Microbial Metabolite, Macro Impact: Urolithin A in the Nexus of Insulin Resistance and Colorectal Tumorigenesis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Urolithin A and Colorectal Cancer
3.1.1. Cell Viability and Apoptosis
3.1.2. Autophagy and Mitophagy
3.1.3. Wnt/β-Catenin Suppression
3.1.4. Chemoresistance
3.1.5. Critical Appraisal and Translational Relevance of Current Evidence
3.2. Urolithin A and Type 2 Diabetes Mellitus
3.2.1. Apoptosis and Cellular Stress vs. Autophagy
| System/ Complication | Model/System | UA Dose/Exposure | Mechanism(s) | Key Findings | References |
|---|---|---|---|---|---|
| Pancreatic β-cell survival | Diabetic mice; MIN6 β-cells | 50 mg/kg/day; 11.5 μg/mL | ↑ Autophagy (LC3II ↑, SQSTM1/p62 ↓); ↓ Caspase-3/1; ΔΨm restoration | ↓ β-cell apoptosis; ↑ β-cell viability and morphology | [48] |
| Obesity/insulin resistance | HFD & ob/ob mice; Adipocytes | 30 mg/kg/day; 20 μM | ↑ BAT thermogenesis; ↑ iWAT browning; ↑ UCP1/PGC-1α; ↑ Dio2 → ↑ T3 | ↓ Adiposity; ↑ Insulin sensitivity | [23] |
| Insulin resistance (liver and skeletal muscle) | DBA/2J mice; Hepatocytes | 0.1% dietary UA; 10 μM | ↑ Mitophagy (PINK1/PRKN); ↑ MFN2; ↑ mtDNA; ↓ Proton leak | ↓ Fasting glucose; ↑ Adiponectin; Improved IPTT/GTT | [53] |
| Diabetic retinopathy | STZ rats; HRECs | 2.5 mg/kg/day; 10 μM | ↑ Nrf2/HO-1; ↓ IL-6, IL-1β, TNF-α; ↓ MDA; ↑ SOD/GSH | ↓ Oxidative stress; ↓ VEGF-mediated leakage; Improved retina | [58] |
| Cognitive dysfunction | T2DM mice; HT22 neurons | 200 mg/kg/day; 5 μM | ↓ ER stress (ATF6, CHOP); ↓ Atp2a3; ↓ Tau phosphorylation | ↑ Cognition; ↓ Apoptosis | [46] |
| Gut barrier dysfunction | T2DM mice; Caco-2 cells | 200 mg/kg; 10 μM | ↑ Tight junctions (ZO-1, Occludin, Claudin 1); ↓ TLR4/Myd88; ↓ NLRP3; ↑ N-glycosylation genes | ↑ Gut integrity; ↓ Inflammation; ↑ Cognition | [59] |
| Podocytopathy (kidney) | Mouse podocytes (HG) | 10 μM (beneficial); (cytotoxic at 100 μM) | ↓ ROS; ↑ Bcl-2; ↑ Autophagy (LC3B, ATG5); ↑ Nephrin | ↑ Podocyte survival | [50] |
| DKD— tubular injury | STZ mice; HK-2 cells | 50 mg/kg/day; 20 μM | ↑ Mitophagy ↓ TRPC6–calpain-1; ↓ Cytokines | ↓ Tubular fibrosis & inflammation | [51] |
| Wound healing (angiogenesis) | STZ rats; EPCs | 25 mg/kg/day; 20 μM | ↑ Mitophagy (Parkin); ↓ ROS ↑ EPC proliferation | ↑ Wound closure; ↑ Neovascularization | [56] |
| Vascular dysfunction (HG) | STZ rats; VSMCs | Juice → UA; 5–40 μM | ↓ AKT phosphorylation ↓ β-catenin (c-Myc, cyclin D1) | ↑ Endothelial function ↓ VSMC proliferation | [54] |
| Diabetic cardiomyopathy | STZ rats (T1D model) | 2.5 mg/kg/day | ↑ SIRT1 → deacetylation of Nrf2, FOXO1, NF-κB; ↑ MnSOD, GSH; ↓ ROS, TNF-α, IL-6 | ↑ Cardiac function; ↓ Fibrosis (TGF-β1, Smad3, Col1A1); ↓ Apoptosis; Effects abolished by SIRT1 inhibitor | [60] |
3.2.2. Glucose Metabolism and Insulin Signaling
3.2.3. Anti-Inflammatory and Antioxidant Effects
3.2.4. Modulating Adipose Tissue and Systemic Health
3.3. Shared Mechanisms of UA’s Role in CRC and T2DM
4. Discussion
- Targeted delivery strategies: Advance UA formulations (e.g., pH-responsive polymers, nanoparticles) to concentrate high doses in colonic tumors while limiting systemic exposure. Nanocarriers designed to release UA in the tumor microenvironment could widen its therapeutic window [92].
- Dose and regimen optimization: Map the dose–response continuum of UA’s effects. Careful titration is needed to separate its anti-CRC (high-dose) and anti-diabetic (low-dose) actions. For example, intermittent high-dose pulses might kill tumor cells while daily low-dose maintains metabolic control.
- Combination therapy trials: Given UA’s ability to sensitize CRC to chemotherapy and to enhance antitumor T-cell responses, testing UA alongside standard chemo/immunotherapies is warranted. Early-phase clinical trials could assess the effects of UA supplementation in CRC patients with metabolic syndrome, monitoring tumor markers, glycemic indices, and immune phenotypes.
- Patient stratification and biomarkers: Identify biomarkers (e.g., gut metabotype, insulin resistance status) to select patients most likely to benefit from UA. Only some individuals efficiently convert ellagitannins to UA.
- Safety assessments: Evaluate UA’s effects in autoimmunity and healthy tissues. Its immune-boosting properties should be tested for safety in autoimmune disease models to ensure it does not exacerbate β-cell autoimmunity. Long-term toxicology should confirm that sustained UA levels (even via targeted delivery) are well tolerated.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| ATG5 | Autophagy related 5 |
| ATRA | All-trans retinoic acid |
| AKT | Protein kinase B (AKT) |
| AMPK | AMP-activated protein kinase |
| BAT | Brown adipose tissue |
| BAX | Bcl-2 associated X protein |
| BCL-2 | B-cell lymphoma 2 (anti-apoptotic protein) |
| CRC | Colorectal cancer |
| DPP-4 | Dipeptidyl peptidase-4 |
| DRP1 | Dynamin-related protein 1 (mitochondrial fission regulator) |
| EPC(s) | Endothelial progenitor cell(s) |
| ER | Endoplasmic reticulum |
| GLUT4 | Glucose transporter type 4 |
| GSH | Glutathione |
| HO-1 | Heme oxygenase-1 |
| IR | Insulin receptor |
| IWAT | Inguinal (subcutaneous) white adipose tissue |
| LC3 | Microtubule-associated protein 1 light chain 3 (autophagy marker) |
| MAM(s) | Mitochondria-associated membranes |
| MCP-1 | Monocyte chemoattractant protein-1 (CCL2) |
| MDA | Malondialdehyde |
| mtROS | Mitochondrial reactive oxygen species |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 (inflammasome) |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OCR | Oxygen consumption rate |
| PI3K | Phosphoinositide 3-kinase. |
| PINK1 | PTEN-induced kinase 1 |
| PRKN (Parkin) | Parkin RBR E3 ubiquitin protein ligase (PRKN) |
| PTP1B | Protein tyrosine phosphatase 1B |
| PSD95 | Postsynaptic density protein 95 |
| PTEN | Phosphatase and tensin homolog (if used in text) |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SQSTM1/p62 | Sequestosome 1 (p62; autophagy adaptor) |
| T2DM | Type 2 diabetes mellitus |
| TGM2 | Transglutaminase 2 |
| TSCM | T memory stem cell (memory-stem CD8+ T cell) |
| UCP1 | Uncoupling protein 1 (thermogenesis marker) |
| UA | Urolithin A |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| ΔΨm | Mitochondrial membrane potential (delta psi m) |
References
- Home. Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 30 August 2025).
- Cancer of the Colon and Rectum—Cancer Stat Facts. SEER. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 22 September 2025).
- CDC. Health and Economic Benefits of Colorectal Cancer Interventions’; National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP): Atlantan, GA, USA, 2025. Available online: https://www.cdc.gov/nccdphp/priorities/colorectal-cancer.html (accessed on 25 July 2025).
- Chen, Z.; Hong, Q. Correlation of serum IGF-1, AGEs and their receptors with the risk of colorectal cancer in patients with type 2 diabetes mellitus. Front. Oncol. 2023, 13, 1125745. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. The Proliferating Role of Insulin and Insulin-Like Growth Factors in Cancer. Trends Endocrinol. Metab. TEM 2010, 21, 610–618. [Google Scholar] [CrossRef]
- Alvarez-Leite, J.I. The Role of Bioactive Compounds in Human Health and Disease. Nutrients 2025, 17, 1170. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Lin, I.-C.; Wu, J.-Y.; Fang, C.-Y.; Wang, S.-C.; Liu, Y.-W.; Ho, S.-T. Absorption and Metabolism of Urolithin A and Ellagic Acid in Mice and Their Cytotoxicity in Human Colorectal Cancer Cells. Evid.-Based Complement. Altern. Med. Ecam 2023, 2023, 8264716. [Google Scholar] [CrossRef]
- Aichinger, G. Natural Dibenzo-α-Pyrones: Friends or Foes? Int. J. Mol. Sci. 2021, 22, 13063. [Google Scholar] [CrossRef]
- He, F.; Bian, Y.; Zhao, Y.; Xia, M.; Liu, S.; Gui, J.; Hou, X.; Fang, Y. In vitro conversion of ellagic acid to urolithin A by different gut microbiota of urolithin metabotype A. Appl. Microbiol. Biotechnol. 2024, 108, 215. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Ares, A.M.; Toribio, L.; García-Villalba, R.; Villalgordo, J.M.; Althobaiti, Y.; Tomás-Barberán, F.A.; Bernal, J. Separation of Isomeric Forms of Urolithin Glucuronides Using Supercritical Fluid Chromatography. J. Agric. Food Chem. 2023, 71, 3033–3039. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S.; Stefańska, J.; Kiss, A.K. Phase II Conjugates of Urolithins Isolated from Human Urine and Potential Role of β-Glucuronidases in Their Disposition. Drug Metab. Dispos. 2017, 45, 657–665. [Google Scholar] [CrossRef]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A.; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef]
- Lin, J.; Zhuge, J.; Zheng, X.; Wu, Y.; Zhang, Z.; Xu, T.; Meftah, Z.; Xu, H.; Wu, Y.; Tian, N.; et al. Urolithin A-induced mitophagy suppresses apoptosis and attenuates intervertebral disc degeneration via the AMPK signaling pathway. Free. Radic. Biol. Med. 2020, 150, 109–119. [Google Scholar] [CrossRef]
- El-Wetidy, M.S.; Ahmad, R.; Rady, I.; Helal, H.; Rady, M.I.; Vaali-Mohammed, M.-A.; Al-Khayal, K.; Traiki, T.B.; Abdulla, M.-H. Urolithin A induces cell cycle arrest and apoptosis by inhibiting Bcl-2, increasing p53-p21 proteins and reactive oxygen species zproduction in colorectal cancer cells. Cell Stress Chaperones 2021, 26, 473–493. [Google Scholar] [CrossRef]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of Fruit Ellagitannin Extracts, Ellagic Acid, and Their Colonic Metabolite, Urolithin A, on Wnt Signaling. J. Agric. Food Chem. 2010, 58, 3965–3969. [Google Scholar] [CrossRef]
- Norden, E.; Heiss, E.H. Urolithin A gains in antiproliferative capacity by reducing the glycolytic potential via the p53/TIGAR axis in colon cancer cells. Carcinogenesis 2019, 40, 93–101. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells. Food Chem. Toxicol. 2020, 139, 111260. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, R.; Vanwinkle, Z.M.; Guo, H.; Vemula, P.K.; Goel, A.; Haribabu, B.; Jala, V.R. Microbial metabolite restricts 5-fluorouracil-resistant colonic tumor progression by sensitizing drug transporters via regulation of FOXO3-FOXM1 axis. Theranostics 2022, 12, 5574–5595. [Google Scholar] [CrossRef]
- Kondo, S.; Adachi, S.-I.; Komatsu, W.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Urolithin A in Cultured L6 Myotubes and Type 2 Diabetic Model KK-A(y)/Ta Mice with Glucose Intolerance. Curr. Issues Mol. Biol. 2024, 46, 1078–1090. [Google Scholar] [CrossRef]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; Núñez-Sánchez, M.A.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; García-Villalba, R.; Tomás-Barberán, F.A.; Espín, J.C. Antiproliferative activity of the ellagic acid-derived gut microbiota isourolithin A and comparison with its urolithin A isomer: The role of cell metabolism. Eur. J. Nutr. 2017, 56, 831–841. [Google Scholar] [CrossRef]
- Tortora, K.; Femia, A.P.; Romagnoli, A.; Sineo, I.; Khatib, M.; Mulinacci, N.; Giovannelli, L.; Caderni, G. Pomegranate By-Products in Colorectal Cancer Chemoprevention: Effects in Apc-Mutated Pirc Rats and Mechanistic Studies In Vitro and Ex Vivo. Mol. Nutr. Food Res. 2018, 62, 1700401. [Google Scholar] [CrossRef]
- Tiwari, A.; Tiwari, V.; Verma, S.; Sharma, A.; Marisetti, A.L.; Kumar, M.; Kumar, G.; Qurtam, A.A.; Nasr, F.A.; Mubarak, M.; et al. Network Pharmacology, Molecular Docking, Simulation Studies of Urolithin A by Inhibiting AKT and Caspase Pathways against Colorectal Cancer. J. Biol. Regul. Homeost. Agents 2024, 38, 5175–5194. [Google Scholar] [CrossRef]
- Orth, J.D.; Loewer, A.; Lahav, G.; Mitchison, T.J. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell 2012, 23, 567–576. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef]
- Gupta, S.; Kass, G.E.; Szegezdi, E.; Joseph, B. The mitochondrial death pathway: A promising therapeutic target in diseases. J. Cell. Mol. Med. 2009, 13, 1004–1033. [Google Scholar] [CrossRef]
- Meza-Sosa, K.F.; Miao, R.; Navarro, F.; Zhang, Z.; Zhang, Y.; Hu, J.J.; Hartford, C.C.R.; Li, X.L.; Pedraza-Alva, G.; Pérez-Martínez, L.; et al. SPARCLE, a p53-induced lncRNA, controls apoptosis after genotoxic stress by promoting PARP-1 cleavage. Mol. Cell 2022, 82, 785–802.e10. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Espín, J.-C.; Tomás-Barberán, F.A.; García-Conesa, M.-T. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol. Nutr. Food Res. 2009, 53, 686–698. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Ginefra, P.; Hope, H.C.; Chiang, Y.-H.; Nutten, S.; Blum, S.; Coukos, G.; Vannini, N. Urolithin-A Promotes CD8+ T Cell-mediated Cancer Immunosurveillance via FOXO1 Activation. Cancer Res. Commun. 2024, 4, 1189–1198. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, F.; Guo, Z.; Zhao, J.; Song, X.; Yang, H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol. Carcinog. 2018, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.-R.; Shi, Z.-Q.; Zhu, H.-P.; Gu, L.-H.; Wang, X.-F.; Yang, Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget 2017, 8, 62759–62768. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.; Petrocelli, V.; Conche, C.; Drachsler, M.; Ziegler, P.K.; Braun, A.; Kress, A.; Nicolas, A.M.; Mohs, K.; Becker, C.; et al. Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity 2022, 55, 2059–2073.e8. [Google Scholar] [CrossRef]
- Giles, R.H.; van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2003, 1653, 1–24. [Google Scholar] [CrossRef]
- Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T. Beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 1999, 155, 1033–1038. [Google Scholar] [CrossRef]
- Jung, A.; Schrauder, M.; Oswald, U.; Knoll, C.; Sellberg, P.; Palmqvist, R.; Niedobitek, G.; Brabletz, T.; Kirchner, T. The Invasion Front of Human Colorectal Adenocarcinomas Shows Co-Localization of Nuclear β-Catenin, Cyclin D1, and p16INK4A and Is a Region of Low Proliferation. Am. J. Pathol. 2001, 159, 1613–1617. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Tomé-Carneiro, J.; Bellesia, A.; Tomás-Barberán, F.A.; Espín, J.C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 2015, 6, 1460–1469. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.T.; Karmokar, A.; González-Sarrías, A.; García-Villalba, R.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Brown, K.; Espín, J.C. In vivo relevant mixed urolithins and ellagic acid inhibit phenotypic and molecular colon cancer stem cell features: A new potentiality for ellagitannin metabolites against cancer. Food Chem. Toxicol. 2016, 92, 8–16. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, Y.H.; Choi, G.E.; Kim, J.S.; Chae, C.W.; Lim, J.R.; Kim, S.Y.; Yoon, J.H.; Cho, J.H.; Lee, S.-J.; et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2021, 28, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Bacskai, B.J.; Calvo-Rodriguez, M. Mitochondria-ER contacts and glucose: The powerhouse of Alzheimer’s disease? Cell Calcium 2021, 97, 102434. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, K.; Bian, J.; Zhang, Y.; Li, J.; Liu, H.; Ye, Y.; Han, L.; Gong, L.; Wang, M. Urolithin A Protects Neuronal Cells against Stress Damage and Apoptosis by Atp2a3 Inhibition. Mol. Nutr. Food Res. 2023, 67, e2300146. [Google Scholar] [CrossRef]
- Mittal, R.; Lemos, J.R.N.; Chapagain, P.; Hirani, K. Interplay of hypoxia, immune dysregulation, and metabolic stress in pathophysiology of type 1 diabetes. Front. Immunol. 2025, 16, 1599321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Halemahebai, G.; Tian, L.; Dong, H.; Aisker, G. Urolithin A, a pomegranate metabolite, protects pancreatic β cells from apoptosis by activating autophagy. J. Ethnopharmacol. 2021, 272, 113628. [Google Scholar] [CrossRef]
- Tuohetaerbaike, B.; Zhang, Y.; Tian, Y.; Zhang, N.N.; Kang, J.; Mao, X.; Zhang, Y.; Li, X. Pancreas protective effects of Urolithin A on type 2 diabetic mice induced by high fat and streptozotocin via regulating autophagy and AKT/mTOR signaling pathway. J. Ethnopharmacol. 2020, 250, 112479. [Google Scholar] [CrossRef]
- Kotewicz, M.; Krauze-Baranowska, M.; Daca, A.; Płoska, A.; Godlewska, S.; Kalinowski, L.; Lewko, B. Urolithins Modulate the Viability, Autophagy, Apoptosis, and Nephrin Turnover in Podocytes Exposed to High Glucose. Cells 2022, 11, 2471. [Google Scholar] [CrossRef]
- Liu, C.-C.; Ji, J.-L.; Wang, Z.; Zhang, X.-J.; Ding, L.; Zhang, Y.; Zhou, Y.; Zhang, D.-J.; Tang, Z.-L.; Cao, J.-Y.; et al. TRPC6-Calpain-1 Axis Promotes Tubulointerstitial Inflammation by Inhibiting Mitophagy in Diabetic Kidney Disease. Kidney Int. Rep. 2024, 9, 3301–3317. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Huang, Q.; Lin, L.; Wang, L.; Wu, Y.; Wu, K.; Gao, R.; Liu, X.; Liu, X.; et al. Mitophagy disorder mediates cardiac deterioration induced by severe hypoglycemia in diabetic mice. Mol. Cell. Endocrinol. 2023, 575, 111994. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Henning, S.M.; Chan, B.; Long, J.; Zhong, J.; Acin-Perez, R.; Petcherski, A.; Shirihai, O.; Heber, D.; et al. Ellagic Acid and Its Microbial Metabolite Urolithin A Alleviate Diet-Induced Insulin Resistance in Mice. Mol. Nutr. Food Res. 2020, 64, e2000091. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Zheng, G.-H.; Qiu, Z. Emblic Leafflower (Phyllanthus emblica L.) Fruits Ameliorate Vascular Smooth Muscle Cell Dysfunction in Hyperglycemia: An Underlying Mechanism Involved in Ellagitannin Metabolite Urolithin A. Evid. Based Complement. Altern. Med. eCAM 2018, 2018, 8478943. [Google Scholar] [CrossRef]
- Dirimanov, S.; Högger, P. Screening of Inhibitory Effects of Polyphenols on Akt-Phosphorylation in Endothelial Cells and Determination of Structure-Activity Features. Biomolecules 2019, 9, 219. [Google Scholar] [CrossRef]
- Fang, X.; Shao, Z.; Ding, H.; Xu, H.; Tu, Z.; Wang, H.; Li, D.; Huang, C.; Jiang, C. Urolithin A enhances diabetic wound healing: Insights from parkin-mediated mitophagy in endothelial progenitor cells. Int. Immunopharmacol. 2025, 155, 114572. [Google Scholar] [CrossRef]
- Tang, W.; Yan, C.; He, S.; Du, M.; Cheng, B.; Deng, B.; Zhu, S.; Li, Y.; Wang, Q. Neuron-targeted overexpression of caveolin-1 alleviates diabetes-associated cognitive dysfunction via regulating mitochondrial fission-mitophagy axis. Cell Commun. Signal. 2023, 21, 357. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Li, K.; Wang, X.; Li, X.; An, M.; Yu, X.; Long, X.; Zhong, R.; Liu, Q.; et al. Urolithin A ameliorates diabetic retinopathy via activation of the Nrf2/HO-1 pathway. Endocr. J. 2022, 69, 971–982. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, K.; Bian, J.; Liu, H.; Zhai, X.; El-Omar, E.; Han, L.; Gong, L.; Wang, M. Urolithin A Attenuates Diabetes-Associated Cognitive Impairment by Ameliorating Intestinal Barrier Dysfunction via N-glycan Biosynthesis Pathway. Mol. Nutr. Food Res. 2022, 66, e2100863. [Google Scholar] [CrossRef]
- Albasher, G.; Alkahtani, S.; Al-Harbi, L.N. Urolithin A prevents streptozotocin-induced diabetic cardiomyopathy in rats by activating SIRT1. Saudi J. Biol. Sci. 2022, 29, 1210–1220. [Google Scholar] [CrossRef]
- Han, L.; Zhao, D.; Li, Y.; Jin, J.; El-Kott, A.F.; Al-Saeed, F.A.; Eldib, A.M. Assessment of the Anti-Breast Cancer Effects of Urolithin with Molecular Docking Studies in the In Vitro Condition: Introducing a Novel Chemotherapeutic Drug. Mol. Biotechnol. 2024, 66, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Goldberg, I.J. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ. Res. 2010, 106, 1449–1458. [Google Scholar] [CrossRef]
- Barone Lumaga, R.; Tagliamonte, S.; De Rosa, T.; Valentino, V.; Ercolini, D.; Vitaglione, P. Consumption of a Sourdough-Leavened Croissant Enriched with a Blend of Fibers Influences Fasting Blood Glucose in a Randomized Controlled Trial in Healthy Subjects. J. Nutr. 2024, 154, 2976–2987. [Google Scholar] [CrossRef]
- Pratama, R.A.; Astina, J.; Parikesit, A.A. In silico Screening of Potential Antidiabetic Phenolic Compounds from Banana (Musa spp.) Peel Against PTP1B Protein. J. Trop. Biodivers. Biotechnol. 2023, 8, 139. [Google Scholar] [CrossRef]
- Tolmie, M.; Bester, M.J.; Serem, J.C.; Nell, M.; Apostolides, Z. The potential antidiabetic properties of green and purple tea [Camellia sinensis (L.) O Kuntze], purple tea ellagitannins, and urolithins. J. Ethnopharmacol. 2023, 309, 116377. [Google Scholar] [CrossRef]
- Moreno Uclés, R.; González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A.; Janes, M.; Cheng, H.; Finley, J.; Greenway, F.; Losso, J.N. Effects of red raspberry polyphenols and metabolites on the biomarkers of inflammation and insulin resistance in type 2 diabetes: A pilot study. Food Funct. 2022, 13, 5166–5176. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef]
- Boakye, Y.D.; Groyer, L.; Heiss, E.H. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Laveriano-Santos, E.P.; Quifer-Rada, P.; Marhuenda-Muñoz, M.; Arancibia-Riveros, C.; Vallverdú-Queralt, A.; Tresserra-Rimbau, A.; Ruiz-León, A.M.; Casas, R.; Estruch, R.; Bodega, P.; et al. Microbial Phenolic Metabolites in Urine Are Inversely Linked to Certain Features of Metabolic Syndrome in Spanish Adolescents. Antioxidants 2022, 11, 2191. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Perše, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? BioMed Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Harata, K.; Madhyastha, H.; Kuribayashi, F. Ellagic acid and its fermentative derivative urolithin A show reverse effects on the gp91-phox gene expression, resulting in opposite alterations in all-trans retinoic acid-induced superoxide generating activity of U937 cells. Biochem. Biophys. Rep. 2021, 25, 100891. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Hao, W.; Yang, H.; Song, X.; Zhao, M.; Peng, S. Preparative isolation and purification of urolithins from the intestinal metabolites of pomegranate ellagitannins by high-speed counter-current chromatography. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2015, 990, 111–117. [Google Scholar] [CrossRef]
- Groestlinger, J.; Seidl, C.; Varga, E.; Del Favero, G.; Marko, D. Combinatory Exposure to Urolithin A, Alternariol, and Deoxynivalenol Affects Colon Cancer Metabolism and Epithelial Barrier Integrity in vitro. Front. Nutr. 2022, 9, 882222. [Google Scholar] [CrossRef] [PubMed]
- Dehal, A.N.; Newton, C.C.; Jacobs, E.J.; Patel, A.V.; Gapstur, S.M.; Campbell, P.T. Impact of Diabetes Mellitus and Insulin Use on Survival After Colorectal Cancer Diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2012, 30, 53–59. [Google Scholar] [CrossRef]
- Lawler, T.; Walts, Z.L.; Steinwandel, M.; Lipworth, L.; Murff, H.J.; Zheng, W.; Warren Andersen, S. Type 2 Diabetes and Colorectal Cancer Risk. JAMA Netw. Open 2023, 6, e2343333. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef]
- Gaur, A.; Maity, R.; Dhali, A.; Biswas, J. Impact of poorly controlled type II diabetes mellitus on chemoresistance in colorectal cancer. World J. Gastroenterol. 2025, 31, 104065. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Gosalbes, M.-J.; Macià, A.; Rubió, L.; Vázquez-Castellanos, J.F.; Jiménez Hernández, N.; Moya, A.; Latorre, A.; Motilva, M.-J. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol. Nutr. Food Res. 2015, 59, 1942–1953. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Functional Deficits in Gut Microbiome of Young and Middle-Aged Adults with Prediabetes Apparent in Metabolizing Bioactive (Poly)phenols. Nutrients 2020, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Liu, S.; Hai, Y.; Yang, G.; Lu, J.; He, F.; Zhao, Y.; Xia, M.; Hou, X.; Fang, Y. Lactococcus garvieae FUA009, a Novel Intestinal Bacterium Capable of Producing the Bioactive Metabolite Urolithin A from Ellagic Acid. Foods 2022, 11, 2621. [Google Scholar] [CrossRef]
- Philip, A.K.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 79–87. [Google Scholar] [CrossRef]
- Vignali, D.; Cantarelli, E.; Bordignon, C.; Canu, A.; Citro, A.; Annoni, A.; Piemonti, L.; Monti, P. Detection and Characterization of CD8+ Autoreactive Memory Stem T Cells in Patients With Type 1 Diabetes. Diabetes 2018, 67, 936–945. [Google Scholar] [CrossRef]
- Karumuru, V.; Dhasmana, A.; Mamidi, N.; Chauhan, S.C.; Yallapu, M.M. Unveiling the potential of Urolithin A in Cancer Therapy: Mechanistic Insights to Future Perspectives of Nanomedicine. Nanotheranostics 2025, 9, 121–143. [Google Scholar] [CrossRef]
 : UA mediated stimulation,
: UA mediated stimulation,  UA mediated inhibition, ┴: inhibition. c.
UA mediated inhibition, ┴: inhibition. c.
 : UA mediated stimulation,
: UA mediated stimulation,  UA mediated inhibition, ┴: inhibition. c.
UA mediated inhibition, ┴: inhibition. c.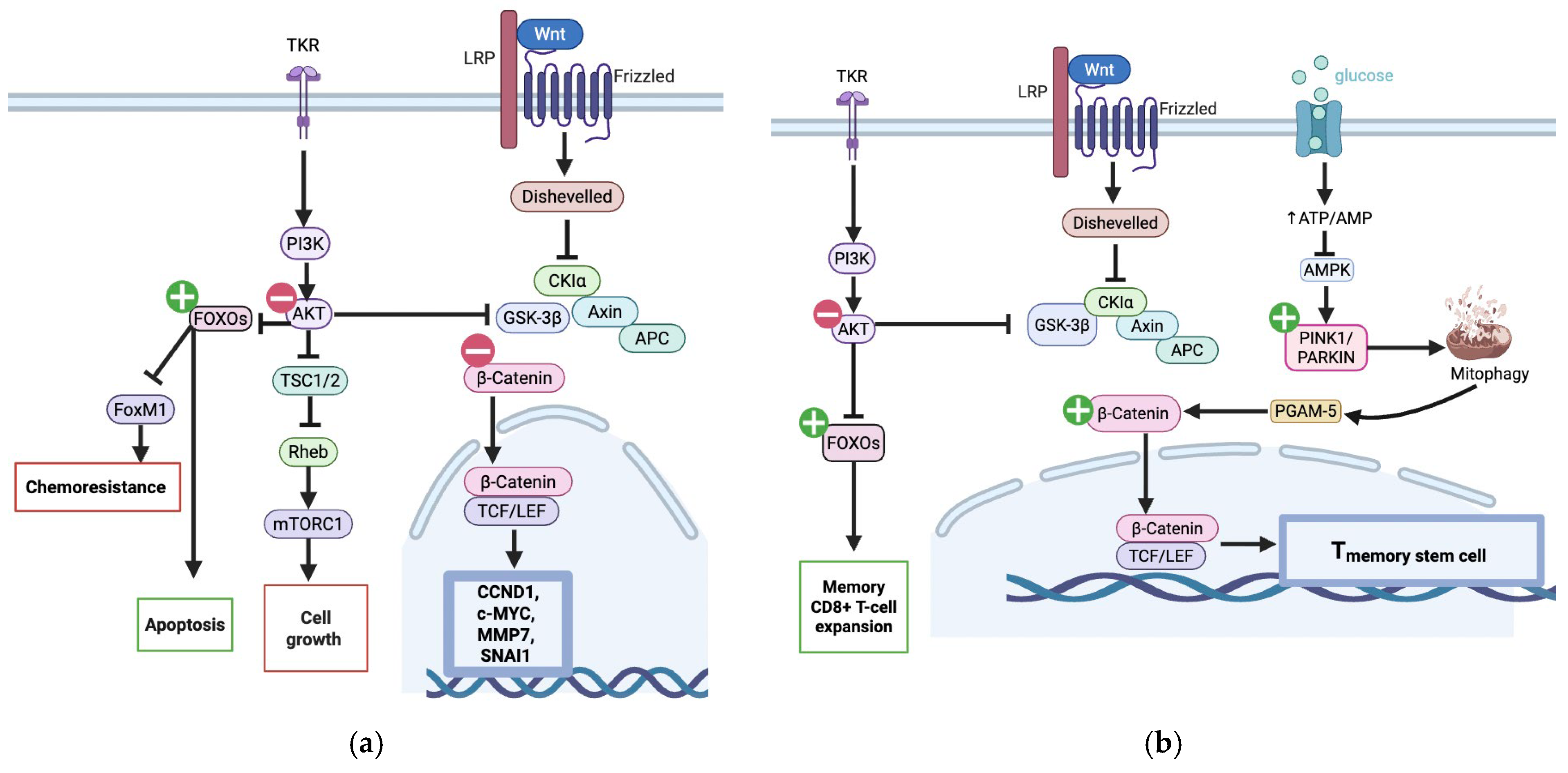
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/oatsiyd accessed on 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/oatsiyd accessed on 8 November 2025).
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/oatsiyd accessed on 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/oatsiyd accessed on 8 November 2025).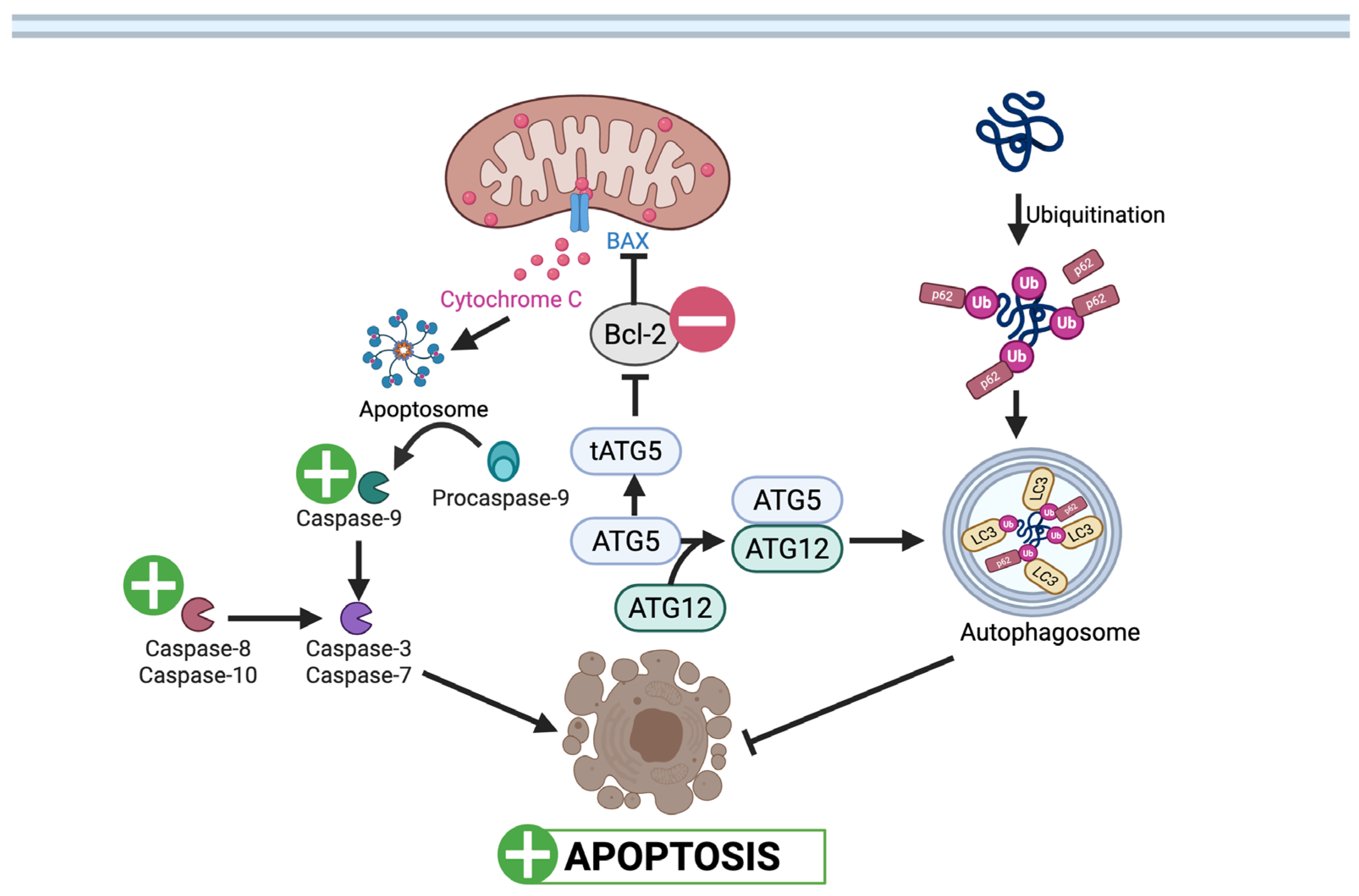
 : UA mediated inhibition (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/x9iuf4l accessed on 8 November 2025).
: UA mediated inhibition (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/x9iuf4l accessed on 8 November 2025).
 : UA mediated inhibition (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/x9iuf4l accessed on 8 November 2025).
: UA mediated inhibition (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/x9iuf4l accessed on 8 November 2025).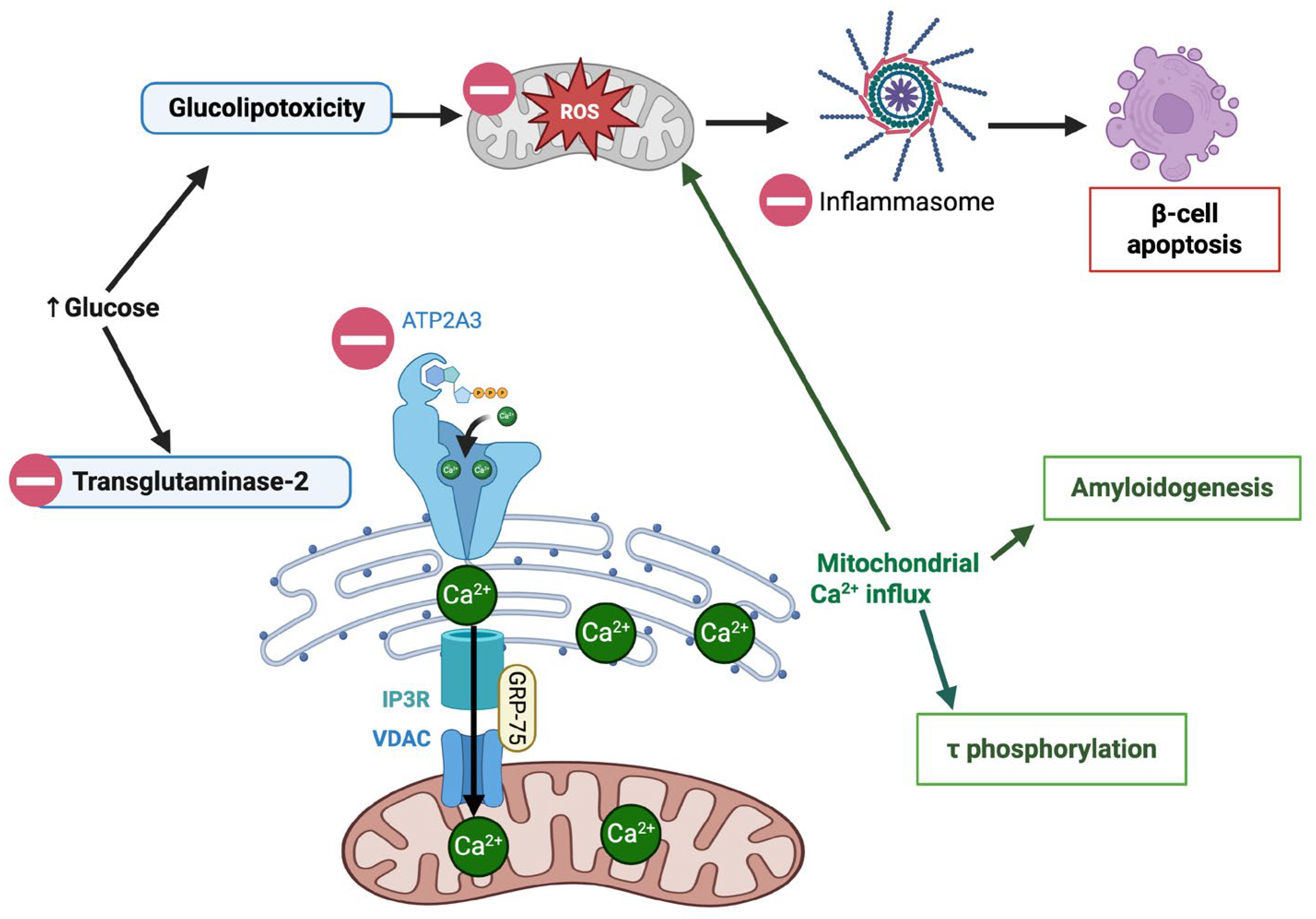
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/0v18m6c 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/0v18m6c 8 November 2025).
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/0v18m6c 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/0v18m6c 8 November 2025).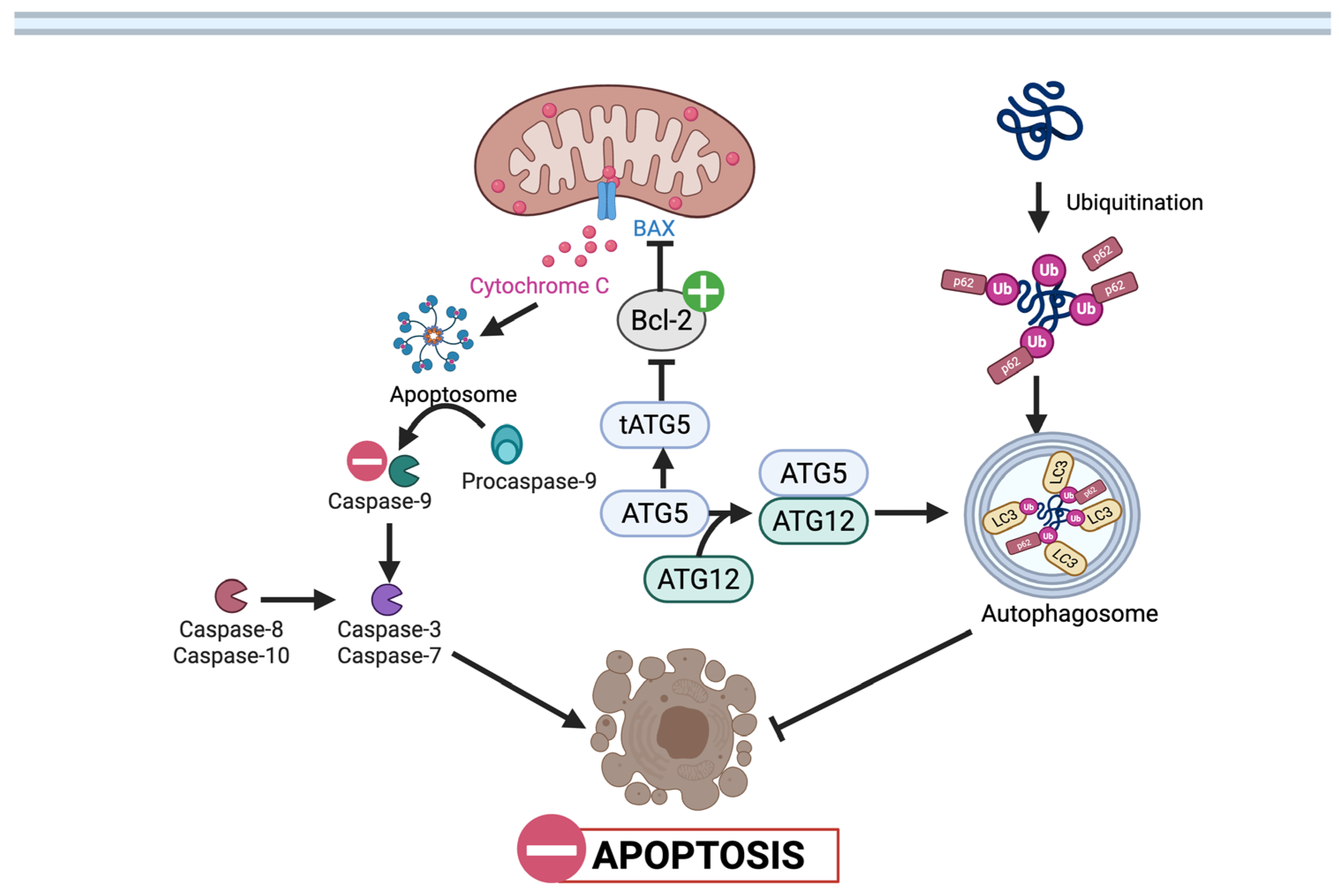
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/3w2bhrn 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/3w2bhrn 8 November 2025).
 : UA mediated stimulation,
: UA mediated stimulation,  : UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/3w2bhrn 8 November 2025).
: UA mediated inhibition, ┴: inhibition. (Created in BioRender. Busselberg, D. (2025) https://BioRender.com/3w2bhrn 8 November 2025).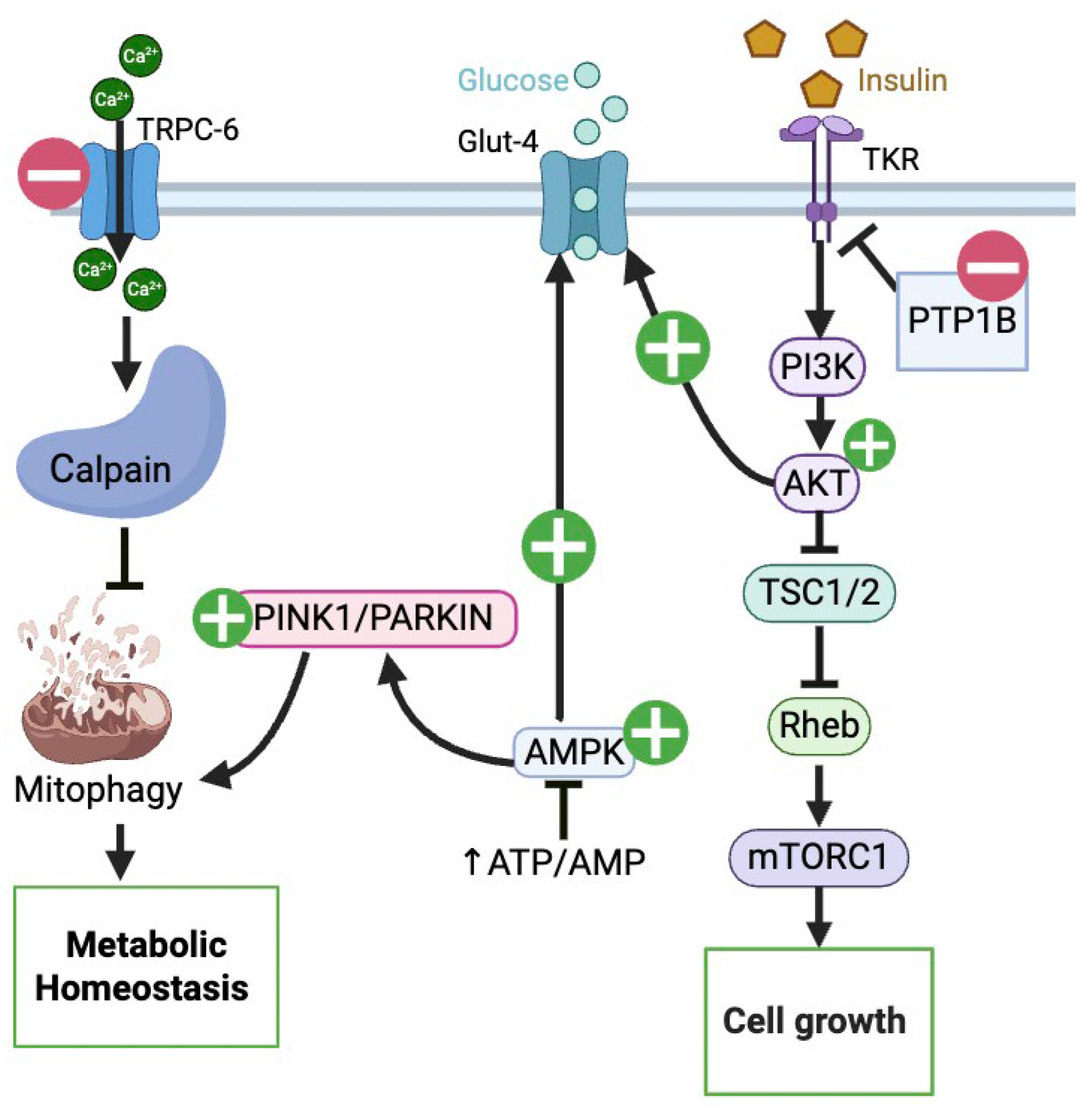
| Study | Model | UA Dose | Main Findings (↑ */↓ **) | Advantages | Limitations |
|---|---|---|---|---|---|
| González-Sarrías et al., 2015 [42] | Caco-2, SW480, HT-29 (human CRC) | 10 µM, 20 µM (with 5-FU) | ↓ IC50 of 5-FU/5′DFUR; chemosensitization | Multiple colon cancer cell lines; Use of concentrations achievable in the colorectum | No in vivo validation; limited to combo treatment (UA + 5-FU) |
| Zhao et al., 2018 [36] | SW620 (human CRC) | 0.05–15 µM | At sub-µM: ↑ Autophagy | Shows activity at sub-micromolar doses—suggests plausible colonic relevancy | Single CRC line only; no animal/human confirmation |
| Norden & Heiss (2019) [18] | HCT116 (human CRC) WT vs. p53−/− | IC50 ≈ 19 µM (72 h) | ↑ p53, ↑ p21; ↓ glycolysis (via p53/TIGAR) | Mechanistic insight into p53 dependency—potential biomarker for response | Effects depend on tumor p53 status; no in vivo or clinical data |
| El-Wetidy et al., 2021 [16] | HT-29, SW480, SW620 (human CRC) | IC50: ~25.5 µM HT-29; 38.1 µM SW480; 53.6 µM SW620 (at 48 h) | G2/M arrest; ↑ p53 & p21; ↓ Bcl-2; ↑ cytochrome-c release; ↑ caspase-3 activation; ↑ Reactive Oxygen Species (ROS) | Multiple cell lines tested. | No in vivo experiments; physiological relevance questionable |
| Ghosh et al., 2022 [21] | SW480, HCT116 (5-FU resistant lines); NRG mice xenografts; AOM/DSS mice | In vitro: 10–50 µM; In vivo: UroA 20–40 mg/kg PO (mice); 5-FU 20 mg/kg i.p | UA ± 5-FU: ↓ Viability, ↓ Invasion, ↓ EMT markers, ↓ drug transporters (MDR1, BCRP, MRP2/7); ↓ tumor growth in xenograft & AOM/DSS models | in vitro and in vivo model tested; immunocompetent and immunodeficient in vivo mouse models tested. | UAS03 is synthetic (not naturally produced by gut microbiota), so human applicability depends on safety/PK in humans. No direct patient data or clinical validation |
| González-Sarrías et al., 2009 [33] | Caco-2 (human) + rat colon (in situ buffer experiments) | 40 µM n = 3 | ↑ Phase I/Phase II detox enzymes (↑ CYP1A1, ↑ UGT1A10); inhibited sulfotransferases (favoring glucuronidation) | It compared in vitro (Caco-2), in situ (rat colon perfusion), and in vivo (rat feeding) models. | Inability to reproduce the in vitro findings in the in vivo animal colon, suggesting the in vitro model (compounds in buffer) lacks physiological relevance for this specific endpoint |
| González-Sarrías et al., 2014 [25] | Caco-2, SW480, HT-29 | 100 µM (aglycones & glucuronides tested) | S and G2/M arrest Uro-A most active. Glucuronidation: ↓ activity | Used multiple cell lines; it directly tested and compared the biological activity of urolithin aglycones against their glucuronide metabolites. | High concentrations; No in vivo testing |
| González-Sarrías et al., 2017 [26] | Caco-2 and CCD18-Co (normal colonic cells) | IC50 ≈50 µM Uro-A, ~70 µM IsoUro-A) | ↓ Proliferation, S/G2 arrest, ↑ Apoptosis at ~50–70 µM; conjugated forms inactive; higher glucuronidation in IsoUro-A | Compares isomeric forms and conjugation rates. | High concentrations; physiologic relevance uncertain; lack of in vivo validation |
| Giménez-Bastida et al., 2020 [19] | HCT116 (p53WT), Caco-2, HT-29 | 10 µM chronic exposure (up to >2 weeks) | ↑ cellular senescence (↑ p53/p21) and anti-clonogenic effects | Focus on long-term low-dose exposure (physiologically relevant colonic concentrations)—strong translational logic | No animal data; emphasis on senescence rather than cell death |
| Núñez-Sánchez et al., 2016 [43] | Caco-2 colonospheres; primary patient-derived CRC CSCs | Mixtures: C1 = 0.85 mM; C2 = 17 mM (UA ≈ 85% of mix) | ↓ colonosphere ↓ ALDH+ | Uses patient-derived CSCs (more clinically relevant) | Concentrations very high; unrealistic physiologic exposure; mixture study obscures single-compound effect; no in vivo data |
| Tortora et al., 2018 [27] | Pirc rats (APC+/− model) + CRC cell lines; ex vivo tissue | Diet-derived UA (pomegranate mesocarp)—in vitro: UA 25 µM + 2.5 mM sodium butyrate | ↓ precancerous lesions (MDF); ↑ apoptosis in lesions | Combines in vitro & in vivo evidence | UA effect shown only with co-treatment (butyrate/diet matrix); small n (rats: n = 4); Pirc model = FAP representation (not general CRC) |
| Tiwari et al., 2024 [28] | HT-29 (human colon adenocarcinoma) | IC50 ≈120 µM | ↓ Viability (IC50 ≈ 120 µM); ↑ p53/p21, ↑ caspase activation; ↓ Bcl-2, AKT1/2 | Detailed pathway predictions and in vitro validation | IC50 extremely high (120 µM)—far above realistic plasma levels; single cell line; no in vivo data |
| Scheme Molecule/Pathway | Effect in CRC | Effect in T2DM | References for CRC | References for T2DM |
|---|---|---|---|---|
| Wnt/β-catenin | In T-cells: ↑ In CRC cells: ↓ | In VSMC: ↓ In β-cells: ↑ | [21,38] | [22,49,54] |
| Apoptosis (p53, p21, caspases, Bax/Bcl-2) | In C.R.C cells: ↑ | In β-cells: ↓ Podocytes: ↓ | [16,18,28] | [48,50] |
| Autophagy & Mitophagy (PINK1/Parkin, ULK1, LC3) | In T cells: ↑ | In β-cells: ↑ In Podocytes: ↑ In EPC: ↑ | [36,38] | [48,51,56] |
| Inflammation (TLR4/Myd88/NLRP3, cytokines) | In CRC cells: ↓ IL-6, ↓ TNF-α | In Retinal cells: ↓ IL-6, ↓ IL-1β, and ↓ TNF-α In Gut/Brain cells: ↓ TLR4/Myd88, ↓ NLRP-3 | [21] | [51,58,59] |
| Oxidative Stress (ROS, Nrf2/HO-1) | In CRC cells: ↓ R.O.S, MDA, ↑ S.O.D. | In β-cells: ↓ MDA, ↓ GSH In retinal cells: ↑ Nrf2/HO-1 | [75] | [49,58] |
| Aryl Hydrocarbon Receptor (AhR) pathway | In colonic cells: ∴↑ gut barrier integrity | In neurons: ↑ ∴↑ neuroprotection | [76] | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, V.; Hornak, S.; Kubatka, P.; Büsselberg, D. Microbial Metabolite, Macro Impact: Urolithin A in the Nexus of Insulin Resistance and Colorectal Tumorigenesis. Nutrients 2025, 17, 3712. https://doi.org/10.3390/nu17233712
Joseph V, Hornak S, Kubatka P, Büsselberg D. Microbial Metabolite, Macro Impact: Urolithin A in the Nexus of Insulin Resistance and Colorectal Tumorigenesis. Nutrients. 2025; 17(23):3712. https://doi.org/10.3390/nu17233712
Chicago/Turabian StyleJoseph, Vennila, Slavomir Hornak, Peter Kubatka, and Dietrich Büsselberg. 2025. "Microbial Metabolite, Macro Impact: Urolithin A in the Nexus of Insulin Resistance and Colorectal Tumorigenesis" Nutrients 17, no. 23: 3712. https://doi.org/10.3390/nu17233712
APA StyleJoseph, V., Hornak, S., Kubatka, P., & Büsselberg, D. (2025). Microbial Metabolite, Macro Impact: Urolithin A in the Nexus of Insulin Resistance and Colorectal Tumorigenesis. Nutrients, 17(23), 3712. https://doi.org/10.3390/nu17233712








