Can Early Nutrition Be Responsible for Future Gut Microbiota Changes and Different Health Outcomes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims
2.2. End Points
2.3. Statistical Analysis
2.4. Fecal Microbiota Analysis
3. Results
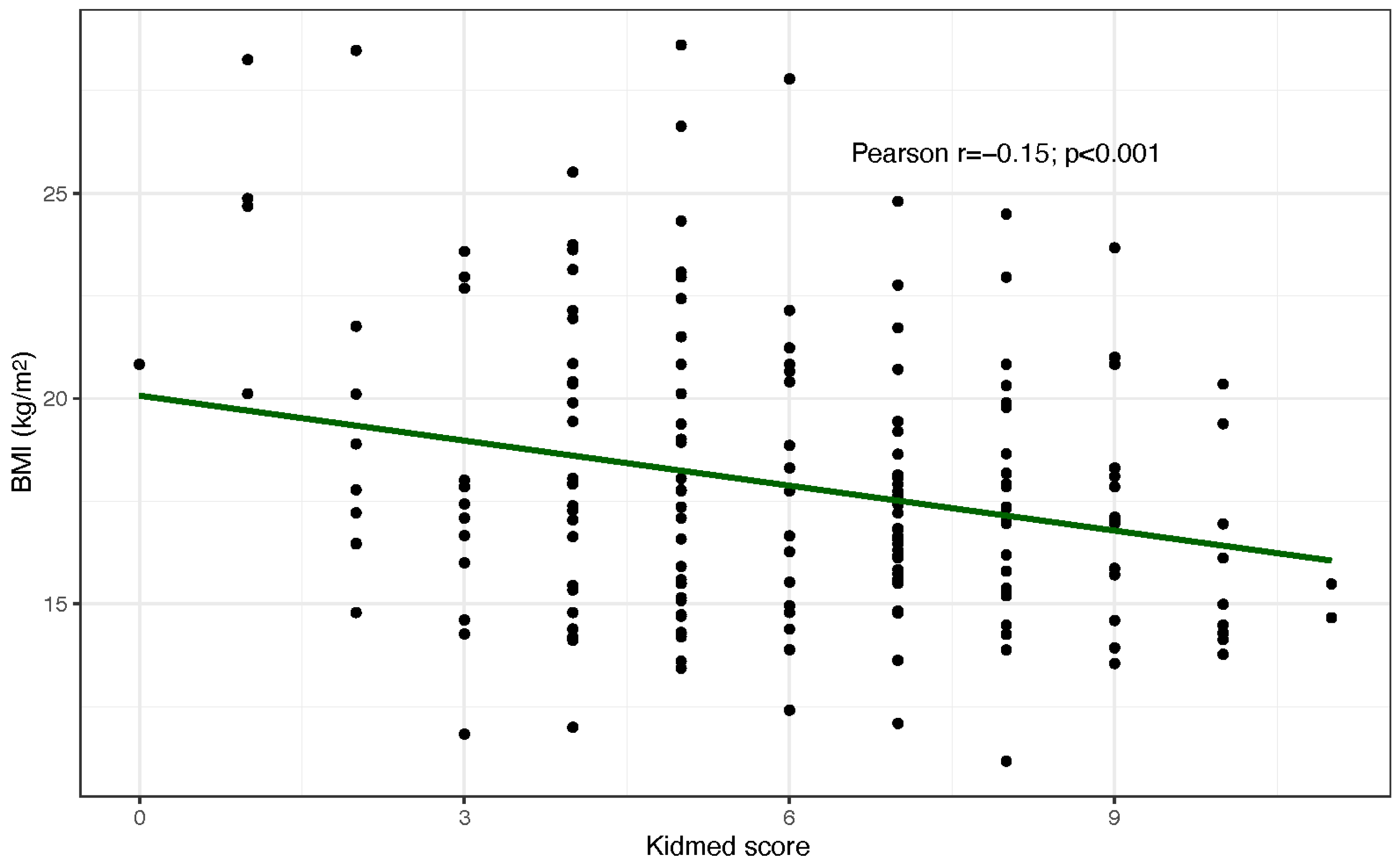
3.1. Mediterranean Diet Adherence
3.2. Anthropometric Measurements
3.3. Non-Communicable Inflammatory Chronic Diseases
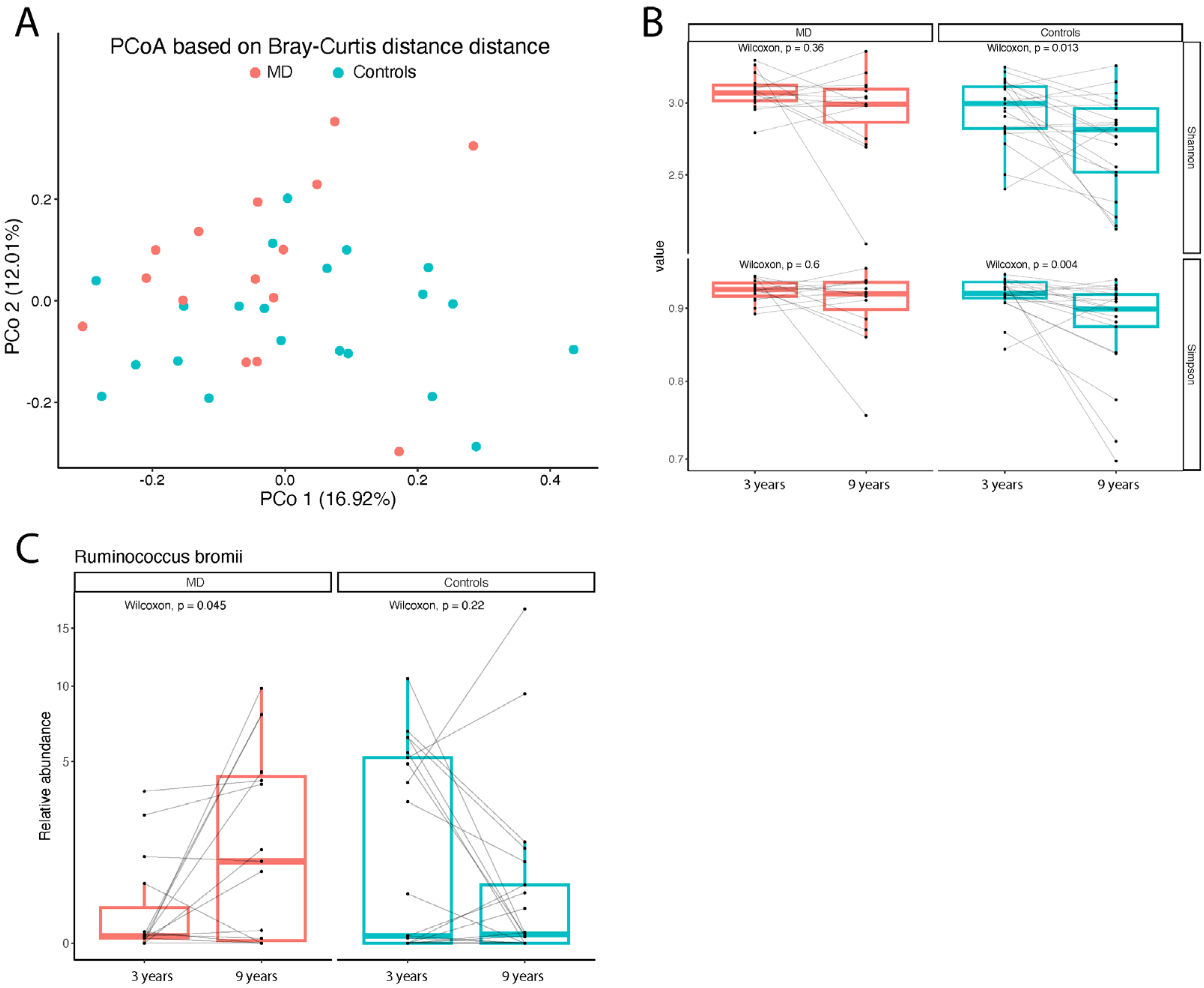
3.4. Gut Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.V.; Allin, K.H.; Poulsen, G.J.; Lee, J.C.; Jess, T. Characterizing the pre-clinical phase of inflammatory bowel disease. Cell Rep. Med. 2023, 21, 101263. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Agrinier, N.; Danese, S.; Peyrin-Biroulet, L. Disease prevention trials in IBD: Feasibility to future outlook. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Rainey-Smith, S.R.; Gu, Y.; Gardener, S.L.; Doecke, J.D.; Villemagne, V.L.; Brown, B.M.; Taddei, K.; Laws, S.M.; Sohrabi, H.R.; Weinborn, M.; et al. Mediterranean diet adherence and rate of cerebral Aβ-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl. Psychiatry 2018, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Timon, E.A.; Herbert, T. Western diets and chronic diseases. Nat. Med. 2024, 30, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bozza, L.; Canale, P.; Chiacchio, M.; Cortese, P.; D’avino, A.; De Giovanni, M.; Iacovo, M.D.; D’onofrio, A.; Federico, A.; et al. The Effect of Weaning with Adult Food Typical of the Mediterranean Diet on Taste Development and Eating Habits of Children: A Randomized Trial. Nutrients 2022, 14, 2486. [Google Scholar] [CrossRef] [PubMed]
- Valentino, V.; De Filippis, F.; Menghi, L.; Gasperi, F.; Ercolini, D. Food Neophobia and scarce olfactory performances are linked to oral microbiota. Food Res. Int. 2022, 155, 111092. [Google Scholar] [CrossRef] [PubMed]
- Bankole, T.; Li, Y. The early-life gut microbiome in common pediatric diseases: Roles and therapeutic implications. Front. Nutr. 2025, 29, 1597206. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Valentino, V.; De Filippis, F.; Marotta, R.; Pasolli, E.; Ercolini, D. Genomic features and prevalence of Ruminococcus species in humans are associated with age, lifestyle, and disease. Cell Rep. 2024, 43, 115018. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Fazio, A.; Curcio, R.; Tucci, P.; Lauria, G.; Cappello, A.R.R.; Dolce, V. Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods 2021, 10, 2062. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.; Simon, G.E.; Hernandez, A.F. Is Learning Worth the Trouble?—Improving Health Care System Partecipation in Embedded Research. N. Engl. J. Med. 2021, 385, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Azzolina, D.; Auricchio, S.; Greco, L.; Auricchio, R. Bayesian Sequential Pragmatic Cluster Randomized Clinical Trial Design for PrEventive Effect of MEditerranean Diet in Children: PEMED Trial Research Protocol. J. Clin. Med. 2025, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Andria, G. Come sta cambiando la ricerca clinica “nel mondo reale”della rivoluzione digitale e dell’intelligenza artificiale. Prospett. Pediatr. 2023, 53, 70–78. Available online: https://prospettiveinpediatria.it/come-sta-cambiando-la-ricerca-clinica-nel-mondo-reale-della-rivoluzione-digitale-e-dellintelligenza-artificiale/ (accessed on 25 October 2025).
- Troncone, R.; Staiano, A.; Greco, L.; Auricchio, S. Cure primarie e ruolo della ricerca: Un’esperienza di “health learning system”. Area Pediatr. 2023, 24, 14–18. [Google Scholar]
| Overall (n = 191; 100%) | Control Group (n = 95; 49.7%) | MD Group (n = 96; 50.3%) | |
|---|---|---|---|
| Gender | |||
| Male | 89 (46.6) | 48 (50.5) | 41 (42.7) |
| Female | 102 (53.4) | 47 (49.5) | 55 (57.3) |
| Age at evaluation | 7.8 ± 0.5 (4.8 to 8.8) | 7.8 ± 0.4 (6.6 to 8.8) | 7.8 ± 0.6 (4.8 to 8.6) |
| Overall (n =191; 100%) | Control Group (n = 95; 49.7%) | MD Group (n = 96; 50.3%) | p | |
|---|---|---|---|---|
| Fruit or freshly squeezed fruit juice every day | 152 (79.6) | 80 (84.2) | 72 (75) | 0.151 |
| More fruit per day | 82 (42.9) | 43 (45.3) | 39 (40.6) | 0.560 |
| Fresh and cooked vegetables once a day | 118 (61.8) | 49 (51.6) | 69 (71.9) | 0.005 |
| More fresh or cooked vegetables per day | 57 (29.8) | 24 (25.3) | 33 (34.4) | 0.206 |
| Fish regularly (at least 2–3 times per week) | 105 (55.3) | 49 (51.6) | 56 (58.9) | 0.381 |
| Fast-food more than once a week. | 23 (12) | 12 (12.6) | 11 (11.5) | 0.828 |
| Legumes more than once a week | 181 (94.8) | 91 (95.8) | 90 (93.8) | 0.747 |
| Pasta and rice almost every day | 189 (99) | 93 (97.9) | 96 (100) | 0.246 |
| Breakfast bread or grain products (cereals) | 85 (44.5) | 39 (41.1) | 46 (47.9) | 0.383 |
| Nuts regularly (at least 2–3 times per week) | 43 (22.6) | 21 (22.3) | 22 (22.9) | >0.99 |
| Olive oil at home | 190 (99.5) | 94 (98.9) | 96 (100) | 0.497 |
| Eats Breakfast | 164 (85.9) | 79 (83.2) | 85 (88.5) | 0.307 |
| Milk and dairy products for breakfast | 133 (69.6) | 67 (70.5) | 66 (68.8) | 0.875 |
| Baked goods and pastries for breakfast | 145 (75.9) | 74 (77.9) | 71 (74) | 0.612 |
| Yogurt and/or a large slice cheese | 55 (28.8) | 27 (28.4) | 28 (29.2) | >0.99 |
| Sweet sugar and sweets several times a day | 64 (33.5) | 30 (31.6) | 34 (35.4) | 0.646 |
| Overall (n = 191; 100%) | Control Group (n = 95; 49.7%) | MD Group (n = 96; 50.3%) | p Value | |
|---|---|---|---|---|
| BMI; kg/m2 | 17.9 ± 3.4 (11.2 to 28.6) | 17.7 ± 3.5 (11.8 to 28.5) | 18.1 ± 3.4 (11.2 to 28.6) | 0.384 |
| Overweight; n (%) | 47 (25) | 21 (22.8) | 26 (27) | 0.613 |
| Obese; n (%) | 8 (4.3) | 6 (6.5) | 2 (2.1) | 0.163 |
| Overall (n = 191; 100%) | Control Group (n = 95; 49.7%) | MD Group (n = 96; 50.3%) | p Value | |
|---|---|---|---|---|
| Asthma | 35 (21.2) | 15 (18.1) | 20 (24.4) | 0.346 |
| Constipation | 7 (4.2) | 5 (6) | 2 (2.4) | 0.443 |
| Irritable Bowel Syndrome | 1 (0.6) | 0 (0) | 1 (1.2) | 0.497 |
| Autism Spectrum Disorders | 4 (2.4) | 4 (4.8) | 0 (0) | 0.12 |
| ADHD | 6 (3.6) | 4 (4.8) | 2 (2.4) | 0.682 |
| Allergic Rhinitis | 14 (8.5) | 9 (10.8) | 5 (6.1) | 0.403 |
| Eczema | 1 (0.6) | 1 (1.2) | 0 (0) | >0.99 |
| Atopic Dermatitis | 15 (9.1) | 9 (10.8) | 6 (7.3) | 0.59 |
| Atopic Dermatitis or Allergic Rhinitis | 27 (16.4) | 16 (19.3) | 11 (13.4) | 0.401 |
| Autism Spectrum Disorders or ADHD | 10 (6.1) | 8 (9.6) | 2 (2.4) | 0.099 |
| At least one chronic disease | 80 (48.5) | 40 (48.2) | 40 (48.8) | >0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Franchis, R.; Bozza, L.; Cortese, P.; D’Antonio, L.; D’Avino, A.; Gasparini, N.; Ippolito, G.; Spadaro, R.; Tedesco, M.; Antignani, A.; et al. Can Early Nutrition Be Responsible for Future Gut Microbiota Changes and Different Health Outcomes? Nutrients 2025, 17, 3721. https://doi.org/10.3390/nu17233721
de Franchis R, Bozza L, Cortese P, D’Antonio L, D’Avino A, Gasparini N, Ippolito G, Spadaro R, Tedesco M, Antignani A, et al. Can Early Nutrition Be Responsible for Future Gut Microbiota Changes and Different Health Outcomes? Nutrients. 2025; 17(23):3721. https://doi.org/10.3390/nu17233721
Chicago/Turabian Stylede Franchis, Raffaella, Luigi Bozza, Paolo Cortese, Lorenzo D’Antonio, Antonio D’Avino, Nicoletta Gasparini, Giorgia Ippolito, Raffaella Spadaro, Mariangela Tedesco, Angelo Antignani, and et al. 2025. "Can Early Nutrition Be Responsible for Future Gut Microbiota Changes and Different Health Outcomes?" Nutrients 17, no. 23: 3721. https://doi.org/10.3390/nu17233721
APA Stylede Franchis, R., Bozza, L., Cortese, P., D’Antonio, L., D’Avino, A., Gasparini, N., Ippolito, G., Spadaro, R., Tedesco, M., Antignani, A., De Filippis, F., Valentino, V., Auricchio, R., Auricchio, S., & Bruzzese, D. (2025). Can Early Nutrition Be Responsible for Future Gut Microbiota Changes and Different Health Outcomes? Nutrients, 17(23), 3721. https://doi.org/10.3390/nu17233721









