Ferritin Mitochondrial (FTMT)-Driven Mitochondrial Ferroptosis in Vascular Smooth Muscle Cells: A Role of NCOA4 in Atherosclerosis Pathogenesis and Modulation by Gualou–Xiebai
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Animals Grouping and Treatment
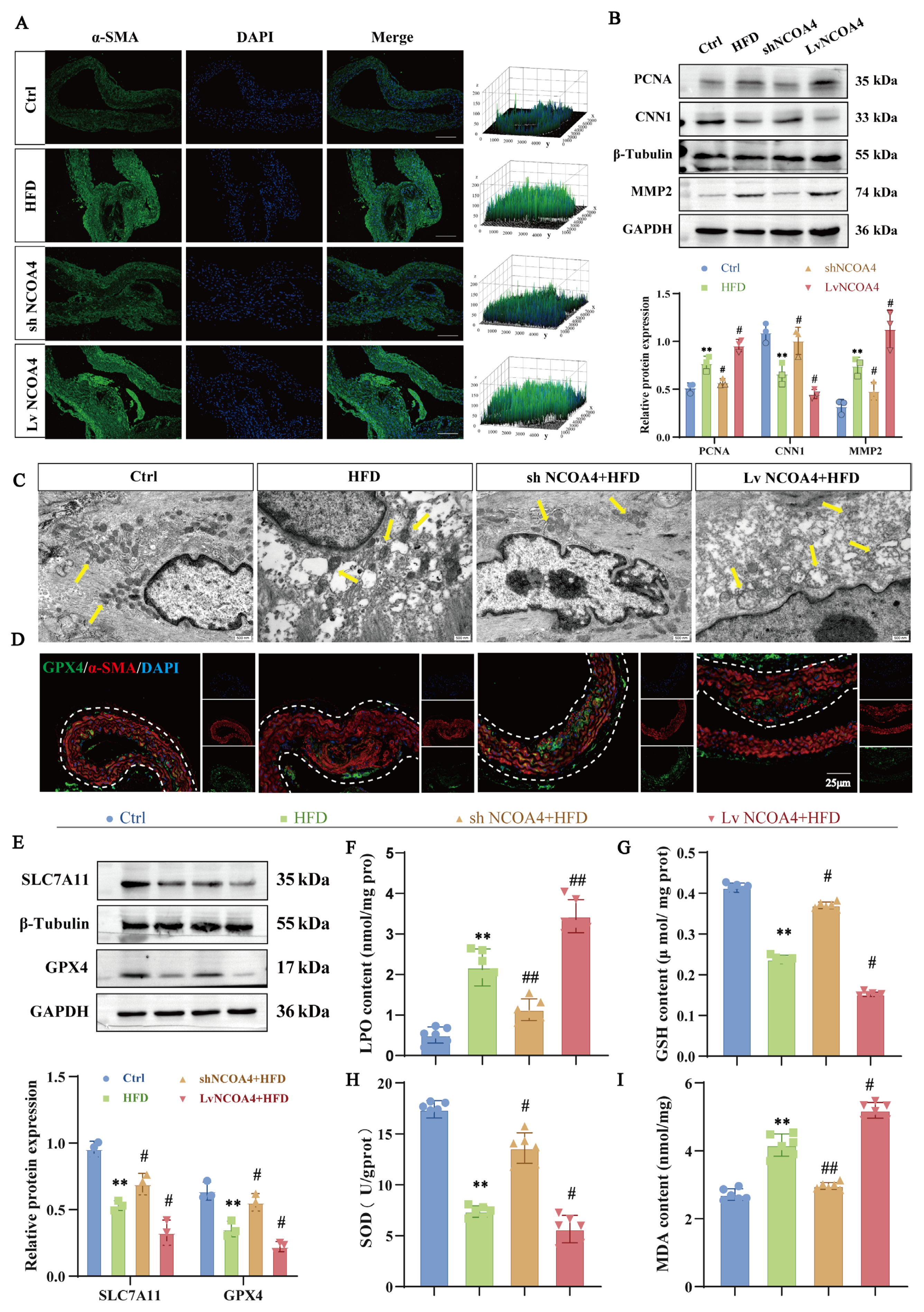
2.4. Lentiviral (Lv)-Infected ApoE−/− Mice
2.5. Preparation of GLXB Extract and In Vivo Administration
2.6. Preparation of GLXB-Containing Serum
2.7. Cell Culture and Groupings
2.8. Transfection of Lentivirus and Plasmid
2.9. Stereomicroscopic Imaging of Aortas
2.10. Hematoxylin-Eosin (HE) Staining
2.11. Immunofluorescence Staining
2.12. 5-Ethynyl-2′-Deoxyuridine (EdU) Analysis
2.13. Migration Assay
2.14. Transwell Migration Assay
2.15. Western Blot Analysis
2.16. Co-Immunoprecipitation (Co-IP) Analysis
2.17. GST Pull-Down Assay
2.18. Biochemical Analysis
2.19. Transmission Electron Microscope (TEM) Analysis
2.20. Flow Cytometry Analysis
2.21. Statistical Analysis
3. Results
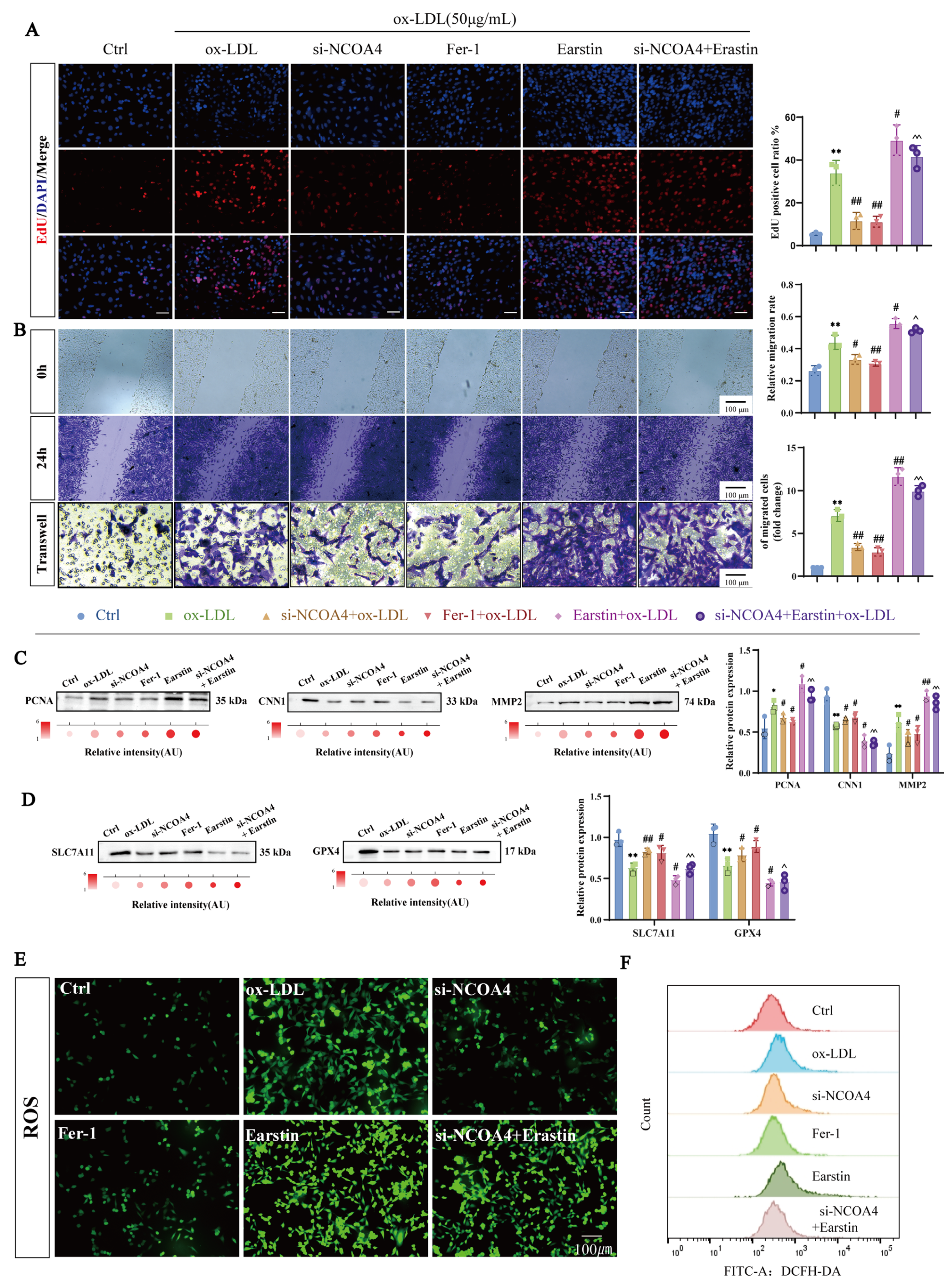
3.1. NCOA4-Mediated Ferroptosis Inhibits the Proliferation and Migration of VSMCs
3.2. NCOA4 Knockdown Reduces ox-LDL-Induced VSMCs Proliferation and Migration by Suppressing Ferroptosis
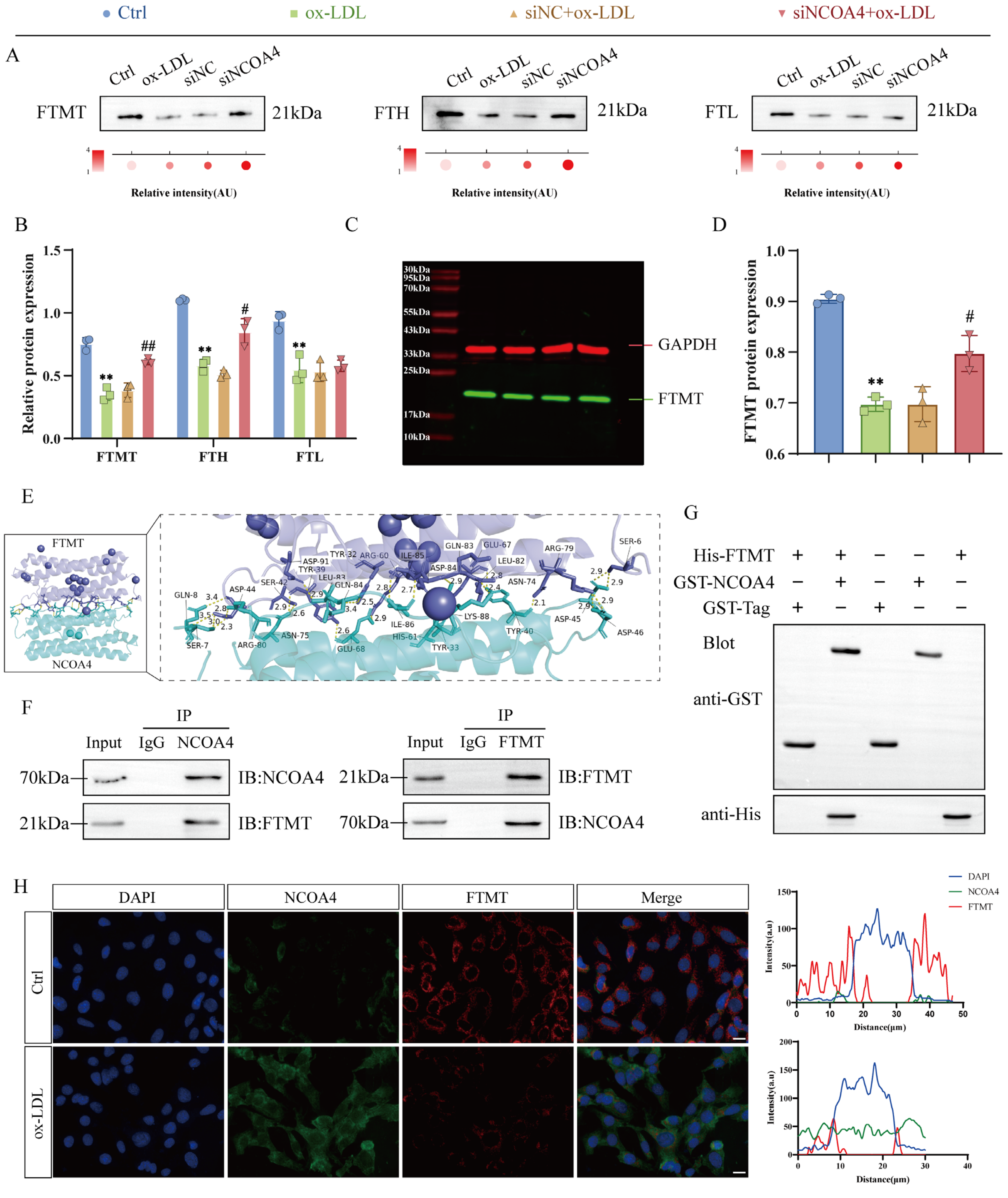
3.3. NCOA4 Facilitates FTMT Degradation via Direct Interaction
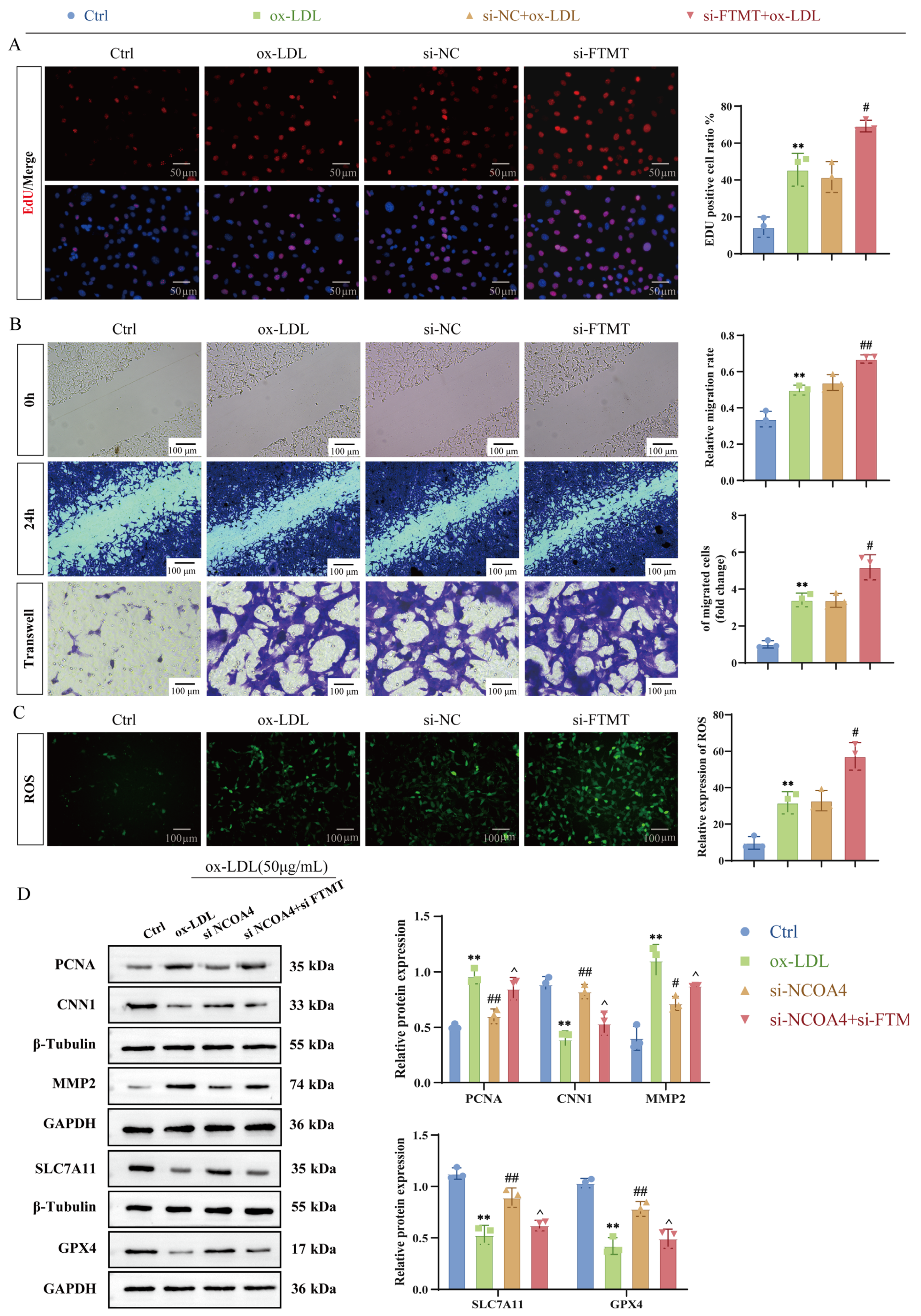
3.4. VSMCs-Specific FTMT Gene Knockout Enhances VSMCs Proliferation and Migration
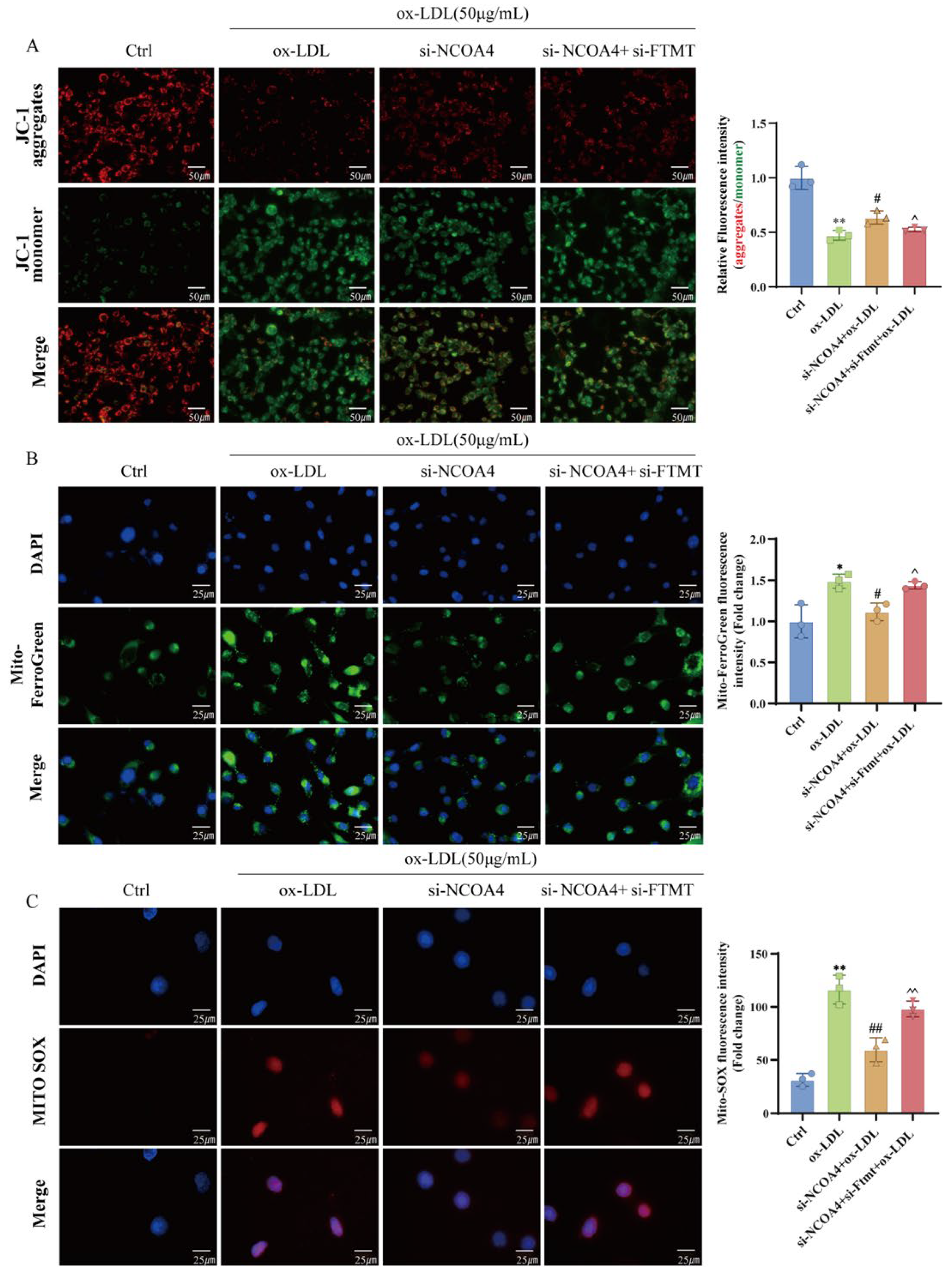
3.5. NCOA4 Induces Mitochondrial Damage in VSMCs via FTMT Suppression and Iron Homeostasis Dysregulation
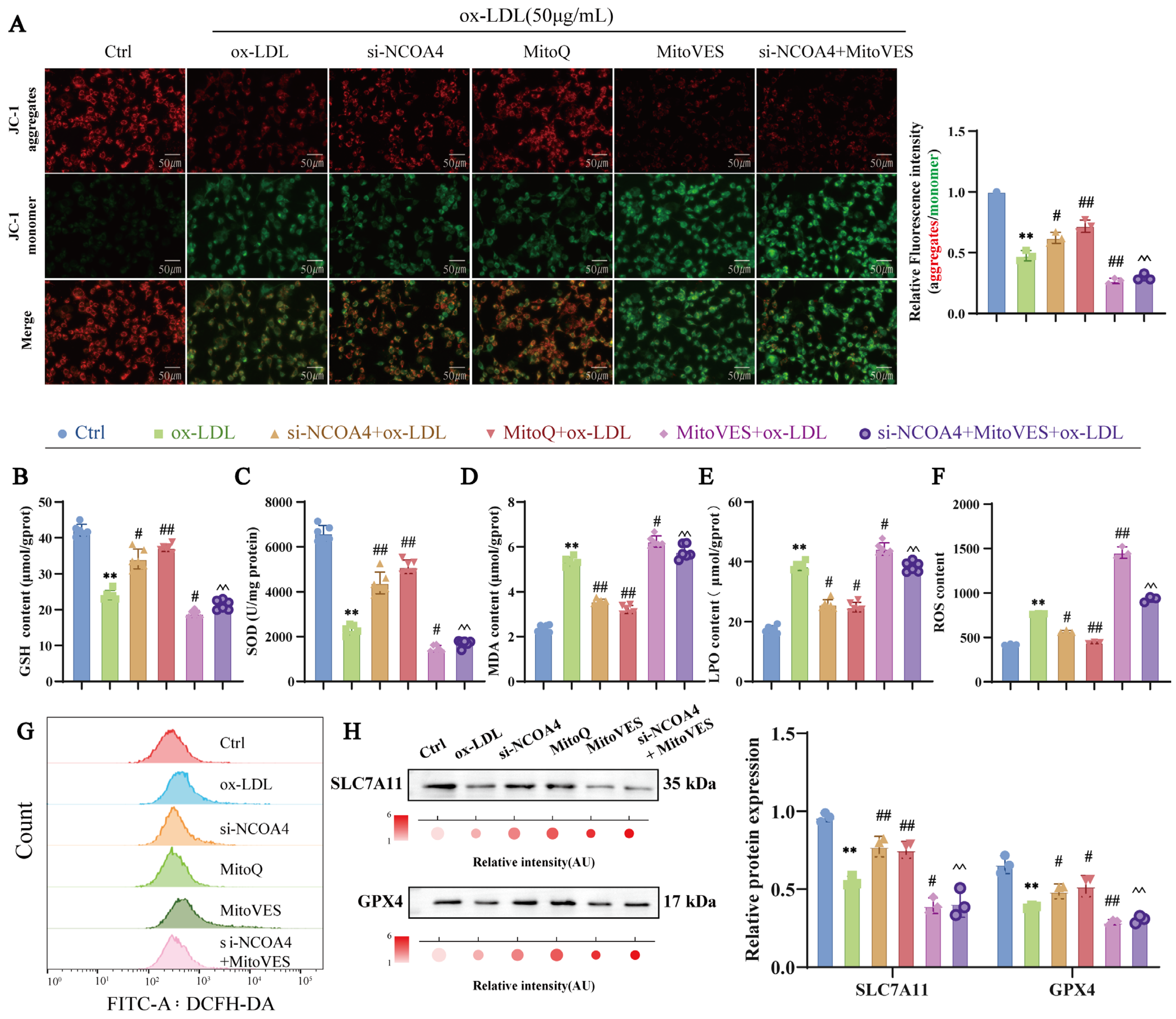
3.6. Silencing NCOA4 Inhibits Ferroptosis in VSMCs via Mitochondrial Protection
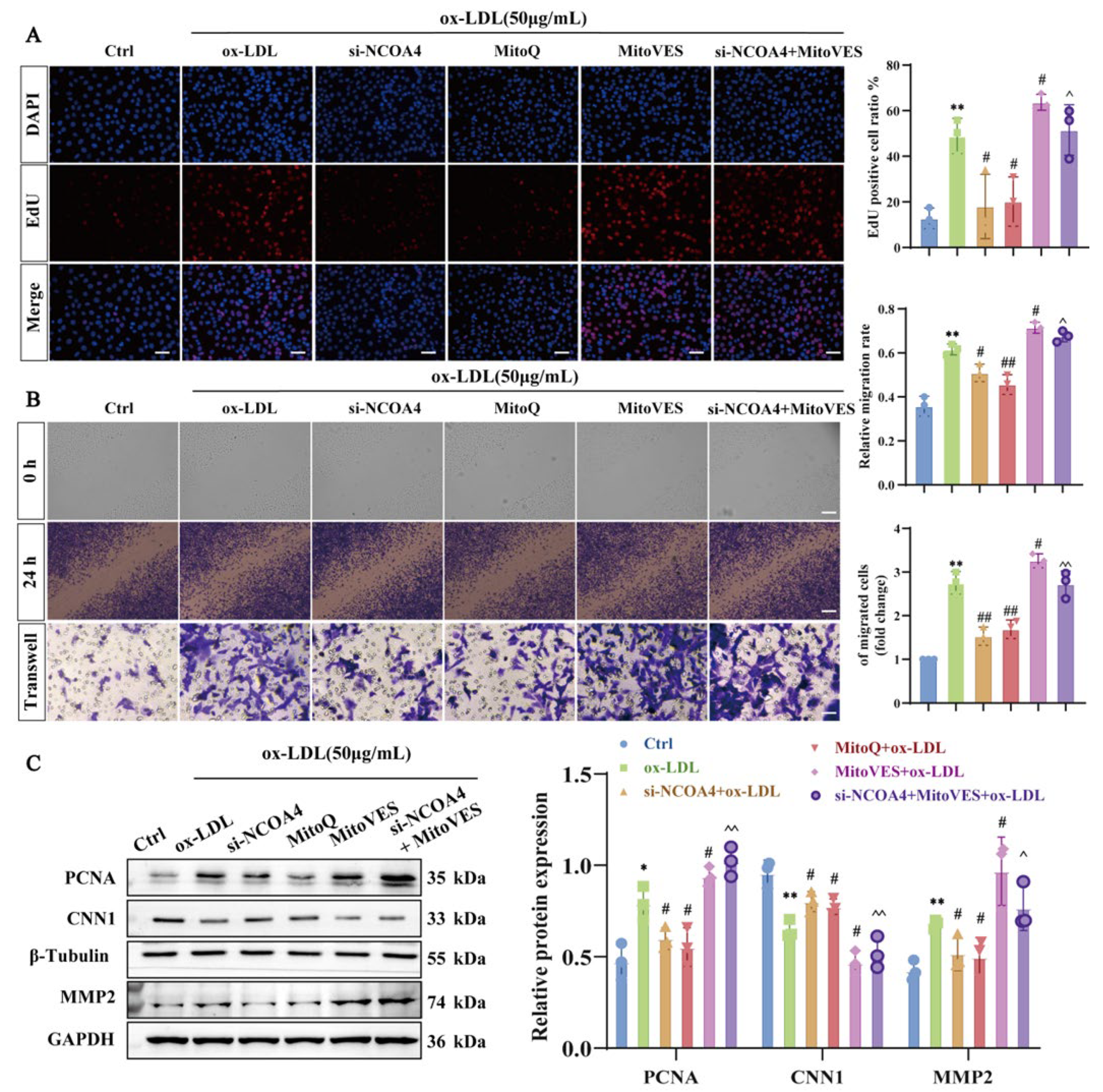
3.7. Silencing NCOA4 Inhibits Proliferation and Migration in VSMCs by Improving Mitochondrial Damage
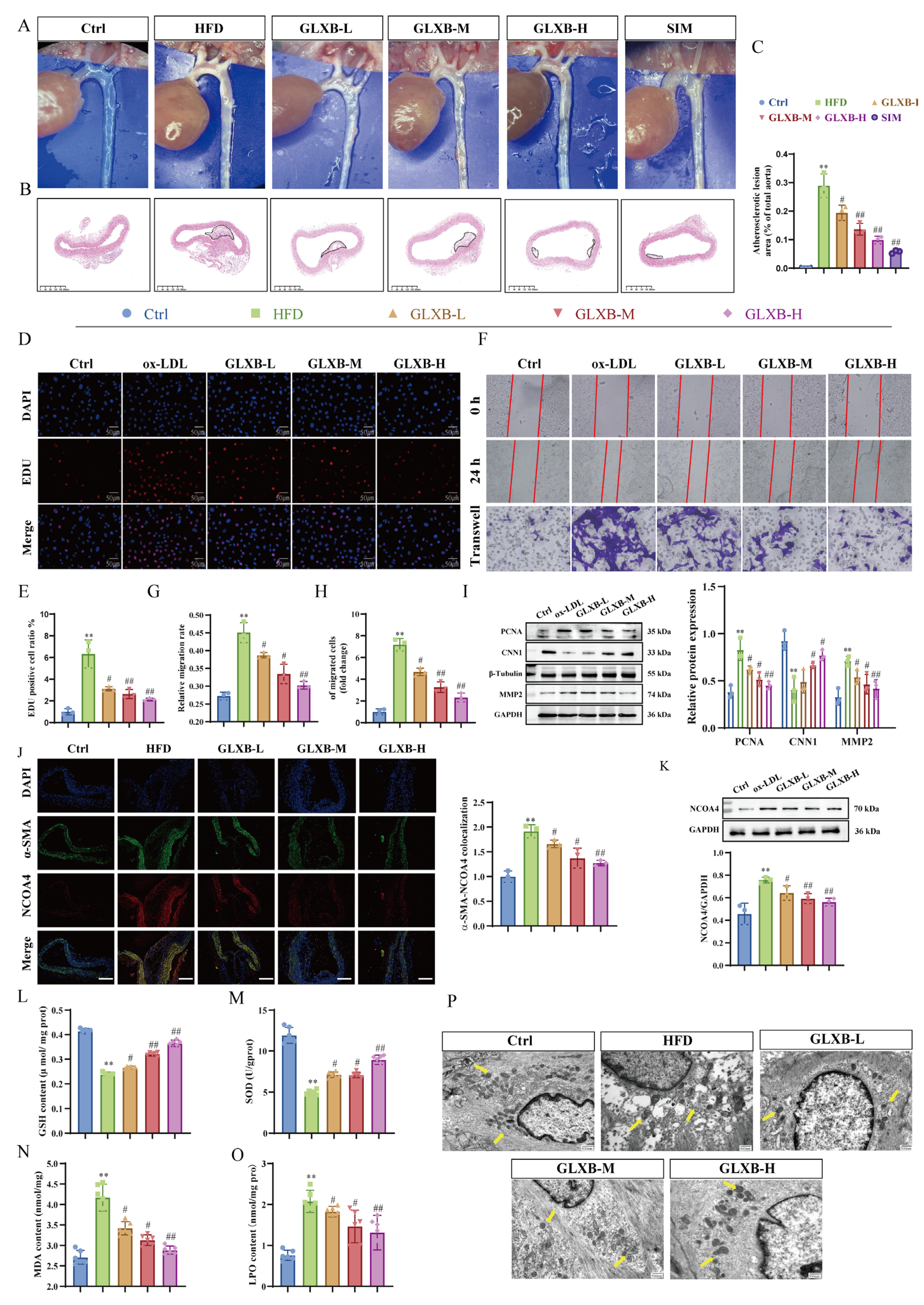
3.8. GLXB Attenuates Atherosclerotic Plaque Formation via NCOA4-Dependent Suppression of Ferroptosis in VSMCs
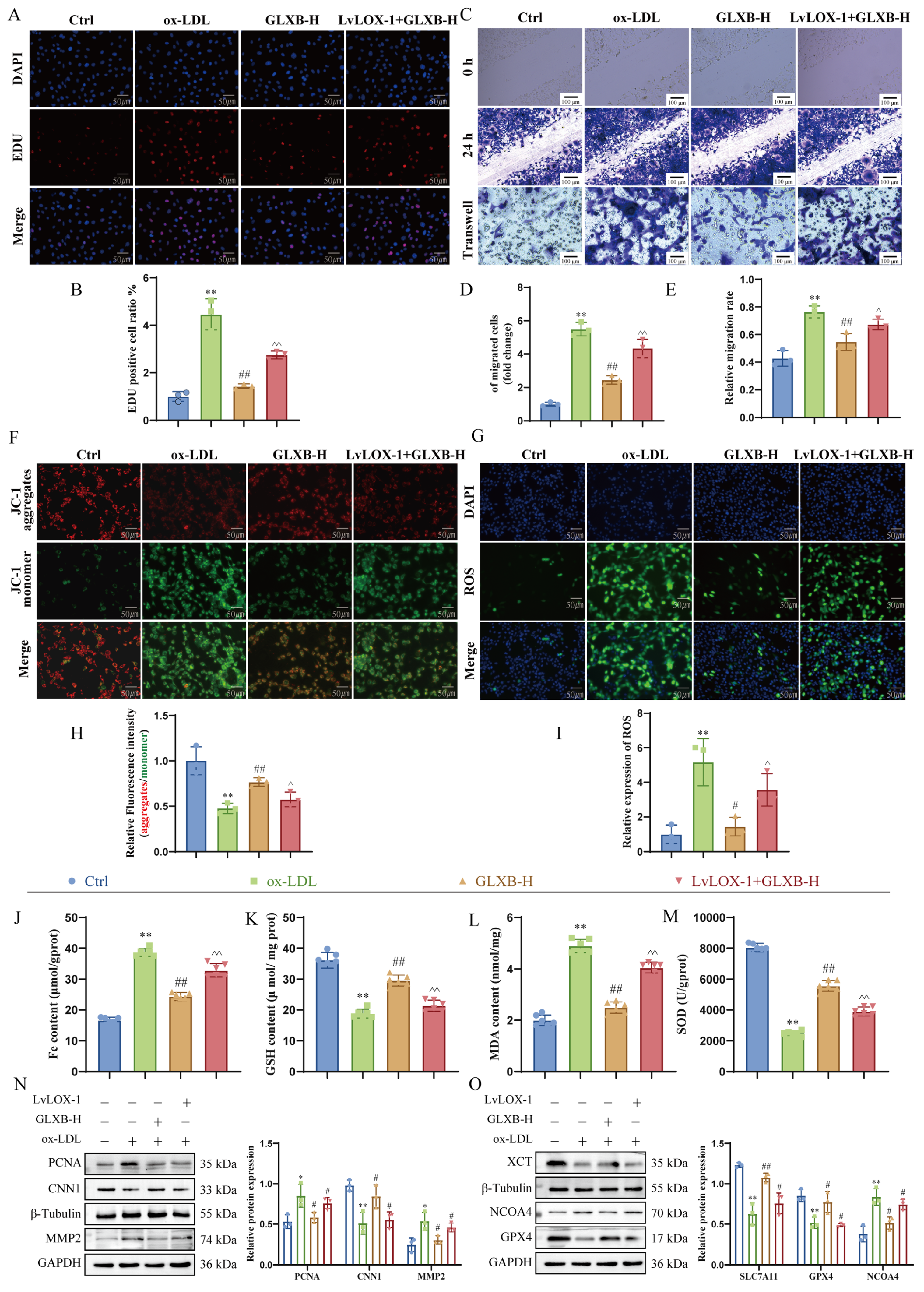
3.9. GLXB-Containing Serum Attenuates VSMCs Proliferation and Migration by Targeting LOX-1 to Suppress NCOA4-Dependent Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | atherosclerosis |
| VSMCs | Vascular smooth muscle cells |
| GLXB | Gualou–Xiebai herb pair |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| NCOA4 | Nuclear receptor Coactivator |
| LOX-1 | Lectin-like ox-LDL receptor 1 |
| LPO | Lipid hydroperoxide |
| Fer-1 | Ferrostatin-1 |
| FTMT | Mitochondrial ferritin |
| MDA | Malondialdehyde |
| ox-LDL | Oxidized low-density lipoprotein |
| ROS | Reactive oxygen species |
| SLC7A11 | Solute carrier family 7 member 11 |
| SOD | Superoxide dismutase |
References
- Bu, L.-L.; Yuan, H.-H.; Xie, L.-L.; Guo, M.-H.; Liao, D.-F.; Zheng, X.-L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 15160. [Google Scholar] [CrossRef]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, W.R.; Shi, W.T.; Tan, J.J.; Zhang, K.Y.; Tang, J.Y.; Chen, X.L.; Zhou, Z.Y. Tribulus terrestris L. extract ameliorates atherosclerosis by inhibition of vascular smooth muscle cell proliferation in ApoE(−/−) mice and A7r5 cells via suppression of Akt/MEK/ERK signaling. J. Ethnopharmacol. 2022, 297, 115547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Zhu, N.; Guo, Z.F.; Kazama, K.; Yi, B.; Tongmuang, N.; Yao, H.; Yang, R.; Zhang, C.; Qin, Y.; Han, L.; et al. Epigenetic regulation of vascular smooth muscle cell phenotypic switch and neointimal formation by PRMT5. Cardiovasc. Res. 2023, 119, 2244–2255. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. Ferroptosis: When metabolism meets cell death. Physiol. Rev. 2025, 105, 651–706. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Liang, F.G.; Zandkarimi, F.; Lee, J.; Axelrod, J.L.; Pekson, R.; Yoon, Y.; Stockwell, B.R.; Kitsis, R.N. OPA1 promotes ferroptosis by augmenting mitochondrial ROS and suppressing an integrated stress response. Mol. Cell 2024, 84, 3098–3114.e3096. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.H.; Li, L.C.; Ye, Z.P.; Liu, J.N.; Chen, Y.H.; Hu, B.X.; Tang, J.H.; Feng, G.K.; Li, Z.M.; et al. Susceptibility of Mitophagy-Deficient Tumors to Ferroptosis Induction by Relieving the Suppression of Lipid Peroxidation. Adv. Sci. 2025, 12, e2412593. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Meng, J.; Yu, Z.; Guo, Y.; Zhang, X.; Yan, Y.; Du, S.; Jin, S.; Li, J.; Yang, H.; et al. Ru single-atom nanozymes targeting ROS-ferroptosis pathways for enhanced endometrial regeneration in intrauterine adhesion therapy. Biomaterials 2025, 315, 122923. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, L.; Wang, B.; Tang, L.; Li, J.; Liu, C.; Huang, Y.; Zhang, Z.; Zhang, D.; Zhang, L.; et al. Remote Ischemic Preconditioning Attenuates Mitochondrial Dysfunction and Ferroptosis of Tubular Epithelial Cells by Inhibiting NOX4-ROS Signaling in Acute Kidney Injury. Int. J. Biol. Sci. 2025, 21, 2313–2329. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Bugg, Z.; Pullin, J.; Moore, G.R.; Svistunenko, D.A.; Le Brun, N.E. Human mitochondrial ferritin exhibits highly unusual iron-O(2) chemistry distinct from that of cytosolic ferritins. Nat. Commun. 2025, 16, 4695. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Ren, Q.; Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.Z. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021, 12, 447. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Liu, Y.; Li, Z.; Bai, H.; Zhao, Y.; Chang, Y.Z. Mitochondrial ferritin alleviates apoptosis by enhancing mitochondrial bioenergetics and stimulating glucose metabolism in cerebral ischemia reperfusion. Redox Biol. 2022, 57, 102475. [Google Scholar] [CrossRef]
- Li, K.; Chen, B.; Xu, A.; Shen, J.; Li, K.; Hao, K.; Hao, R.; Yang, W.; Jiang, W.; Zheng, Y.; et al. TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 2022, 56, 102451. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Del Rey, M.Q.; Kapner, K.S.; Zhang, H.; Gikandi, A.; Malcolm, C.; Poupault, C.; Kuljanin, M.; John, K.M.; Biancur, D.E.; et al. NCOA4-Mediated Ferritinophagy Is a Pancreatic Cancer Dependency via Maintenance of Iron Bioavailability for Iron-Sulfur Cluster Proteins. Cancer Discov. 2022, 12, 2180–2197. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Liu, J.; Li, Z.; Bao, Y.; Sun, X.; Zhao, W.; Zhou, A.; Wu, H. NCOA4 linked to endothelial cell ferritinophagy and ferroptosis: A key regulator aggravate aortic endothelial inflammation and atherosclerosis. Redox Biol. 2025, 79, 103465. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhao, Z.D.; Kong, P.Y.; Gao, L.; Yu, Y.N.; Liu, J.; Wang, P.Q.; Li, B.; Zhang, X.X.; Yang, L.Q.; et al. A comparative pharmacogenomic analysis of three classic TCM prescriptions for coronary heart disease based on molecular network modeling. Acta Pharmacol. Sin. 2020, 41, 735–744. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, L.; Wang, Y.; Liu, J.; Liu, Z.; Li, Z.; Zhou, A.; Wu, H. Gualou-Xiebai herb pair and its active ingredients act against atherosclerosis by suppressing VSMC-derived foam cell formation via regulating P2RY12-mediated lipophagy. Phytomedicine 2024, 128, 155341. [Google Scholar] [CrossRef]
- Yan, L.L.; Zhang, W.Y.; Wei, X.H.; Yan, L.; Pan, C.S.; Yu, Y.; Fan, J.Y.; Liu, Y.Y.; Zhou, H.; Han, J.Y.; et al. Gualou Xiebai Decoction, a Traditional Chinese Medicine, Prevents Cardiac Reperfusion Injury of Hyperlipidemia Rat via Energy Modulation. Front. Physiol. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.Y.; Yu, Y.; Cheng, C.S.; Han, J.Y.; Yao, X.S.; Zhou, H. Discovery of the mechanisms and major bioactive compounds responsible for the protective effects of Gualou Xiebai Decoction on coronary heart disease by network pharmacology analysis. Phytomedicine 2019, 56, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The Anti-atherosclerotic Effect of Paeonol against Vascular Smooth Muscle Cell Proliferation by Up-regulation of Autophagy via the AMPK/mTOR Signaling Pathway. Front. Pharmacol. 2017, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, T.T.; Zhuang, M.; Tan, C.Y.; Xie, T.H.; Cai, J.; Yao, Y.; Zhu, L. Iron derived from NCOA4-mediated ferritinophagy causes cellular senescence via the cGAS-STING pathway. Cell Death Discov. 2023, 9, 419. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, P.; Li, S.; Zhang, J.; Wang, D.; Wang, Z.; Zhu, L.; Wang, L. Mitochondrial GCN5L1 acts as a novel regulator for iron homeostasis to promote sorafenib sensitivity in hepatocellular carcinoma. J. Transl. Med. 2024, 22, 593. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- López-Grueso, M.J.; Lagal, D.J.; García-Jiménez, Á.F.; Tarradas, R.M.; Carmona-Hidalgo, B.; Peinado, J.; Requejo-Aguilar, R.; Bárcena, J.A.; Padilla, C.A. Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells. Redox Biol. 2020, 37, 101737. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Ouyang, W.; Zhang, W.; Yang, L.; Lin, X.; Dai, M.; Hu, H.; Tang, H.; Liu, H.; Xia, J.; et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021, 41, 101936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bao, Y.; Liu, Z.; Liu, J.; Li, Z.; Sun, X.; Zhou, A.; Wu, H. Gualou-Xiebai herb pair ameliorate atherosclerosis in HFD-induced ApoE(−/−) mice and inhibit the ox-LDL-induced injury of HUVECs by regulating the Nrf2-mediated ferroptosis. J. Ethnopharmacol. 2024, 326, 117892. [Google Scholar] [CrossRef] [PubMed]
- Aherrahrou, R.; Guo, L.; Nagraj, V.P.; Aguhob, A.; Hinkle, J.; Chen, L.; Yuhl Soh, J.; Lue, D.; Alencar, G.F.; Boltjes, A.; et al. Genetic Regulation of Atherosclerosis-Relevant Phenotypes in Human Vascular Smooth Muscle Cells. Circ. Res. 2020, 127, 1552–1565. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, L.; Huo, S.; Peng, D.; Gou, J.; Shi, W.; Tao, J.; Jiang, T.; Jiang, Y.; Wang, Q.; et al. STAT3 signaling promotes cardiac injury by upregulating NCOA4-mediated ferritinophagy and ferroptosis in high-fat-diet fed mice. Free Radic. Biol. Med. 2023, 201, 111–125. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, X.; Zou, Y.; Zhang, B.; Jian, Y.; Wu, Q.; Chen, S.; Zhang, X. Hypoxia Aggravates Neuron Ferroptosis in Early Brain Injury Following Subarachnoid Hemorrhage via NCOA4-Meditated Ferritinophagy. Antioxidants 2023, 12, 2097. [Google Scholar] [CrossRef]
- Qi, X.; Song, A.; Ma, M.; Wang, P.; Zhang, X.; Lu, C.; Zhang, J.; Zheng, S.; Jin, H. Curcumol inhibits ferritinophagy to restrain hepatocyte senescence through YAP/NCOA4 in non-alcoholic fatty liver disease. Cell Prolif. 2021, 54, e13107. [Google Scholar] [CrossRef]
- Jin, L.; Yu, B.; Wang, H.; Shi, L.; Yang, J.; Wu, L.; Gao, C.; Pan, H.; Han, F.; Lin, W.; et al. STING promotes ferroptosis through NCOA4-dependent ferritinophagy in acute kidney injury. Free Radic. Biol. Med. 2023, 208, 348–360. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Gou, F.; Wang, J.; Fan, Q.; Zhao, D.; Yang, L. Oxidative stress and mitochondrial impairment: Key drivers in neurodegenerative disorders. Ageing Res. Rev. 2025, 104, 102667. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Song, Y.; Gao, M.; Wei, B.; Huang, X.; Yang, Z.; Zou, J.; Guo, Y. Mitochondrial ferritin alleviates ferroptosis in a kainic acid-induced mouse epilepsy model by regulating iron homeostasis: Involvement of nuclear factor erythroid 2-related factor 2. CNS Neurosci. Ther. 2024, 30, e14663. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Yin, B.; Guo, Y.; Liu, Y.; Zhao, Y.; Wang, Y.; Cao, Y.; Feng, J.; Leng, J.; et al. BaiJiu Increases Nitric Oxide Bioactivity of Chinese Herbs Used to Treat Coronary Artery Disease Through the NO3−-NO2−-NO Pathway. J. Cardiovasc. Pharmacol. 2019, 74, 348–354. [Google Scholar] [CrossRef]
- Ding, Y.F.; Peng, Y.R.; Shen, H.; Shu, L.; Wei, Y.J. Gualou Xiebai decoction inhibits cardiac dysfunction and inflammation in cardiac fibrosis rats. BMC Complement. Altern. Med. 2016, 16, 49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Gao, J.; Liu, Z.; Zhou, A.; Wu, H. Ferritin Mitochondrial (FTMT)-Driven Mitochondrial Ferroptosis in Vascular Smooth Muscle Cells: A Role of NCOA4 in Atherosclerosis Pathogenesis and Modulation by Gualou–Xiebai. Nutrients 2025, 17, 3713. https://doi.org/10.3390/nu17233713
Zhu L, Gao J, Liu Z, Zhou A, Wu H. Ferritin Mitochondrial (FTMT)-Driven Mitochondrial Ferroptosis in Vascular Smooth Muscle Cells: A Role of NCOA4 in Atherosclerosis Pathogenesis and Modulation by Gualou–Xiebai. Nutrients. 2025; 17(23):3713. https://doi.org/10.3390/nu17233713
Chicago/Turabian StyleZhu, Li, Jun Gao, Zijian Liu, An Zhou, and Hongfei Wu. 2025. "Ferritin Mitochondrial (FTMT)-Driven Mitochondrial Ferroptosis in Vascular Smooth Muscle Cells: A Role of NCOA4 in Atherosclerosis Pathogenesis and Modulation by Gualou–Xiebai" Nutrients 17, no. 23: 3713. https://doi.org/10.3390/nu17233713
APA StyleZhu, L., Gao, J., Liu, Z., Zhou, A., & Wu, H. (2025). Ferritin Mitochondrial (FTMT)-Driven Mitochondrial Ferroptosis in Vascular Smooth Muscle Cells: A Role of NCOA4 in Atherosclerosis Pathogenesis and Modulation by Gualou–Xiebai. Nutrients, 17(23), 3713. https://doi.org/10.3390/nu17233713






