High Prevalence and Clinical Associations of Vitamin D Deficiency in Inflammatory Bowel Disease: Evidence from a Tertiary Center Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Measurement of Vitamin D Levels
2.4. Ethics
2.5. Statistical Analysis
2.6. AI/Editing Statement
3. Results
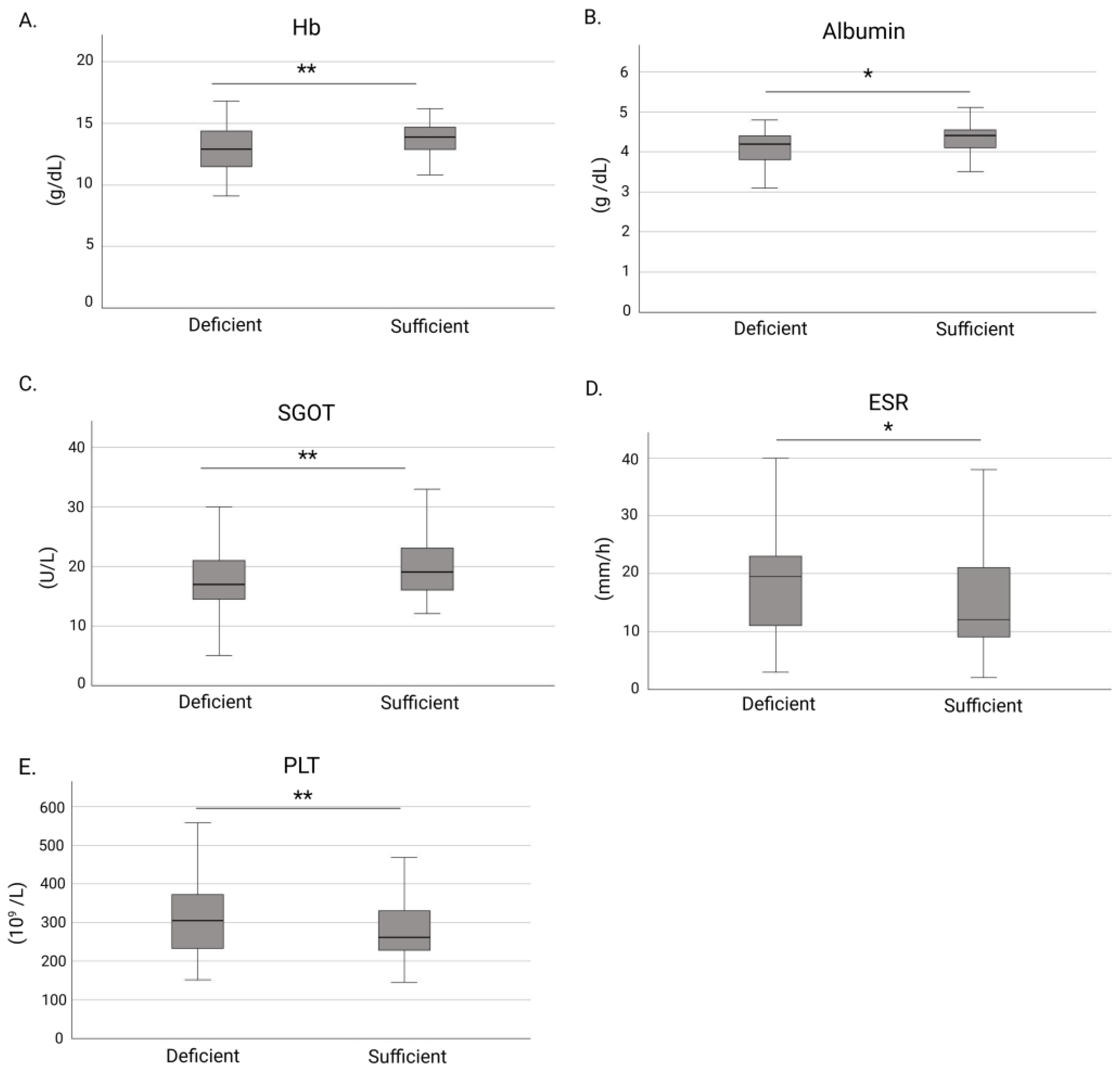
3.1. Associations Between Vitamin D Deficiency and Clinical Characteristics in IBD
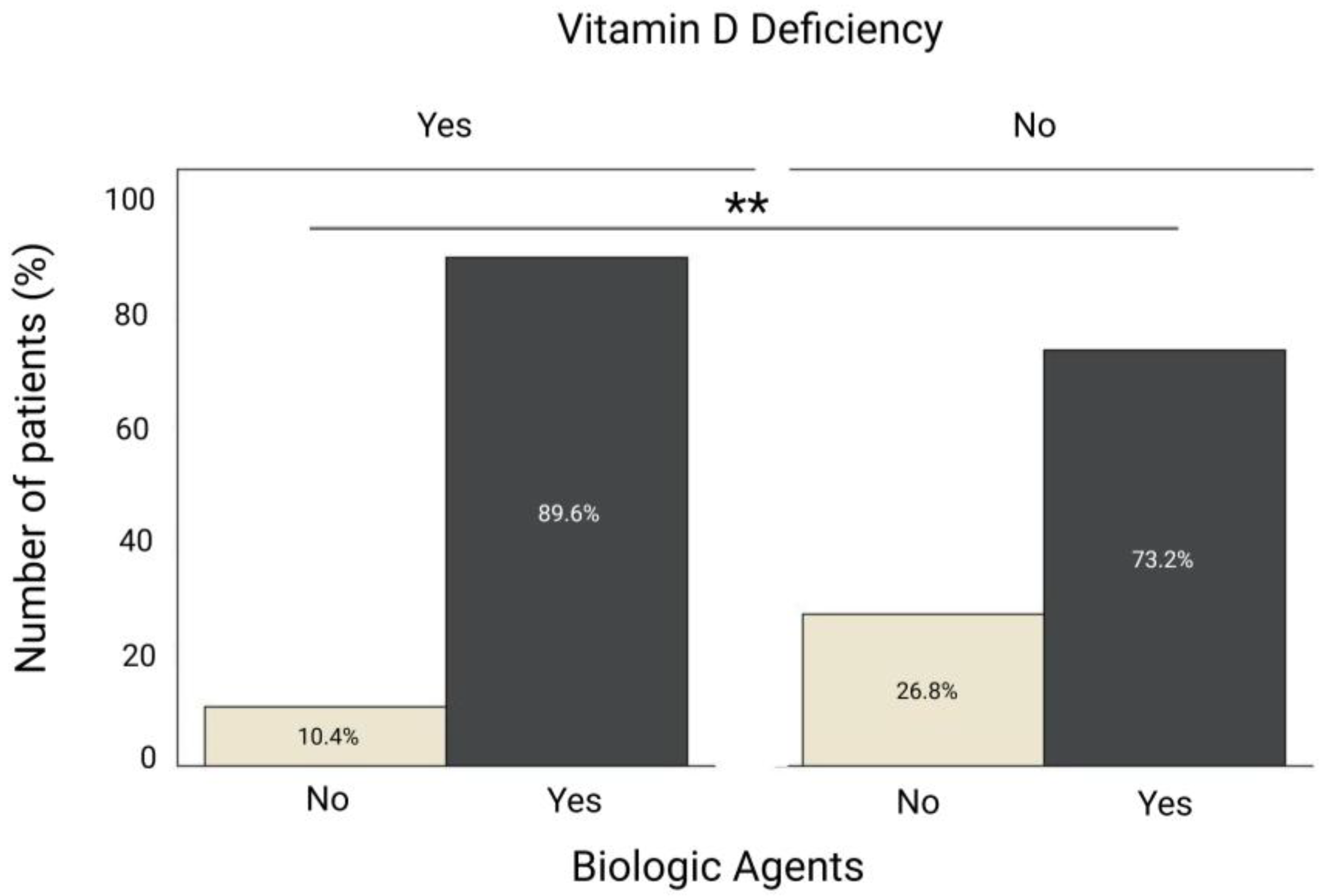
3.2. Comparison of Biologic Therapy Use Between IBD Patients with and Without Vitamin D Deficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- de Souza Pereira, P.; Neto, M.A.d.F.L. Prevalence of Vitamin D Deficiency in Patients with Inflammatory Bowel Disease. J. Coloproctology 2025, 45, e1–e5. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Koppelman, L.J.M.; Oyugi, A.A.; Maljaars, P.W.J.; van der Meulen-de Jong, A.E. Modifiable Factors Influencing Disease Flares in Inflammatory Bowel Disease: A Literature Overview of Lifestyle, Psychological, and Environmental Risk Factors. J. Clin. Med. 2025, 14, 2296. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The Association of Vitamin D Status with Disease Activity in a Cohort of Crohn’s Disease Patients in Canada. Nutrients 2017, 9, 1112. [Google Scholar] [CrossRef]
- Kafentzi, T.; Tsounis, E.P.; Tourkochristou, E.; Avramopoulou, E.; Aggeletopoulou, I.; Geramoutsos, G.; Sotiropoulos, C.; Pastras, P.; Thomopoulos, K.; Theocharis, G.; et al. Genetic Polymorphisms (ApaI, FokI, BsmI, and TaqI) of the Vitamin D Receptor (VDR) Influence the Natural History and Phenotype of Crohn’s Disease. Int. J. Mol. Sci. 2025, 26, 1848. [Google Scholar] [CrossRef]
- Fatahi, S.; Alyahyawi, N.; Albadawi, N.; Mardali, F.; Dara, N.; Sohouli, M.H.; Prabahar, K.; Rohani, P.; Koushki, N.; Sayyari, A.; et al. The association between vitamin D status and inflammatory bowel disease among children and adolescents: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1007725. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. JPEN J. Parenter. Enteral Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Binkley, N.; Sempos, C.T. Standardizing vitamin D assays: The way forward. J. Bone Miner. Res. 2014, 29, 1709–1714. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- EFSA. Panel on Dietetic Products, Nutrition and Allergies. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Cantorna, M.T. Vitamin D and autoimmunity: Is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc. Soc. Exp. Biol. Med. 2000, 223, 230–233. [Google Scholar] [CrossRef]
- Cantorna, M.T.; McDaniel, K.; Bora, S.; Chen, J.; James, J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med. 2014, 239, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Koureta, E.; Karatzas, P.; Kanellopoulos, P.; Papapanagiotou, A.; Lekakis, V.; Bamias, G.; Koutsoumpas, A.; Karamanolis, G.; Vlachogiannakos, J.; Papavassiliou, A.G.; et al. The importance of vitamin D levels in patients with inflammatory bowel disease. J. Physiol. Biochem. 2025, 81, 729–739. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Hansen, T.I.; Gubatan, J.M.; Jensen, K.B.; Rejnmark, L. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol. 2019, 10, 394–400. [Google Scholar] [CrossRef]
- Lu, J.; Yu, F.; Huang, J.; Yu, H.; Li, F.; Le, Z.; Cheng, Y.; Zhang, Q.; Li, G.; Xie, X.; et al. Hypocholesterolemia and Inflammatory Biomarkers Act as Predictors of Severe Vitamin D Deficiency in Patients With Crohn’s Disease: A Clinical Analysis of 862 Patients in China. Front. Nutr. 2022, 9, 806887. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, S.; Yao, L.; Cao, Q.; Shao, B. Association of vitamin D and platelet-to-lymphocyte ratio in treatment escalation risk for newly diagnosed Crohn’s disease adults. Nutr. J. 2025, 24, 49. [Google Scholar] [CrossRef]
- Xu, C.; Song, Z.; Hu, L.T.; Tong, Y.H.; Hu, J.Y.; Shen, H. Abnormal platelet parameters in inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 214. [Google Scholar] [CrossRef]
- Yan, M.; Wang, Z.; Qiu, Z.; Cui, Y.; Xiang, Q. Platelet signaling in immune landscape: Comprehensive mechanism and clinical therapy. Biomark. Res. 2024, 12, 164. [Google Scholar] [CrossRef]
- Silvagno, F.; De Vivo, E.; Attanasio, A.; Gallo, V.; Mazzucco, G.; Pescarmona, G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE 2010, 5, e8670. [Google Scholar] [CrossRef] [PubMed]
- Johny, E.; Jala, A.; Nath, B.; Alam, M.J.; Kuladhipati, I.; Das, R.; Borkar, R.M.; Adela, R. Vitamin D Supplementation Modulates Platelet-Mediated Inflammation in Subjects With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Immunol. 2022, 13, 869591. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.N.; Yıldız, A. The relationship between ulcerative colitis activity and vitamin D, mean platelet volume and platelet distribution width. Anatol. Curr. Med. J. 2022, 4, 374–379. [Google Scholar] [CrossRef]
- Cappello, M.; Randazzo, C.; Bravatà, I.; Licata, A.; Peralta, S.; Craxì, A.; Almasio, P.L. Liver Function Test Abnormalities in Patients with Inflammatory Bowel Diseases: A Hospital-based Survey. Clin. Med. Insights Gastroenterol. 2014, 7, 25–31. [Google Scholar] [CrossRef]
- Cheng, Y.W.; McLean, R.; Sewell, J.L.; Huang, C.Y.; Khalili, M. Inflammatory bowel disease type influences development of elevated liver enzymes. JGH Open 2022, 6, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.T.; Elangovan, H.; Stokes, R.A.; Gunton, J.E. Vitamin D and the Liver-Correlation or Cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Triantos, C. Vitamin D and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Novel Mechanistic Insights. Int. J. Mol. Sci. 2024, 25, 4901. [Google Scholar] [CrossRef]
- Lee, S.-b.; Jin, M.H.; Yoon, J.-H. The contribution of vitamin D insufficiency to the onset of steatotic liver disease among individuals with metabolic dysfunction. Sci. Rep. 2024, 14, 6714. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Guan, C.; Cheng, N.; Wang, X.; Liu, Y.; Chen, J.; Wang, C. Vitamin D receptor agonists inhibit liver fibrosis by disrupting the interaction between hepatic stellate cells and neutrophil extracellular traps. Biochem. Pharmacol. 2025, 240, 117059. [Google Scholar] [CrossRef]
- Abramovitch, S.; Dahan-Bachar, L.; Sharvit, E.; Weisman, Y.; Ben Tov, A.; Brazowski, E.; Reif, S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 2011, 60, 1728–1737. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, H.; Zhang, Y.; Sun, M.; Sun, H.; Pei, Y. Evaluating the predictive effect of vitamin D on clinical outcomes of infliximab-treated Crohn’s disease patients. Front. Immunol. 2025, 16, 1651209. [Google Scholar] [CrossRef]
- Hizarcioglu-Gulsen, H.; Kaplan, J.L.; Moran, C.J.; Israel, E.J.; Lee, H.; Winter, H. The Impact of Vitamin D on Response to Anti-tumor Necrosis Factor-α Therapy in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e125–e131. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.V.; Gopal, S.; Shetty, A.J.; Shenoy, S.; Tantry, B.V.; Unnikrishnan, B.; Holla, R.; Anand, R. Predictive accuracy of fecal calprotectin in assessing clinical activity and disease severity in patients with Ulcerative Colitis and Crohn’s disease. BMC Gastroenterol. 2025, 25, 429. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, M.; Qian, W.; Ling, F.; Chen, Y.; Li, S.; Cheng, Y.; Zhu, L. Clinical value of fecal calprotectin for evaluating disease activity in patients with Crohn’s disease. Front. Physiol. 2023, 14, 1186665. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 97 (53) |
| Female | 86 (47) |
| IBD type | |
| Crohn Disease Ulcerative Colitis | 93 (50.5) |
| 84 (45.7) | |
| Indeterminate Colitis | 7 (3.8) |
| IBD family history | 9 (5) |
| Smoking | |
| Current | 62 (34.3) |
| Never | 97 (53.6) |
| Prior | 22 (12) |
| Vitamin D deficiency | 67 (36.4) |
| IBD hospitalization | 75 (46.9) |
| IBD surgery | 29 (15.8) |
| IBD treatment | |
| None Amino salicylates Immunomodulators Corticosteroids Biologic Agents | 7 |
| 67 | |
| 16 | |
| 16 | |
| 142 | |
| Baseline characteristics | Median (IQR) |
| Age (years) | 45 (34–61) |
| BMI | 24.28 (21.70–27.77) |
| Vitamin D (ng/mL) | 23.82 (15.95–30.58) |
| Mayo score (for UC) | 1 (0–5.5) |
| CDAI score (for CD) | 61.50 (29.00–103.25) |
| HBI score (for CD) | 2 (1–4) |
| Hb (g/dL) | 13.6 (12.2–14.6) |
| WBC (109/L) | 6825 (5700–8970) |
| PLT (109/L) | 277 (231–351) |
| CRP (mg/L) | 0.4 (0.2–1.18) |
| ESR (mm/h) | 15 (10–26) |
| SGOT (U/L) | 19 (15–24) |
| SGPT (U/L) | 19 (13–26) |
| γ-GT (U/L) | 16 (12–26) |
| Albumin (g/dL) | 4.2 (4.0–4.5) |
| Fecal calprotectin (μg/g) | 194 (39–350) |
| Variable | Univariate Analysis | OR (95% CI) | Multivariate Analysis | aOR (95% CI) |
|---|---|---|---|---|
| Age (years) | 0.515 | 1.006 (0.988–1.024) | ||
| BMI | 0.213 | 1.047 (0.974–1.126) | ||
| IBD type (CD vs. UC) | 0.117 | 1.625 (0.886–2.980) | ||
| IBD surgery | 0.188 | 0.580 (0.257–1.306) | ||
| Biologic Agents | 0.012 | 0.319 (0.131–0.775) | 0.038 | 0.374 (0.148–0.946) |
| Number of Biologic Agents | 0.217 | 0.791 (0.545–1.148) | ||
| Hb (g/dL) | 0.008 | 1.289 (1.070–1.553) | 0.317 | 1.097 (0.915–1.314) |
| WBC (109/L) | 0.611 | 1.000 (1.000–1.000) | ||
| PLT (109/L) | 0.001 | 0.994 (0.991–0.998) | 0.024 | 0.996 (0.992–0.999) |
| CRP (mg/L) | 0.068 | 0.873 (0.754–1.010) | ||
| ESR (mm/h) | 0.057 | 0.982 (0.964–1.001) | ||
| SGOT (U/L) | 0.010 | 1.061 (1.014–1.109) | 0.036 | 1.050 (1.003–1.098) |
| SGPT (U/L) | 0.284 | 1.012 (0.990–1.035) | ||
| Albumin (g/dL) | 0.473 | 1.053 (0.919–1.170) | ||
| Mayo score | 0.094 | 0.889 (0.775–1.020) | ||
| CDAI score | 0.748 | 0.999 (0.992–1.006) | ||
| HBI score | 0.185 | 0.916 (0.805–1.043) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafentzi, T.; Pastras, P.; Aggeletopoulou, I.; Tsounis, E.P.; Geramoutsos, G.; Kimiskidis, N.; Bali, M.; Thomopoulos, K.; Diamantopoulou, G.; Theocharis, G.; et al. High Prevalence and Clinical Associations of Vitamin D Deficiency in Inflammatory Bowel Disease: Evidence from a Tertiary Center Cohort. Nutrients 2025, 17, 3698. https://doi.org/10.3390/nu17233698
Kafentzi T, Pastras P, Aggeletopoulou I, Tsounis EP, Geramoutsos G, Kimiskidis N, Bali M, Thomopoulos K, Diamantopoulou G, Theocharis G, et al. High Prevalence and Clinical Associations of Vitamin D Deficiency in Inflammatory Bowel Disease: Evidence from a Tertiary Center Cohort. Nutrients. 2025; 17(23):3698. https://doi.org/10.3390/nu17233698
Chicago/Turabian StyleKafentzi, Theodora, Ploutarchos Pastras, Ioanna Aggeletopoulou, Efthymios P. Tsounis, Georgios Geramoutsos, Nikitas Kimiskidis, Maria Bali, Konstantinos Thomopoulos, Georgia Diamantopoulou, Georgios Theocharis, and et al. 2025. "High Prevalence and Clinical Associations of Vitamin D Deficiency in Inflammatory Bowel Disease: Evidence from a Tertiary Center Cohort" Nutrients 17, no. 23: 3698. https://doi.org/10.3390/nu17233698
APA StyleKafentzi, T., Pastras, P., Aggeletopoulou, I., Tsounis, E. P., Geramoutsos, G., Kimiskidis, N., Bali, M., Thomopoulos, K., Diamantopoulou, G., Theocharis, G., & Triantos, C. (2025). High Prevalence and Clinical Associations of Vitamin D Deficiency in Inflammatory Bowel Disease: Evidence from a Tertiary Center Cohort. Nutrients, 17(23), 3698. https://doi.org/10.3390/nu17233698









