Association of Mediterranean Diet Scores with Psychological Distress in Pregnancy: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
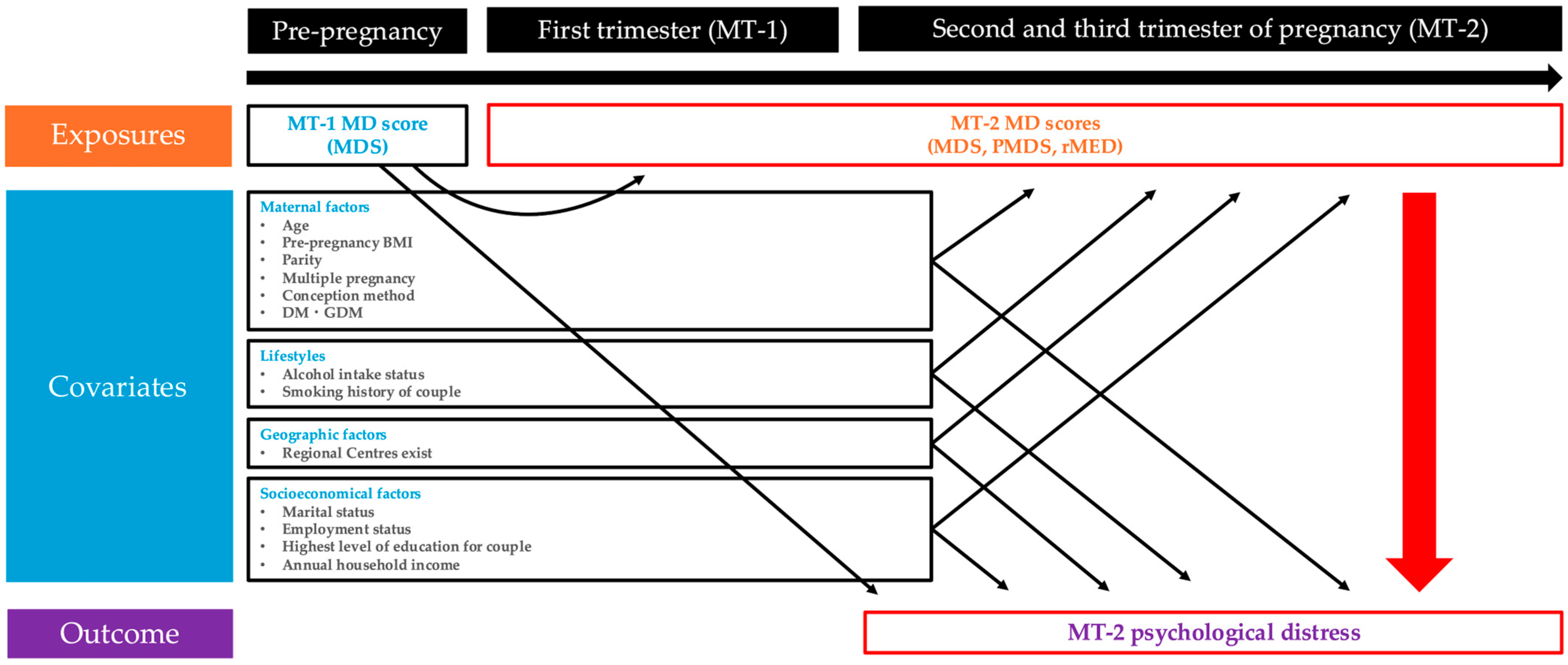
2.1. Study Design
2.2. Exposure (MD Score)
2.3. Outcome (Psychological Distress)
2.4. Collection and Classification of Other Variables
2.5. Statistical Analysis
3. Results
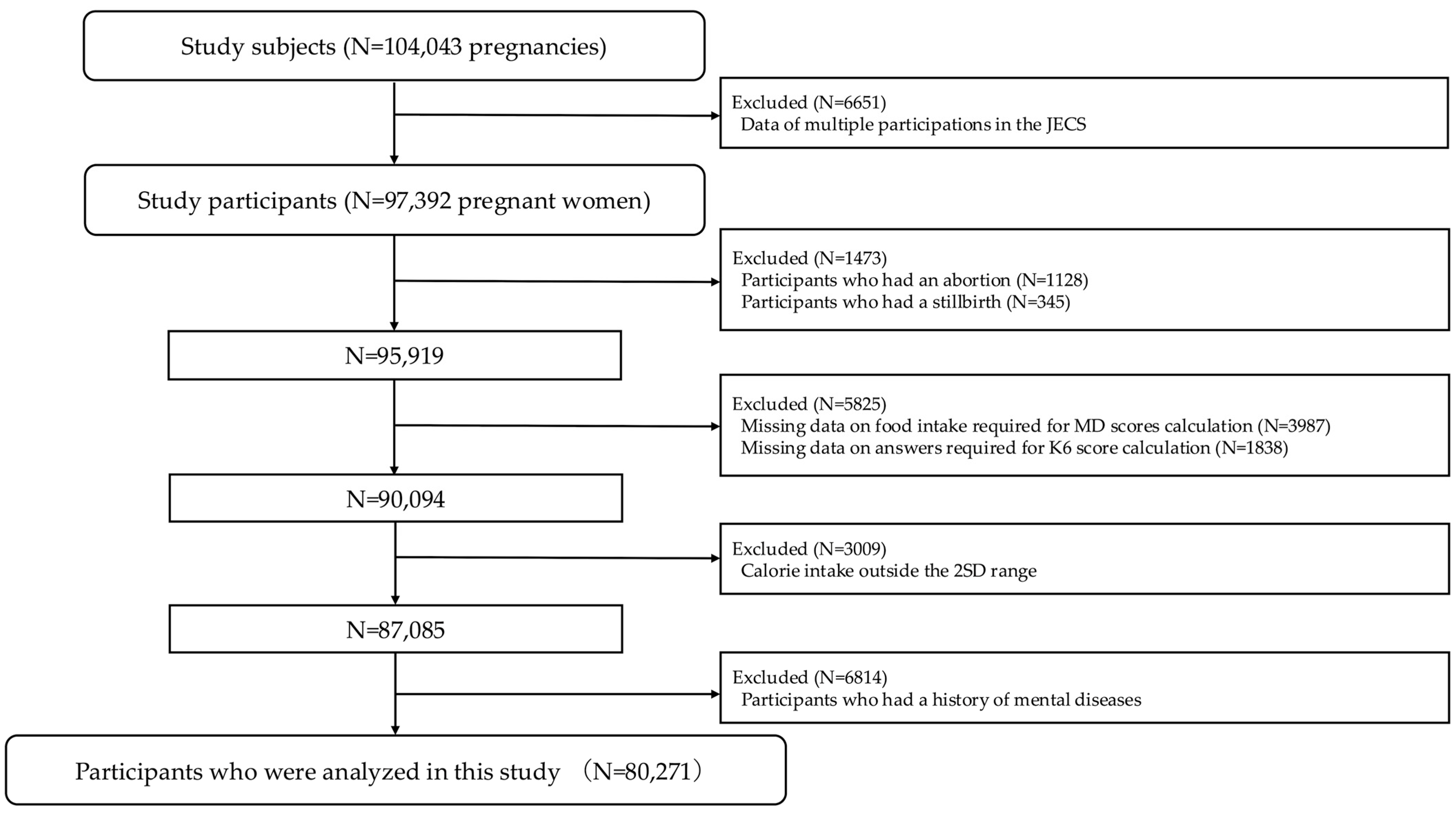
3.1. Participant Selection
3.2. Characteristics of the Study Participants
3.3. Main Results
3.3.1. Association Between MD Scores and K6 Points
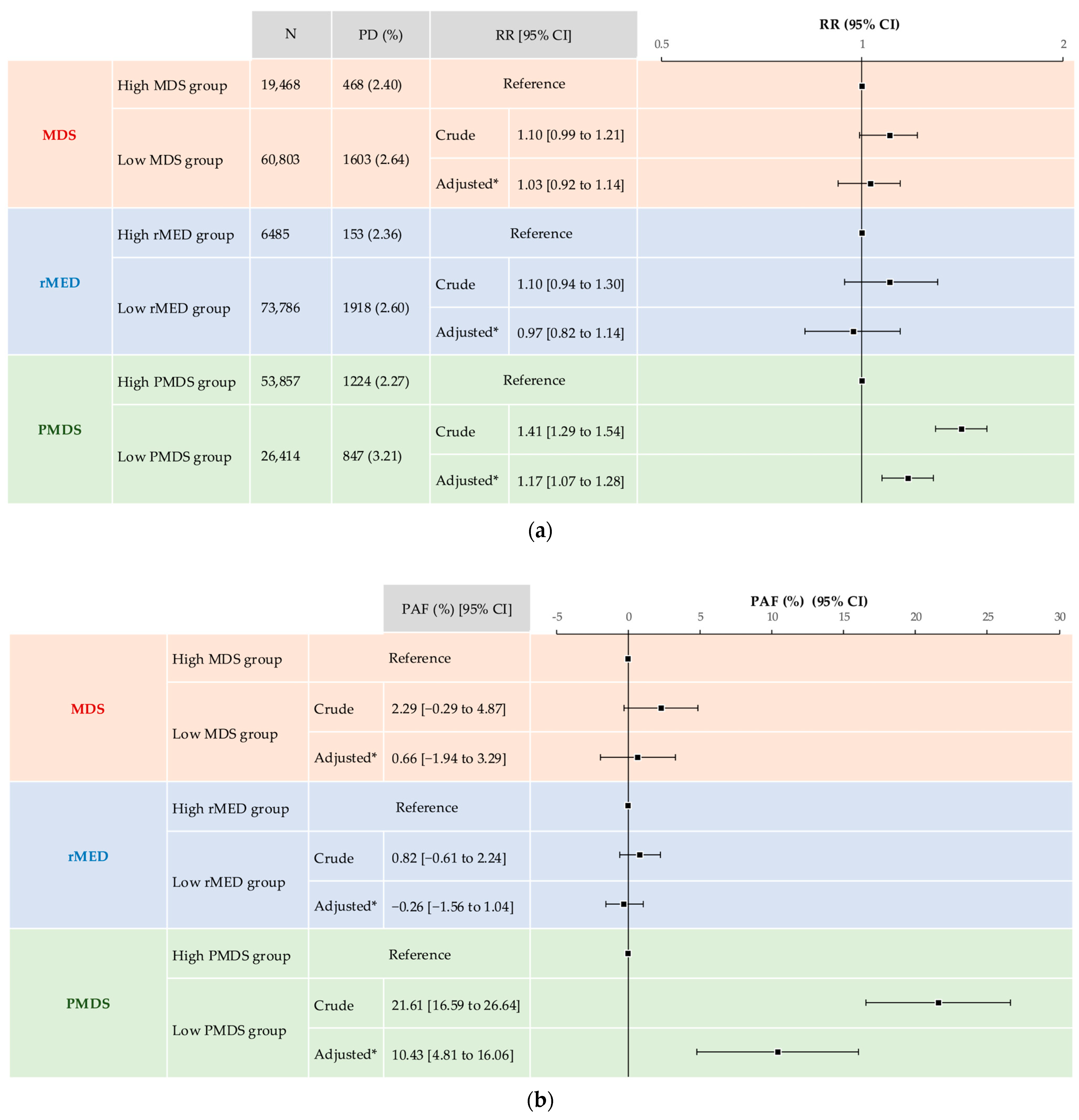
3.3.2. Association Between MD Scores and Psychological Distress
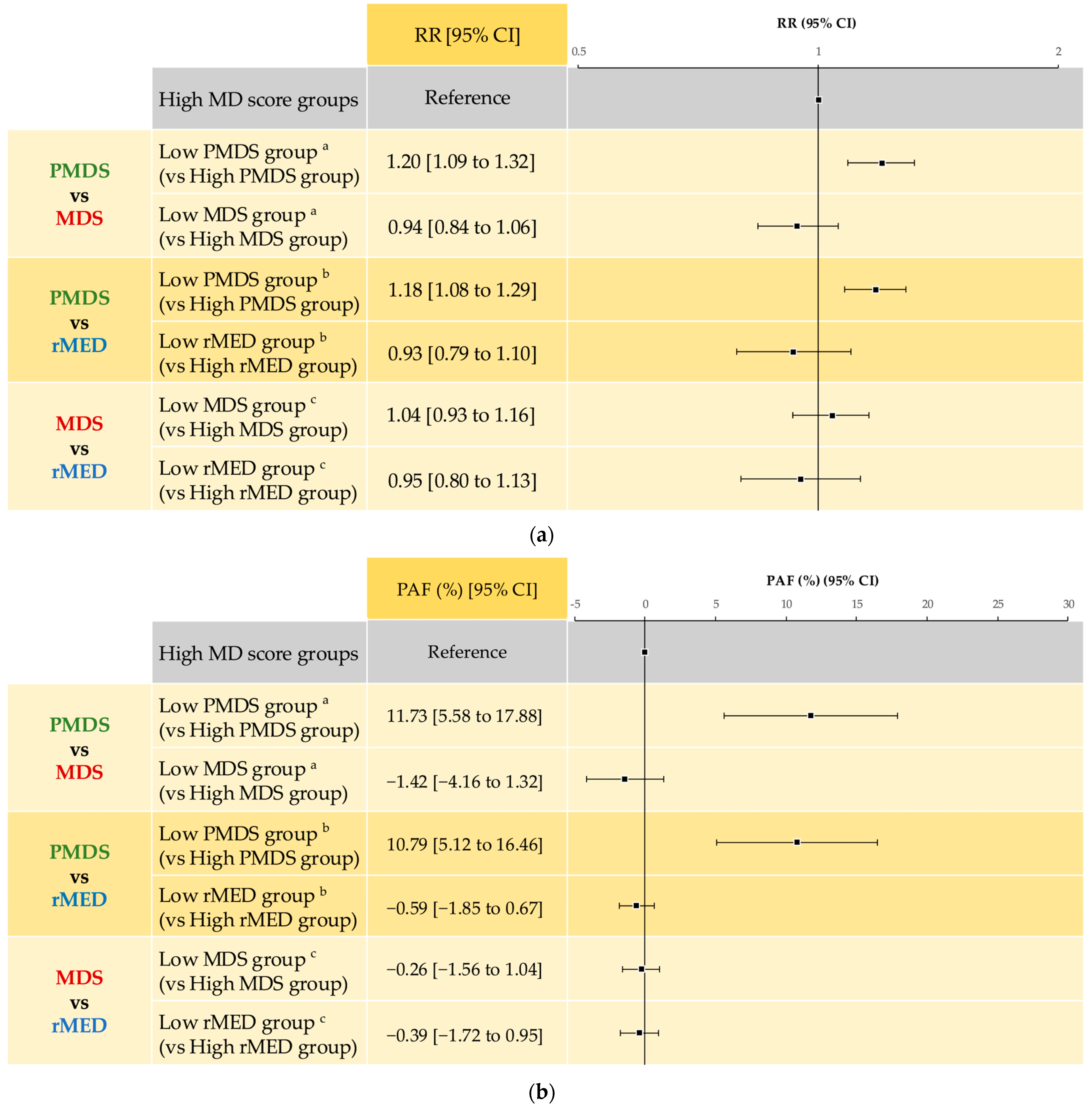
3.3.3. Comparison of Each MD Score in Psychological Distress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Orr, S.T.; James, S.A.; Blackmore Prince, C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am. J. Epidemiol. 2002, 156, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.; Creveuil, C.; Marks, M.N.; Conroy, S.; Herlicoviez, M.; Dreyfus, M.; Tordjman, S. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosom. Med. 2006, 68, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.; Ortenstrand, A.; Hjelmstedt, A. Antenatal depressive symptoms and preterm birth: A prospective study of a Swedish national sample. Birth 2011, 38, 10–16. [Google Scholar] [CrossRef]
- Straub, H.; Adams, M.; Kim, J.J.; Silver, R.K. Antenatal depressive symptoms increase the likelihood of preterm birth. Am. J. Obstet. Gynecol. 2012, 207, 329.e1–329.e4. [Google Scholar] [CrossRef]
- Neggers, Y.; Goldenberg, R.; Cliver, S.; Hauth, J. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet. Gynecol. Scand. 2006, 85, 277–285. [Google Scholar] [CrossRef]
- Rahman, A.; Bunn, J.; Lovel, H.; Creed, F. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr. Scand. 2007, 115, 481–486. [Google Scholar] [CrossRef]
- Kurki, T.; Hiilesmaa, V.; Raitasalo, R.; Mattila, H.; Ylikorkala, O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet. Gynecol. 2000, 95, 487–490. [Google Scholar] [CrossRef]
- Field, T. Prenatal depression effects on early development: A review. Infant Behav. Dev. 2011, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.G.; Heron, J.; Golding, J.; Glover, V. Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. J. Child Psychol. Psychiatry 2003, 44, 1025–1036. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Ben-Shlomo, Y.; Heron, J.; Golding, J.; Adams, D.; Glover, V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol. Psychiatry 2005, 58, 211–217. [Google Scholar] [CrossRef]
- Sharp, H.; Hill, J.; Hellier, J.; Pickles, A. Maternal antenatal anxiety, postnatal stroking and emotional problems in children: Outcomes predicted from pre- and postnatal programming hypotheses. Psychol. Med. 2015, 45, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Dietz, P.M.; Williams, S.B.; Callaghan, W.M.; Bachman, D.J.; Whitlock, E.P.; Hornbrook, M.C. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am. J. Psychiatry 2007, 164, 1515–1520. [Google Scholar] [CrossRef]
- Kitamura, T.; Yoshida, K.; Okano, T.; Kinoshita, K.; Hayashi, M.; Toyoda, N.; Ito, M.; Kudo, N.; Tada, K.; Kanazawa, K.; et al. Multicentre prospective study of perinatal depression in Japan: Incidence and correlates of antenatal and postnatal depression. Arch. Womens Ment. Health 2006, 9, 121–130. [Google Scholar] [CrossRef]
- Leigh, B.; Milgrom, J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry 2008, 8, 24. [Google Scholar] [CrossRef]
- Robertson, E.; Grace, S.; Wallington, T.; Stewart, D.E. Antenatal risk factors for postpartum depression: A synthesis of recent literature. Gen. Hosp. Psychiatry 2004, 26, 289–295. [Google Scholar] [CrossRef]
- Kokubu, M.; Okano, T.; Sugiyama, T. Postnatal depression, maternal bonding failure, and negative attitudes towards pregnancy: A longitudinal study of pregnant women in Japan. Arch. Womens Ment. Health 2012, 15, 211–216. [Google Scholar] [CrossRef]
- Kitamura, T.; Ohashi, Y.; Kita, S.; Haruna, M.; Kubo, R. Depressive mood, bonding failure, and abusive parenting among mothers with three-month-old babies in a Japanese community. Open J. Psychiatry 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Chong, M.F.; Wong, J.X.; Colega, M.; Chen, L.W.; van Dam, R.M.; Tan, C.S.; Lim, A.L.; Cai, S.; Broekman, B.F.; Lee, Y.S.; et al. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J. Psychiatr. Res. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- da Rocha, C.M.; Kac, G. High dietary ratio of omega-6 to omega-3 polyunsaturated acids during pregnancy and prevalence of post-partum depression. Matern. Child Nutr. 2012, 8, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Fish and fat intake and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Psychiatr. Res. 2013, 47, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Seaweed consumption and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. BMC Pregnancy Childbirth 2014, 14, 301. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Dietary patterns and depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Affect. Disord. 2018, 225, 552–558. [Google Scholar] [CrossRef]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 1: Micronutrients. J. Nutr. Health Aging 2006, 10, 377–385. [Google Scholar]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Barkoukis, H. Importance of understanding food consumption patterns. J. Am. Diet. Assoc. 2007, 107, 234–236. [Google Scholar] [CrossRef]
- Wirfält, E.; Drake, I.; Wallström, P. What do review papers conclude about food and dietary patterns? Food Nutr. Res. 2013, 57, 20523. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. UNESCO Intangible Heritage Lists: Mediterranean Diet. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 30 October 2025).
- Whalen, K.A.; Judd, S.; McCullough, M.L.; Flanders, W.D.; Hartman, T.J.; Bostick, R.M. Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with All-Cause and Cause-Specific Mortality in Adults. J. Nutr. 2017, 147, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. Mediterranean diet and type 2 diabetes. Diabetes/Metab. Res. Rev. 2014, 30 (Suppl. S1), 34–40. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Casas, I.; Nakaki, A.; Pascal, R.; Castro-Barquero, S.; Youssef, L.; Genero, M.; Benitez, L.; Larroya, M.; Boutet, M.L.; Casu, G.; et al. Effects of a Mediterranean Diet Intervention on Maternal Stress, Well-Being, and Sleep Quality throughout Gestation—The IMPACT-BCN Trial. Nutrients 2023, 15, 2362. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Baena-Garcia, L.; Migueles, J.H.; Henriksson, P.; Lof, M.; Aparicio, V.A. Associations of Mediterranean diet with psychological ill-being and well-being throughout the pregnancy course: The GESTAFIT project. Qual. Life Res. 2022, 31, 2705–2716. [Google Scholar] [CrossRef]
- Jacovides, C.; Papadopoulou, S.K.; Pavlidou, E.; Dakanalis, A.; Alexatou, O.; Vorvolakos, T.; Lechouritis, E.; Papacosta, E.; Chrysafi, M.; Mitsiou, M.; et al. Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 2096. [Google Scholar] [CrossRef]
- Oddo, V.M.; Moise, C.; Welke, L.; Bernabe, B.P.; Maki, P.; Koenig, M.D.; Pezley, L.; Xia, Y.; Tussing-Humphreys, L. Mediterranean Diet Adherence and Depressive Symptoms among a Nationally Representative Sample of Pregnant Women in the United States. J. Nutr. 2023, 153, 3041–3048. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Pavlidou, E.; Dakanalis, A.; Antasouras, G.; Vorvolakos, T.; Mentzelou, M.; Serdari, A.; Pandi, A.L.; Spanoudaki, M.; Alexatou, O.; et al. Postpartum Depression Is Associated with Maternal Sociodemographic and Anthropometric Characteristics, Perinatal Outcomes, Breastfeeding Practices, and Mediterranean Diet Adherence. Nutrients 2023, 15, 3853. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, P.; Amerikanou, C.; Vezou, C.; Gioxari, A.; Kaliora, A.C.; Skouroliakou, M. Improving Adherence to the Mediterranean Diet in Early Pregnancy Using a Clinical Decision Support System; A Randomised Controlled Clinical Trial. Nutrients 2023, 15, 432. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Tampourlou, M.; Chrysohoou, C.; Nomikos, T.; Antonopoulou, S.; Stefanadis, C. The association between adherence to the Mediterranean diet and adiponectin levels among healthy adults: The ATTICA study. J. Nutr. Biochem. 2010, 21, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef]
- Nakano, K.; Kuraoka, S.; Oda, M.; Ohba, T.; Mitsubuchi, H.; Nakamura, K.; Katoh, T.; the Japan Environment and Children’s Study (JECS) Group. Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study. Nutrients 2023, 15, 1772. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef]
- Kessler, R.C.; Andrews, G.; Colpe, L.J.; Hiripi, E.; Mroczek, D.K.; Normand, S.L.; Walters, E.E.; Zaslavsky, A.M. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol. Med. 2002, 32, 959–976. [Google Scholar] [CrossRef]
- Kessler, R.C.; Barker, P.R.; Colpe, L.J.; Epstein, J.F.; Gfroerer, J.C.; Hiripi, E.; Howes, M.J.; Normand, S.L.; Manderscheid, R.W.; Walters, E.E.; et al. Screening for serious mental illness in the general population. Arch. Gen. Psychiatry 2003, 60, 184–189. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Kawakami, N.; Saitoh, M.; Ono, Y.; Nakane, Y.; Nakamura, Y.; Tachimori, H.; Iwata, N.; Uda, H.; Nakane, H.; et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int. J. Methods Psychiatr. Res. 2008, 17, 152–158. [Google Scholar] [CrossRef]
- Hayasaka, K.; Tomata, Y.; Aida, J.; Watanabe, T.; Kakizaki, M.; Tsuji, I. Tooth loss and mortality in elderly Japanese adults: Effect of oral care. J. Am. Geriatr. Soc. 2013, 61, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, A.; Kuriyama, S.; Nakaya, N.; Ohmori-Matsuda, K.; Kakizaki, M.; Sone, T.; Nagai, M.; Sugawara, Y.; Nitta, A.; Tomata, Y.; et al. Green tea consumption is associated with lower psychological distress in a general population: The Ohsaki Cohort 2006 Study. Am. J. Clin. Nutr. 2009, 90, 1390–1396. [Google Scholar] [CrossRef]
- Nakaya, N.; Kogure, M.; Saito-Nakaya, K.; Tomata, Y.; Sone, T.; Kakizaki, M.; Tsuji, I. The association between self-reported history of physical diseases and psychological distress in a community-dwelling Japanese population: The Ohsaki Cohort 2006 Study. Eur. J. Public Health 2014, 24, 45–49. [Google Scholar] [CrossRef]
- Watanabe, Z.; Iwama, N.; Nishigori, H.; Nishigori, T.; Mizuno, S.; Sakurai, K.; Ishikuro, M.; Obara, T.; Tatsuta, N.; Nishijima, I.; et al. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: The Japan Environment and Children’s Study. J. Affect. Disord. 2016, 190, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- SAS Institute Inc. 58775-Estimating Nonlinear Combinations of Model Parameters. Available online: https://support.sas.com/kb/58/775.html (accessed on 24 April 2025).
- Lin, C.K.; Chen, S.T. Estimation and application of population attributable fraction in ecological studies. Environ. Health 2019, 18, 52. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Altman, D.G. Population attributable fraction. BMJ 2018, 360, k757. [Google Scholar] [CrossRef]
- Arch, J.J. Pregnancy-specific anxiety: Which women are highest and what are the alcohol-related risks? Compr. Psychiatry 2013, 54, 217–228. [Google Scholar] [CrossRef]
- Henderson, J.; Redshaw, M. Anxiety in the perinatal period: Antenatal and postnatal influences and women’s experience of care. J. Reprod. Infant Psychol. 2013, 31, 465–478. [Google Scholar] [CrossRef]
- Madhavanprabhakaran, G.K.; D’Souza, M.S.; Nairy, K.S. Prevalence of pregnancy anxiety and associated factors. Int. J. Afr. Nurs. Sci. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Mazzolani, B.C.; Smaira, F.I.; Esteves, G.P.; Santo André, H.C.; Amarante, M.C.; Castanho, D.; Campos, K.; Benatti, F.B.; Pinto, A.J.; Roschel, H.; et al. Influence of Body Mass Index on Eating Habits and Food Choice Determinants Among Brazilian Women During the COVID-19 Pandemic. Front. Nutr. 2021, 8, 664240. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.H.; Huang, C.L.; French, S.A. Factors associated with women’s and children’s body mass indices by income status. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 536–542. [Google Scholar] [CrossRef]
- Lynn, F.A.; Alderdice, F.A.; Crealey, G.E.; McElnay, J.C. Associations between maternal characteristics and pregnancy-related stress among low-risk mothers: An observational cross-sectional study. Int. J. Nurs. Stud. 2011, 48, 620–627. [Google Scholar] [CrossRef]
- Alipour, Z.; Lamyian, M.; Hajizadeh, E. Anxiety and fear of childbirth as predictors of postnatal depression in nulliparous women. Women Birth 2012, 25, e37–e43. [Google Scholar] [CrossRef]
- Fisher, J.; Stocky, A. Maternal perinatal mental health and multiple births: Implications for practice. Twin Res. 2003, 6, 506–513. [Google Scholar] [CrossRef]
- Otani-Matsuura, A.; Sugiura-Ogasawara, M.; Ebara, T.; Matsuki, T.; Tamada, H.; Yamada, Y.; Omori, T.; Kato, S.; Kano, H.; Kaneko, K.; et al. Depression symptoms during pregnancy and postpartum in patients with recurrent pregnancy loss and infertility: The Japan environment and children’s study. J. Reprod. Immunol. 2022, 152, 103659. [Google Scholar] [CrossRef]
- Brown, H.K.; Cairncross, Z.F.; Lipscombe, L.L.; Wilton, A.S.; Dennis, C.L.; Ray, J.G.; Guttmann, A.; Vigod, S.N. Prepregnancy Diabetes and Perinatal Mental Illness: A Population-Based Latent Class Analysis. Am. J. Epidemiol. 2020, 189, 573–582. [Google Scholar] [CrossRef]
- Lee, K.W.; Ching, S.M.; Devaraj, N.K.; Chong, S.C.; Lim, S.Y.; Loh, H.C.; Abdul Hamid, H. Diabetes in Pregnancy and Risk of Antepartum Depression: A Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2020, 17, 3767. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, C.; Colpe, L.; Hoffman, V.; Warren, L.K. Prevalence of serious psychological distress and mental health treatment in a national sample of pregnant and postpartum women. Matern. Child Health J. 2015, 19, 204–216. [Google Scholar] [CrossRef]
- Zuckerman, B.; Amaro, H.; Bauchner, H.; Cabral, H. Depressive symptoms during pregnancy: Relationship to poor health behaviors. Am. J. Obstet. Gynecol. 1989, 160, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Daalderop, L.A.; Lagendijk, J.; Steegers, E.A.P.; El Marroun, H.; Posthumus, A.G. Psychological distress during pregnancy and adverse maternal and perinatal health outcomes: The role of socioeconomic status. Int. J. Gynaecol. Obstet. 2023, 163, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Bedaso, A.; Adams, J.; Peng, W.; Xu, F.; Sibbritt, D. An examination of the association between marital status and prenatal mental disorders using linked health administrative data. BMC Pregnancy Childbirth 2022, 22, 735. [Google Scholar] [CrossRef]
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; De Graaf, R.; Demyttenaere, K.; Gasquet, I.; De Girolamo, G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007, 6, 168–176. [Google Scholar]
- Cucó, G.; Fernández-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef]
- van der Loo, M. Package ‘Simputation’. Available online: https://cran.r-project.org/web/packages/simputation/simputation.pdf (accessed on 30 April 2025).
- National Tax Agency Japan. 2023 Survey of Private Sector Salaries in Japan; National Tax Agency: Tokyo, Japan, 2024. [Google Scholar]
- Gao, S.Y.; Wu, Q.J.; Sun, C.; Zhang, T.N.; Shen, Z.Q.; Liu, C.X.; Gong, T.T.; Xu, X.; Ji, C.; Huang, D.H.; et al. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: A systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 2018, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Wang, L.; Gao, L.; Xu, B.; Xiong, Y. Selective Serotonin Reuptake Inhibitors (SSRIs) and the Risk of Congenital Heart Defects: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2015, 4, 001681. [Google Scholar] [CrossRef] [PubMed]
- Levinson-Castiel, R.; Merlob, P.; Linder, N.; Sirota, L.; Klinger, G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch. Pediatr. Adolesc. Med. 2006, 160, 173–176. [Google Scholar] [CrossRef]
- Grigoriadis, S.; Vonderporten, E.H.; Mamisashvili, L.; Tomlinson, G.; Dennis, C.L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; Ross, L.E. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: Systematic review and meta-analysis. BMJ 2014, 348, f6932. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X.; Sim, W.S.; Lim, D.Y.; Yeo, W.S. Selective Serotonin Reuptake Inhibitors and Persistent Pulmonary Hypertension of the Newborn: An Update Meta-Analysis. J. Womens Health 2019, 28, 331–338. [Google Scholar] [CrossRef]
- Masarwa, R.; Bar-Oz, B.; Gorelik, E.; Reif, S.; Perlman, A.; Matok, I. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: A systematic review, meta-analysis, and network meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 57.e1–57.e13. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Intake of dairy products and calcium and prevalence of depressive symptoms during pregnancy in Japan: A cross-sectional study. BJOG 2015, 122, 336–343. [Google Scholar] [CrossRef]
- Deutschenbaur, L.; Beck, J.; Kiyhankhadiv, A.; Mühlhauser, M.; Borgwardt, S.; Walter, M.; Hasler, G.; Sollberger, D.; Lang, U.E. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 325–333. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants: Fact or fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E., Jr.; Marangell, L.B.; Richardson, A.J.; Lake, J.; et al. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
| Variables | All Participants N = 80,271 | MDS | rMED | PMDS | |||
|---|---|---|---|---|---|---|---|
| Low MDS N = 60,803 | High MDS N = 19,468 | Low rMED N = 73,786 | High rMED N = 6485 | Low PMDS N = 26,414 | High PMDS N = 53,857 | ||
| Age at MT-1, y | 31 (27, 34) | 31 (27, 34) | 31 (28, 35) | 31 (27, 34) | 32 (29, 35) | 30 (26, 33) | 31 (28, 35) |
| Age at MT-1, N (%) | |||||||
| <20 years | 905 (1.1) | 730 (1.2) | 175 (0.9) | 865 (1.2) | 40 (0.6) | 504 (1.9) | 401 (0.7) |
| 20–34.9 years | 59,906 (74.6) | 45,853 (75.4) | 14,053 (72.2) | 55,491 (75.2) | 4415 (68.1) | 20,877 (79.0) | 39,029 (72.5) |
| ≥35 years | 19,446 (24.2) | 14,209 (23.4) | 5237 (26.9) | 17,416 (23.6) | 2030 (31.3) | 5029 (19.0) | 14,417 (26.8) |
| Missing | 14 (0.02) | 11 (0.02) | 3 (0.02) | 14 (0.02) | 0 (0.0) | 4 (0.02) | 10 (0.02) |
| Pre-pregnancy BMI, kg/m2 | 20.5 (19.1, 22.5) | 20.5 (19.1, 22.5) | 20.5 (19.1, 22.5) | 20.5 (19.1, 22.5) | 20.5 (19.1, 22.6) | 20.5 (19.1, 22.6) | 20.5 (19.1, 22.4) |
| Pre-pregnancy BMI, N (%) | |||||||
| Underweight (<18.5 kg/m2) | 12,919 (16.1) | 9699 (16.0) | 3220 (16.5) | 11,875 (16.1) | 1044 (16.1) | 4427 (16.8) | 8492 (15.8) |
| Normal range (18.5–24.9 kg/m2) | 58,972 (73.5) | 44,807 (73.7) | 14,165 (72.8) | 54,264 (73.5) | 4708 (72.6) | 18,921 (71.6) | 40,051 (74.4) |
| Overweight (≥25.0 kg/m2) | 8347 (10.4) | 6270 (10.3) | 2077 (10.7) | 7616 (10.3) | 731 (11.3) | 3053 (11.6) | 5294 (9.8) |
| Missing | 33 (0.04) | 27 (0.04) | 6 (0.03) | 31 (0.04) | 2 (0.03) | 13 (0.05) | 20 (0.04) |
| GWG, kg | 8.2 (6.0, 10.4) | 8.2 (6.1, 10.5) | 8.1 (6.0, 10.3) | 8.2 (6.1, 10.5) | 7.9 (5.8, 10.0) | 8.3 (6.1, 10.7) | 8.1 (6.0, 10.3) |
| Parity, N (%) | |||||||
| Multiparity | 44,851 (55.9) | 32,918 (54.1) | 11,933 (61.3) | 40,956 (55.5) | 3895 (60.1) | 14,020 (53.1) | 30,831 (57.2) |
| Nulliparity | 33,427 (41.6) | 26,320 (43.3) | 7107 (36.5) | 30,985 (42.0) | 2442 (37.7) | 11,720 (44.4) | 21,707 (40.3) |
| Missing | 1993 (2.5) | 1565 (2.6) | 428 (2.2) | 1845 (2.5) | 148 (2.3) | 674 (2.6) | 1319 (2.4) |
| Multiple pregnancy, N (%) | |||||||
| Singleton | 79,480 (99.0) | 60,202 (99.0) | 19,278 (99.0) | 73,075 (99.0) | 6405 (98.8) | 26,176 (99.1) | 53,304 (99.0) |
| Multiple | 791 (1.0) | 601 (1.0) | 190 (1.0) | 711 (1.0) | 80 (1.2) | 238 (0.9) | 553 (1.0) |
| Conception method, N (%) | |||||||
| Spontaneous pregnancy | 74,307 (92.6) | 56,335 (92.7) | 17,972 (92.3) | 68,401 (92.7) | 5906 (91.1) | 24,950 (94.5) | 49,357 (91.6) |
| Non-ART | 3058 (3.8) | 2336 (3.8) | 722 (3.7) | 2784 (3.8) | 274 (4.2) | 794 (3.0) | 2264 (4.2) |
| ART | 2549 (3.2) | 1860 (3.1) | 689 (3.5) | 2267 (3.1) | 282 (4.3) | 560 (2.1) | 1989 (3.7) |
| Missing | 357 (0.4) | 272 (0.4) | 85 (0.4) | 334 (0.5) | 23 (0.4) | 110 (0.4) | 247 (0.5) |
| DM/GDM, N (%) | 2350 (2.9) | 1769 (2.9) | 581 (3.0) | 2141 (2.9) | 209 (3.2) | 785 (3.0) | 1565 (2.9) |
| Missing | 377 (0.5) | 290 (0.5) | 87 (0.4) | 348 (0.5) | 29 (0.4) | 122 (0.5) | 255 (0.5) |
| Alcohol intake status, N (%) | |||||||
| Never or Quit drinking before | 71,856 (89.5) | 54,611 (89.8) | 17,245 (88.6) | 66,108 (89.6) | 5748 (88.6) | 23,865 (90.3) | 47,991 (89.1) |
| Continue drinking | 8090 (10.1) | 5931 (9.8) | 2159 (11.1) | 7370 (10.0) | 720 (11.1) | 2406 (9.1) | 5684 (10.6) |
| Missing | 325 (0.4) | 261 (0.4) | 64 (0.3) | 308 (0.4) | 17 (0.3) | 143 (0.5) | 182 (0.3) |
| Smoking history, N (%) | |||||||
| Maternal | |||||||
| Not currently smoking | 76,257 (95.0) | 57,711 (94.9) | 18,546 (95.3) | 70,000 (94.9) | 6257 (96.5) | 24,516 (92.8) | 51,741 (96.1) |
| Currently smoking | 3462 (4.3) | 2660 (4.4) | 802 (4.1) | 3270 (4.4) | 192 (3.0) | 1674 (6.3) | 1788 (3.3) |
| Missing | 552 (0.7) | 432 (0.7) | 120 (0.6) | 516 (0.7) | 36 (0.6) | 224 (0.8) | 328 (0.6) |
| Paternal | |||||||
| Not currently smoking | 42,148 (52.5) | 31,811 (52.3) | 10,337 (53.1) | 38,350 (52.0) | 3798 (58.6) | 12,287 (46.5) | 29,861 (55.4) |
| Currently smoking | 36,603 (45.6) | 27,818 (45.8) | 8785 (45.1) | 34,023 (46.1) | 2580 (39.8) | 13,488 (51.1) | 23,115 (42.9) |
| Missing | 1520 (1.9) | 1174 (1.9) | 346 (1.8) | 1413 (1.9) | 107 (1.6) | 639 (2.4) | 881 (1.6) |
| Marital status, N (%) | |||||||
| Married | 76,485 (95.3) | 57,818 (95.1) | 18,667 (95.9) | 70,213 (95.2) | 6272 (96.7) | 24,620 (93.2) | 51,865 (96.3) |
| Unmarried | 2846 (3.5) | 2227 (3.7) | 619 (3.2) | 2682 (3.6) | 164 (2.5) | 1334 (5.1) | 1512 (2.8) |
| Divorced or widowed | 611 (0.8) | 489 (0.8) | 122 (0.6) | 575 (0.8) | 36 (0.6) | 308 (1.2) | 303 (0.6) |
| Missing | 329 (0.4) | 269 (0.4) | 60 (0.3) | 316 (0.4) | 13 (0.2) | 152 (0.6) | 177 (0.3) |
| Employment status, N (%) | |||||||
| Not working | 28,108 (35.0) | 21,122 (34.7) | 6986 (35.9) | 25,806 (35.0) | 2302 (35.5) | 8740 (33.1) | 19,368 (36.0) |
| Working | 49,637 (61.8) | 37,764 (62.1) | 11,873 (61.0) | 45,673 (61.9) | 3964 (61.1) | 16,830 (63.7) | 32,807 (60.9) |
| Missing | 2526 (3.1) | 1917 (3.2) | 609 (3.1) | 2307 (3.1) | 219 (3.4) | 844 (3.2) | 1682 (3.1) |
| Highest education level, N (%) | |||||||
| Maternal | |||||||
| Junior high school | 3446 (4.3) | 2669 (4.4) | 777 (4.0) | 3256 (4.4) | 190 (2.9) | 1682 (6.4) | 1764 (3.3) |
| High school | 24,799 (30.9) | 18,902 (31.1) | 5897 (30.3) | 23,140 (31.4) | 1659 (25.6) | 9664 (36.6) | 15,135 (28.1) |
| College | 51,759 (64.5) | 39,026 (64.2) | 12,733 (65.4) | 47,152 (63.9) | 4607 (71.0) | 14,964 (56.7) | 36,795 (68.3) |
| Missing | 267 (0.3) | 206 (0.3) | 61 (0.3) | 238 (0.3) | 29 (0.4) | 104 (0.4) | 163 (0.3) |
| Paternal | |||||||
| Junior high school | 5504 (6.9) | 4263 (7.0) | 1241 (6.4) | 5197 (7.0) | 307 (4.7) | 2528 (9.6) | 2976 (5.5) |
| High school | 28,931 (36.0) | 21,947 (36.1) | 6984 (35.9) | 26,735 (36.2) | 2196 (33.9) | 10,501 (39.8) | 18,430 (34.2) |
| College | 45,096 (56.2) | 34,002 (55.9) | 11,094 (57.0) | 41,160 (55.8) | 3936 (60.7) | 13,052 (49.4) | 32,044 (59.5) |
| Missing | 740 (0.9) | 591 (1.0) | 149 (0.8) | 694 (0.9) | 46 (0.7) | 333 (1.3) | 407 (0.8) |
| Annual household income, N (%) | |||||||
| <200 × 104 JPY | 3984 (5.0) | 3115 (5.1) | 869 (4.5) | 3732 (5.1) | 252 (3.9) | 1873 (7.1) | 2111 (3.9) |
| 200–399 × 104 JPY | 25,388 (31.6) | 19,420 (31.9) | 5968 (30.7) | 23,600 (32.0) | 1788 (27.6) | 9412 (35.6) | 15,976 (29.7) |
| 400–599 × 104 JPY | 24,913 (31.0) | 18,701 (30.8) | 6212 (31.9) | 22,868 (31.0) | 2045 (31.5) | 7588 (28.7) | 17,325 (32.2) |
| ≥600 × 104 JPY | 20,521 (25.6) | 15,416 (25.4) | 5105 (26.2) | 18,564 (25.2) | 1957 (30.2) | 5385 (20.4) | 15,136 (28.1) |
| Missing | 5465 (6.8) | 4151 (6.8) | 1314 (6.7) | 5022 (6.8) | 443 (6.8) | 2156 (8.2) | 3309 (6.1) |
| Regions where Regional Centers exist, N (%) | |||||||
| Hokkaido | 6531 (8.1) | 5116 (8.4) | 1415 (7.3) | 6155 (8.3) | 376 (5.8) | 2123 (8.0) | 4408 (8.2) |
| Tohoku | 17,631 (22.0) | 13,056 (21.5) | 4575 (23.5) | 15,886 (21.5) | 1745 (26.9) | 5612 (21.2) | 12,019 (22.3) |
| Kanto | 9592 (11.9) | 7434 (12.2) | 2158 (11.1) | 8868 (12.0) | 724 (11.2) | 2887 (10.9) | 6705 (12.4) |
| Chubu | 14,644 (18.2) | 10,800 (17.8) | 3844 (19.7) | 13,272 (18.0) | 1372 (21.2) | 4335 (16.4) | 10,309 (19.1) |
| Kinki | 13,286 (16.6) | 10,399 (17.1) | 2887 (14.8) | 12,489 (16.9) | 797 (12.3) | 4391 (16.6) | 8895 (16.5) |
| Chugoku | 2324 (2.9) | 1731 (2.8) | 593 (3.0) | 2093 (2.8) | 231 (3.6) | 694 (2.6) | 1630 (3.0) |
| Shikoku | 5586 (7.0) | 4158 (6.8) | 1428 (7.3) | 5139 (7.0) | 447 (6.9) | 2139 (8.1) | 3447 (6.4) |
| Kyusyu-Okinawa | 10,677 (13.3) | 8109 (13.3) | 2568 (13.2) | 9884 (13.4) | 793 (12.2) | 4233 (16.0) | 6444 (12.0) |
| MDS at MT-2, points | 4 (3, 4) | ||||||
| MDS at MT-2, N (%) | |||||||
| Low MDS | 60,803 (75.7) | - | - | 58,896 (79.8) | 1907 (29.4) | 26,085 (98.8) | 34,718 (64.5) |
| High MDS | 19,468 (24.3) | - | - | 14,890 (20.2) | 4578 (70.6) | 329 (1.2) | 19,139 (35.5) |
| rMED at MT-2, points | 8 (6, 9) | ||||||
| rMED at MT-2, N (%) | |||||||
| Low rMED | 73,786 (91.9) | 58,896 (96.9) | 14,890 (76.5) | - | - | 25,815 (97.7) | 47,971 (89.1) |
| High rMED | 6485 (8.1) | 1907 (3.1) | 4578 (23.5) | - | - | 599 (2.3) | 5886 (10.9) |
| PMDS at MT-2, points | 4 (3, 5) | ||||||
| PMDS at MT-2, N (%) | |||||||
| Low PMDS | 26,414 (32.9) | 26,085 (42.9) | 329 (1.7) | 25,815 (35.0) | 599 (9.2) | - | - |
| High PMDS | 53,857 (67.1) | 34,718 (57.1) | 19,139 (98.3) | 47,971 (65.0) | 5886 (90.8) | - | - |
| Total K6 Score at MT-2 | All Participants N = 80,271 | Low MDS N = 60,803 | High MDS N = 19,468 | p | Low rMED N = 73,786 | High rMED N = 6485 | p | Low PMDS N = 26,414 | High PMDS N = 53,857 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| ≤4, N (%) | 58,569 (73.0) | 44,095 (72.5) | 14,474 (74.3) | 53,755 (72.9) | 4814 (74.2) | 18,622 (70.5) | 39,947 (74.2) | |||
| 5–9, N (%) | 14,630 (18.2) | 11,205 (18.4) | 3425 (17.6) | 13,494 (18.3) | 1136 (17.5) | 5015 (19.0) | 9615 (17.9) | |||
| 10–12, N (%) | 5001 (6.2) | 3900 (6.4) | 1101 (5.7) | 4619 (6.3) | 382 (5.9) | 1930 (7.3) | 3071 (5.7) | |||
| ≥13, N (%) (Psychological Distress) | 2071 (2.6) | 1603 (2.6) | 468 (2.4) | 0.08 | 1918 (2.6) | 153 (2.4) | 0.24 | 847 (3.2) | 1224 (2.3) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Watanabe, Z.; Iwama, N.; Kumagai, N.; Hamada, H.; Karumai-Mori, H.; Izumi, S.; Yokoyama, E.; Takahashi, Y.; Sato, T.; et al. Association of Mediterranean Diet Scores with Psychological Distress in Pregnancy: The Japan Environment and Children’s Study. Nutrients 2025, 17, 3697. https://doi.org/10.3390/nu17233697
Takahashi Y, Watanabe Z, Iwama N, Kumagai N, Hamada H, Karumai-Mori H, Izumi S, Yokoyama E, Takahashi Y, Sato T, et al. Association of Mediterranean Diet Scores with Psychological Distress in Pregnancy: The Japan Environment and Children’s Study. Nutrients. 2025; 17(23):3697. https://doi.org/10.3390/nu17233697
Chicago/Turabian StyleTakahashi, Yuri, Zen Watanabe, Noriyuki Iwama, Natsumi Kumagai, Hirotaka Hamada, Hikaru Karumai-Mori, Seiya Izumi, Emi Yokoyama, Yasuno Takahashi, Takeki Sato, and et al. 2025. "Association of Mediterranean Diet Scores with Psychological Distress in Pregnancy: The Japan Environment and Children’s Study" Nutrients 17, no. 23: 3697. https://doi.org/10.3390/nu17233697
APA StyleTakahashi, Y., Watanabe, Z., Iwama, N., Kumagai, N., Hamada, H., Karumai-Mori, H., Izumi, S., Yokoyama, E., Takahashi, Y., Sato, T., Toratani, J., Tagami, K., Tomita, H., Tachibana, M., Ishikuro, M., Obara, T., Metoki, H., Suzuki, T., Miura, Y., ... The Japan Environment and Children’s Study Group. (2025). Association of Mediterranean Diet Scores with Psychological Distress in Pregnancy: The Japan Environment and Children’s Study. Nutrients, 17(23), 3697. https://doi.org/10.3390/nu17233697






