingestion: Links to Gut Microbiota Composition in Subjects With and Without Metabolic Syndrome. Nutrients, 17(23), 3719. https://doi.org/10.3390/nu17233719








