The Combination of Diosgenin, Vitamin D, and α-Lactalbumin Normalizes the Menstrual Cycle in Women with PCOS of Phenotype D: A Pilot Clinical Study
Abstract
1. Introduction
2. Materials and Methods
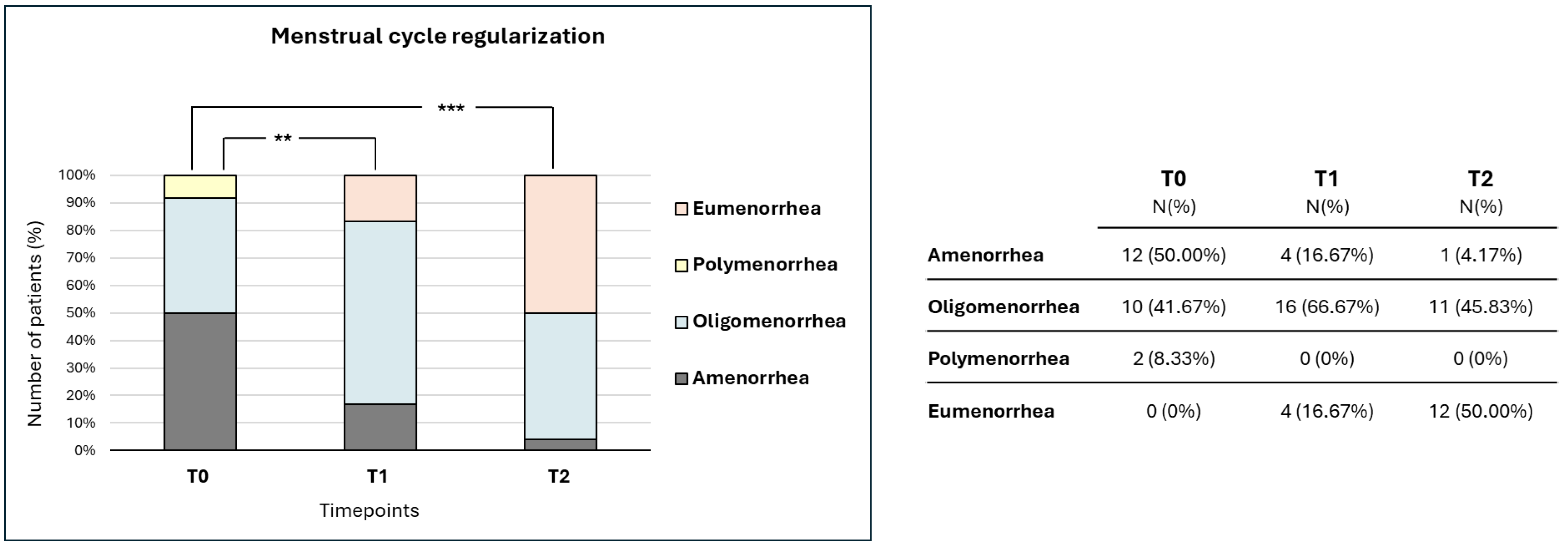
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stener-Victorin, E.; Teede, H.; Norman, R.J.; Legro, R.; Goodarzi, M.O.; Dokras, A.; Laven, J.; Hoeger, K.; Piltonen, T.T. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2024, 10, 27. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Myers, S.H.; Oliva, M.M.; Nordio, M.; Unfer, V. PCOS phenotype focus: Phenotype D under the magnifying glass. Arch. Gynecol. Obs. 2024, 309, 2307–2313. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreale, H.F. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334–352. [Google Scholar] [CrossRef]
- Ramezanali, F.; Ashrafi, M.; Hemat, M.; Arabipoor, A.; Jalali, S.; Moini, A. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes: The predictive value of anti-Müllerian hormone. Reprod. Biomed. Online 2016, 32, 503–512. [Google Scholar] [CrossRef] [PubMed]
- De Zegher, F.; Ibáñez, L. Leader vs follower in the tango of polycystic ovary syndrome: Insulin resistance vs androgen excess. Acta Obstet. Et Gynecol. Scand. 2024, 103, 1680. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Lizneva, D.; Kirubakaran, R.; Mykhalchenko, K.; Suturina, L.; Chernukha, G.; Diamond, M.P.; Azziz, R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: Systematic review and meta-analysis. Fertil. Steril. 2016, 106, 1510–1520. [Google Scholar] [CrossRef]
- Unfer, V.; Dinicola, S.; Russo, M. A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? Int. J. Mol. Sci. 2023, 24, 6213. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Rodriguez-Martinez, M.A.; Spoletini, I.; Regidor, P.A. Obesity and contraceptive use: Impact on cardiovascular risk. ESC Heart Fail. 2022, 9, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxidative Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, R.; Nair, A.K.; Maniyan, G.; Vijaya, R.; Nair, R.V.; Nair, J.H.; Kumar, S.N.; Sasidharan, S. Exploring the molecular mechanism of Dioscorea alata L. for the treatment of menstrual disorders using network pharmacology and molecular docking. Heliyon 2025, 11, e42582. [Google Scholar] [CrossRef]
- Hilario-Martínez, J.C.; Huerta, A.; Amaro-López, J.C.; Alatriste, V.; Santos, M.G.D.L.; Martínez, I.; Bernès, S.; Sandoval-Ramírez, J.; Merino, G.; Luna, F.; et al. Stereoselective synthesis of (26R)-26-hydroxydiosgenin and its effect on the regulation of rat ovarian function. Bioorg. Chem. 2021, 115, 105189. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V.; Paolino, S.; Gotelli, E. Involvement of the secosteroid vitamin D in autoimmune rheumatic diseases and COVID-19. Nat. Rev. Rheumatol. 2023, 19, 265–287. [Google Scholar] [CrossRef]
- Kim, C.H. A functional relay from progesterone to vitamin D in the immune system. DNA Cell Biol. 2015, 34, 379–382. [Google Scholar] [CrossRef]
- Wojtusik, J.; Johnson, P.A. Vitamin D Regulates Anti-Mullerian Hormone Expression in Granulosa Cells of the Hen1. Biol. Reprod. 2012, 86, 93. [Google Scholar] [CrossRef]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef]
- Butt, M.S.; Saleem, J.; Aiman, S.; Zakar, R.; Sadique, I.; Fischer, F. Serum anti-Müllerian hormone as a predictor of polycystic ovarian syndrome among women of reproductive age. BMC Women’s Health 2022, 22, 199. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Chen, H.; Guan, K.; Qi, X.; Wang, R.; Ma, Y. α-Lactalbumin ameliorates hepatic lipid metabolism in high-fat-diet induced obese C57BL/6J mice. J. Funct. Foods 2020, 75, 104253. [Google Scholar] [CrossRef]
- Suturina, L.; Belkova, N.; Igumnov, I.; Lazareva, L.; Danusevich, I.; Nadeliaeva, I.; Sholokhov, L.; Rashidova, M.; Belenkaya, L.; Belskikh, A.; et al. Polycystic Ovary Syndrome and Gut Microbiota: Phenotype Matters. Life 2022, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Van Keizerswaard, J.; de Loos, A.L.D.; Louwers, Y.V.; Laven, J.S. Changes in individual polycystic ovary syndrome phenotypical characteristics over time: A long-term follow-up study. Fertil. Steril. 2022, 117, 1059–1066. [Google Scholar] [CrossRef]
- Wiser, A.; Shehata, F.; Holzer, H.; Hyman, J.H.; Shalom-Paz, E.; Son, W.Y.; Tulandi, T. Effect of high LH/FSH ratio on women with polycystic ovary syndrome undergoing in vitro maturation treatment. J. Reprod. Med. 2013, 58, 219–223. [Google Scholar]
- Myers, S.H.; Russo, M.; Dinicola, S.; Forte, G.; Unfer, V. Questioning PCOS phenotypes for reclassification and tailored therapy. Trends Endocrinol. Metab. 2023, 34, 694–703. [Google Scholar] [CrossRef]
- Tehrani, F.R.; Rashidi, H.; Khomami, M.B.; Tohidi, M.; Azizi, F. The prevalence of metabolic disorders in various phenotypes of polycystic ovary syndrome: A community based study in Southwest of Iran. Reprod. Biol. Endocrinol. 2014, 12, 89. [Google Scholar] [CrossRef]
- Farhadi-Azar, M.; Behboudi-Gandevani, S.; Rahmati, M.; Mahboobifard, F.; Khalili Pouya, E.; Ramezani Tehrani, F.; Azizi, F. The Prevalence of Polycystic Ovary Syndrome, Its Phenotypes and Cardio-Metabolic Features in a Community Sample of Iranian Population: Tehran Lipid and Glucose Study. Front. Endocrinol. 2022, 13, 825528. [Google Scholar] [CrossRef]
- Krentowska, A.; Kowalska, I. Metabolic syndrome and its components in different phenotypes of polycystic ovary syndrome. Diabetes Metab. Res. Rev. 2022, 38, e3464. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E628–E637. [Google Scholar] [CrossRef]
- Unfer, V.; Russo, M.; Aragona, C.; Bilotta, G.; Montanino Oliva, M.; Bizzarri, M. Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes. Biomedicines 2023, 11, 1759. [Google Scholar] [CrossRef]
- Gupta, M.; Yadav, R.; Mahey, R.; Agrawal, A.; Upadhyay, A.; Malhotra, N.; Bhatla, N. Correlation of body mass index (BMI), anti-mullerian hormone (AMH), and insulin resistance among different polycystic ovary syndrome (PCOS) phenotypes—A cross-sectional study. Gynecol. Endocrinol. 2019, 35, 970–973. [Google Scholar] [CrossRef]
- Polak, A.M.; Adamska, A.; Krentowska, A.; Łebkowska, A.; Hryniewicka, J.; Adamski, M.; Kowalska, I. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J. Clin. Med. 2020, 9, 732. [Google Scholar] [CrossRef]
- Jamil, A.S.; Alalaf, S.K.; Al-Tawil, N.G.; Al-Shawaf, T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch. Gynecol. Obstet. 2016, 293, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Afradiasbagharani, P.; Hosseini, E.; Allahveisi, A.; Bazrafkan, M. The insulin-like growth factor and its players: Their functions, significance, and consequences in all aspects of ovarian physiology. Middle East Fertil. Soc. J. 2022, 27, 27. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, H.; Yang, F.; Shang, W.; Zeng, S. Effects of IGF-1 on the Three-Dimensional Culture of Ovarian Preantral Follicles and Superovulation Rates in Mice. Biology 2022, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Tian, X.; Ruan, Y.; Liu, Y.; Bian, D.; Ma, C.; Yu, C.; Feng, M.; Wang, F.; Gao, L.; et al. Diosgenin induces apoptosis in IGF-1-stimulated human thyrocytes through two caspase-dependent pathways. Biochem. Biophys. Res. Commun. 2012, 418, 347–352. [Google Scholar] [CrossRef]
- Völzke, H.; Friedrich, N.; Schipf, S.; Haring, R.; Lüdemann, J.; Nauck, M.; Dörr, M.; Brabant, G.; Wallaschofski, H. Association between serum insulin-like growth factor-I levels and thyroid disorders in a population-based study. J. Clin. Endocrinol. Metab. 2007, 92, 4039–4045. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Gell, J.S. Alternative Medicine. In Clinical Gynecology; Bieber, E.J., Sanfilippo, J.S., Horowitz, I.R., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2006; Chapter 6; pp. 71–78. [Google Scholar]
- Monastra, G.; De Grazia, S.; De Luca, L.; Vittorio, S.; Unfer, V. Vitamin D: A steroid hormone with progesterone-like activity. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2502–2512. [Google Scholar]
- Matsumoto, H.; Sakai, K.; Iwashita, M. Insulin-like growth factor binding protein-1 induces decidualization of human endometrial stromal cells via alpha5beta1 integrin. Mol. Hum. Reprod. 2008, 14, 485–489. [Google Scholar] [CrossRef]
- Young, C.H.; Snow, B.; DeVore, S.B.; Mohandass, A.; Nemmara, V.V.; Thompson, P.R.; Thyagarajan, B.; Navratil, A.M.; Cherrington, B.D. Progesterone stimulates histone citrullination to increase IGFBP1 expression in uterine cells. Reproduction 2021, 162, 117–127. [Google Scholar] [CrossRef]
- Bae, J.H.; Song, D.K.; Im, S.S. Regulation of IGFBP-1 in Metabolic Diseases. J. Lifestyle Med. 2013, 3, 73–79. [Google Scholar]
- Fujii, K.; Matsukawa, T. Saponins and sterols. 8. Saponin of Dioscorea tokoro Makino. J. Pharm. Soc. Jpn. 1936, 56, 408–414. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Munshi, M.; Kumar, P. Diosgenin: Chemistry, extraction, quantification and health benefits. Food Chem. Adv. 2023, 2, 100170. [Google Scholar] [CrossRef]
- Shawky, E.; Nassra, R.A.; El-Alkamy, A.M.T.; Sallam, S.M.; El Sohafy, S.M. Unraveling the mechanisms of Fenugreek seed for managing different gynecological disorders: Steroidal saponins and isoflavones revealed as key bioactive metabolites. J. Pharm. Biomed. Anal. 2024, 238, 115865. [Google Scholar] [CrossRef]
- Shen, M.; Qi, C.; Kuang, Y.-P.; Yang, Y.; Lyu, Q.-F.; Long, H.; Yan, Z.-G.; Lu, Y.-Y. Observation of the influences of diosgenin on aging ovarian reserve and function in a mouse model. Eur. J. Med. Res. 2017, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Seto, K. The effect of Dioscorea esculenta powder on prostaglandin E(2) and cytochrome c oxidase subunit 2 levels, menstrual pain, and premenstrual syndrome in young women: A randomized double-blind controlled trial. Nutr. Health 2022, 30, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, B.; Wang, H.; Qian, Q. Dioscin Ameliorates Polycystic Ovary Syndrome by Inhibiting PI3K/Akt Pathway-Mediated Proliferation and Apoptosis of Ovarian Granulosa Cells. Curr. Top. Nutraceutical Res. 2020, 18, 331–336. [Google Scholar] [CrossRef]
- Li, M.; Hu, S.; Sun, J.; Zhang, Y. The role of vitamin D3 in follicle development. J. Ovarian Res. 2024, 17, 148. [Google Scholar] [CrossRef]
- Łagowska, K. The Relationship between Vitamin D Status and the Menstrual Cycle in Young Women: A Preliminary Study. Nutrients 2018, 10, 1729. [Google Scholar] [CrossRef]
- Alessandri, G.; Mancabelli, L.; Fontana, F.; Lepore, E.; Forte, G.; Burratti, M.; Ventura, M.; Turroni, F. Disclosing α-lactalbumin impact on the intestinal and vaginal microbiota of women suffering from polycystic ovary syndrome. Microb. Biotechnol. 2024, 17, e14540. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Gober, H.J.; Leung, W.T.; Huang, Z.; Pan, X.; Li, C.; Zhang, N.; Wang, L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed. Pharmacother. 2021, 133, 110958. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Delavari, B.; Saboury, A.A.; Atri, M.S.; Ghasemi, A.; Bigdeli, B.; Khammari, A.; Maghami, P.; Moosavi-Movahedi, A.A.; Haertlé, T.; Goliaei, B. Alpha-lactalbumin: A new carrier for vitamin D3 food enrichment. Food Hydrocoll. 2015, 45, 124–131. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Kauffman, A.S. The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS). Med. Sci. 2019, 7, 84. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Adekunbi, D.; Liu, Y.; Long, H.; Wang, L.; Lyu, Q.; Kuang, Y.; O’Byrne, K.T. Hypothalamic effects of progesterone on regulation of the pulsatile and surge release of luteinising hormone in female rats. Sci. Rep. 2017, 7, 8096. [Google Scholar] [CrossRef]
| Baseline Values (T0) | Values at 3 Months (T1) | Values at 6 Months (T2) | p-Value T1 vs. T0 | p-Value T2 vs. T0 | p-Value T2 vs. T1 | Effect Size T1 vs. T0 | Effect Size T2 vs. T0 | Effect Size T2 vs. T1 | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 26.5 [24–30.25] | ||||||||
| Weight (kg) | 61.5 [57.75–65] | 62 [57.75–65] | 61 [58–64.25] | 0.8779 | 0.6719 | 0.4673 | 0.01 | 0.01 | 0.03 |
| BMI (kg/m2) | 22.39 [20.88–23.76] | 22.5 [21.38–23.58] | 22.45 [20.88–23.53] | 0.5879 | 0.7969 | 0.4263 | 0.03 | 0.01 | 0.04 |
| Testosterone (nmol/L) | 2.00 [1.59–2.22] | 1.81 [1.6–2.1] | 1.95 [1.55–2.22] | 0.0071 | 0.1289 | 0.3493 | 0.262 | 0.156 | 0.098 |
| SHBG (nmol/L) | 61.50 [52–75.25] | 53.25 [46–57.63] | 63 [52.68–78.75] | 0.0155 | 0.2866 | 0.0011 | 0.21 | 0.111 | 0.308 |
| FAI | 3.55 [3.31–4.1] | 3.67 [3.02–4.04] | 3.09 [2.29–3.63] | 0.598 | 0.0366 | 0.0428 | 0.055 | 0.21 | 0.204 |
| HOMA | 1.64 [1.20–2.06] | 1.90 [1.66–2.07] | 1.58 [1.12–1.76] | 0.0312 | 0.3566 | 0.0004 | 0.216 | 0.096 | 0.328 |
| LH (mU/ml) | 8.10 [2.78–11.4] | 6.45 [5.78–7.33] | 3.1 [2.8–4.13] | 0.3566 | 0.0057 | <0.0001 | 0.096 | 0.268 | 0.407 |
| FSH (mU/ml) | 4.25 [3.08–5.75] | 4.2 [3.78–4.5] | 3.55 [3–4.3] | 0.3416 | 0.0236 | 0.1326 | 0.099 | 0.212 | 0.155 |
| LH/FSH | 1.26 [0.86–2.13] | 1.67 [1.39–1.97] | 0.93 [0.82–1.15] | 0.2617 | 0.0616 | <0.0001 | 0.117 | 0.19 | 0.402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Porcaro, G.; Aragona, C.; Bilotta, G.; Di Liberto, M.; Unfer, V. The Combination of Diosgenin, Vitamin D, and α-Lactalbumin Normalizes the Menstrual Cycle in Women with PCOS of Phenotype D: A Pilot Clinical Study. Nutrients 2025, 17, 3695. https://doi.org/10.3390/nu17233695
Russo M, Porcaro G, Aragona C, Bilotta G, Di Liberto M, Unfer V. The Combination of Diosgenin, Vitamin D, and α-Lactalbumin Normalizes the Menstrual Cycle in Women with PCOS of Phenotype D: A Pilot Clinical Study. Nutrients. 2025; 17(23):3695. https://doi.org/10.3390/nu17233695
Chicago/Turabian StyleRusso, Michele, Giuseppina Porcaro, Cesare Aragona, Gabriele Bilotta, Massimo Di Liberto, and Vittorio Unfer. 2025. "The Combination of Diosgenin, Vitamin D, and α-Lactalbumin Normalizes the Menstrual Cycle in Women with PCOS of Phenotype D: A Pilot Clinical Study" Nutrients 17, no. 23: 3695. https://doi.org/10.3390/nu17233695
APA StyleRusso, M., Porcaro, G., Aragona, C., Bilotta, G., Di Liberto, M., & Unfer, V. (2025). The Combination of Diosgenin, Vitamin D, and α-Lactalbumin Normalizes the Menstrual Cycle in Women with PCOS of Phenotype D: A Pilot Clinical Study. Nutrients, 17(23), 3695. https://doi.org/10.3390/nu17233695







