Protective Role of Ginsenoside F1-Enriched Extract (SGB121) in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Ginsenoside SGB121
2.3. Animal Tests
2.3.1. Experimental Design for Animal Study
2.3.2. Measurement of Body Weight and Composition
2.3.3. Measurement of Organ Weight
2.3.4. Measurement of Hepatic Malondialdehyde (MDA) Levels
2.3.5. Measurement of Hepatic Total Glutathione (GSH)
2.3.6. Measurement of Hepatic and Serum Triglyceride and Cholesterol Levels
2.3.7. Measurement of Serum AST and ALT Activities
2.3.8. Measurement of Fasting Blood Glucose, Insulin, and HOMA-IR
2.3.9. Measurement of Hepatic Lipid Accumulation Using Histological Analysis
2.4. Measurement of DPPH and Superoxide Radical Scavenging Activities of F1 and SGB121 Under Cell-Free Conditions
2.5. Cell Line
2.6. Measurement of Cell Viability Following F1 and SGB121 Treatment in HepG2 Cells Using the MTT Assay
2.7. Assessment of FFA-Induced Oxidative Stress and Lipid Accumulation
2.7.1. Measurement of Intracellular ROS
2.7.2. Quantification of Lipid Accumulation
2.7.3. Measurement of Gene Expression Levels in HepG2 Cells Using Quantitative Real-Time PCR
2.7.4. Measurement of Target Protein Expression in HepG2 Cells
2.8. Statistical Analysis
3. Results
3.1. Animal Experiment for SGB121
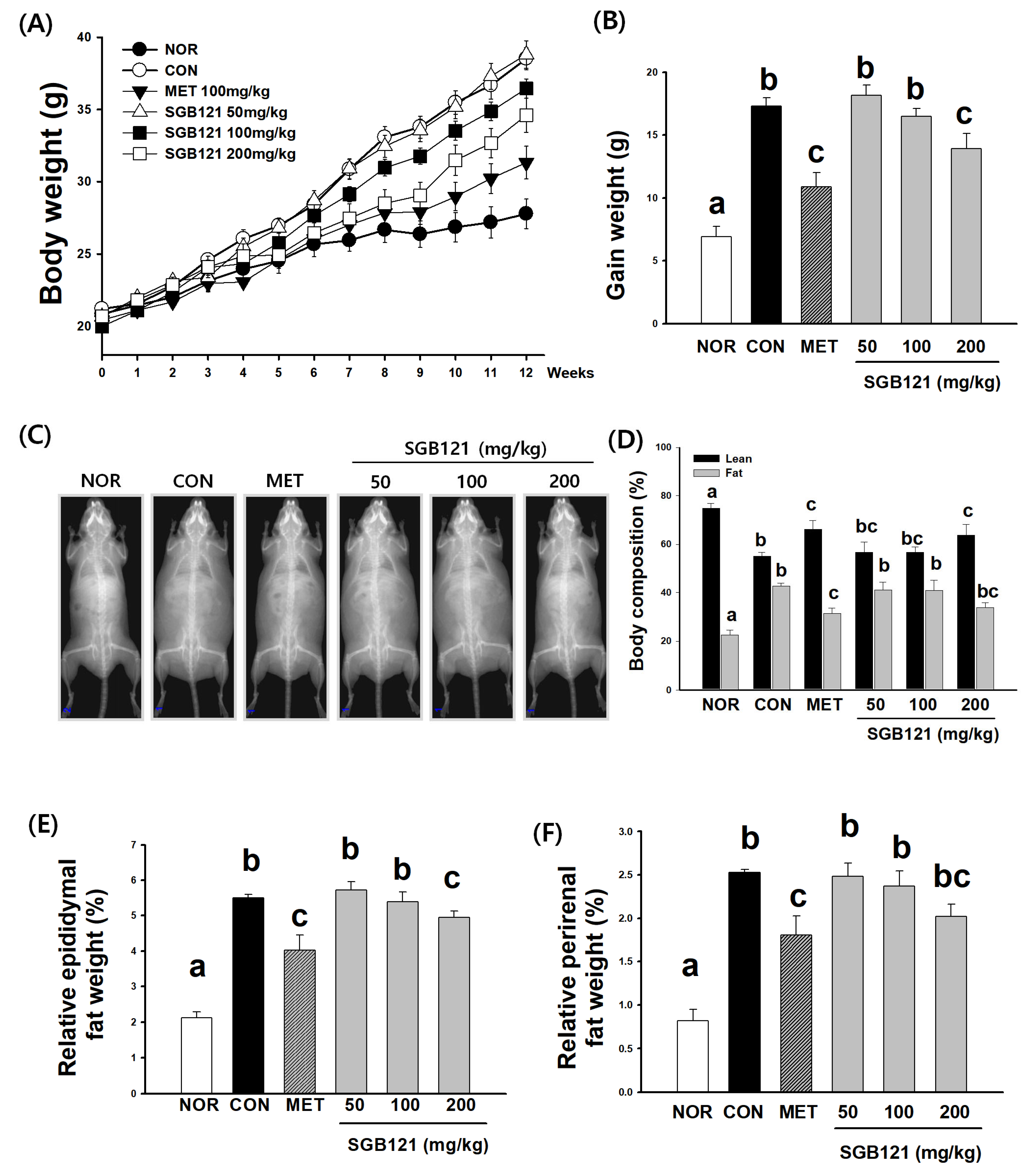
3.1.1. Effects of SGB121 on Body Weight of HFHC Diet-Induced MAFLD Mice
3.1.2. Reduction in Adiposity and Preservation of Lean Mass by SGB121
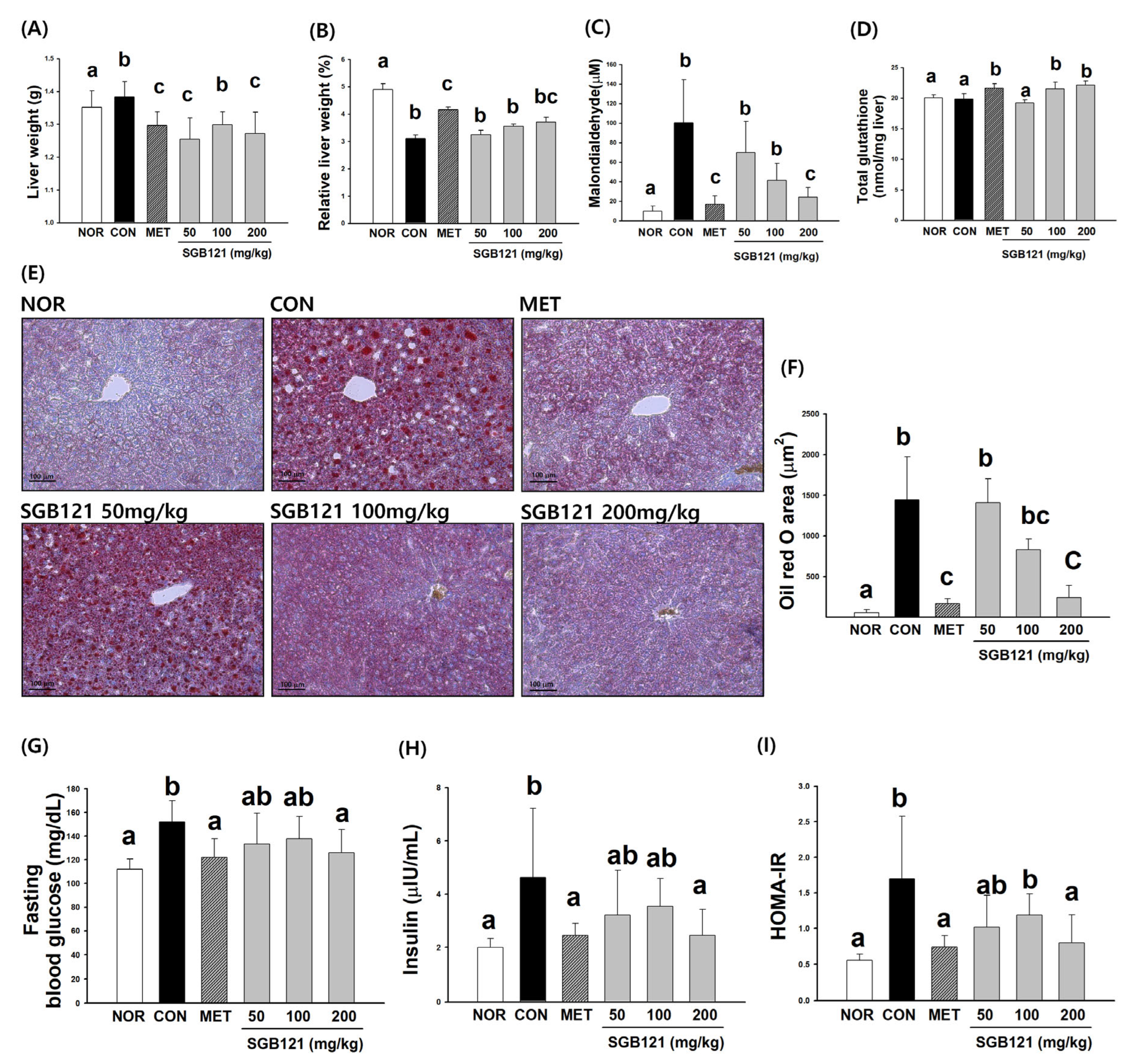
3.1.3. Hepatoprotective Effects of SGB121 via Reduction of Lipid Accumulation and Oxidative Stress
3.1.4. Improvement in Glucose Homeostasis and Insulin Sensitivity by SGB121
3.1.5. Improvement in Lipid Metabolism and Liver Enzyme Levels by SGB121
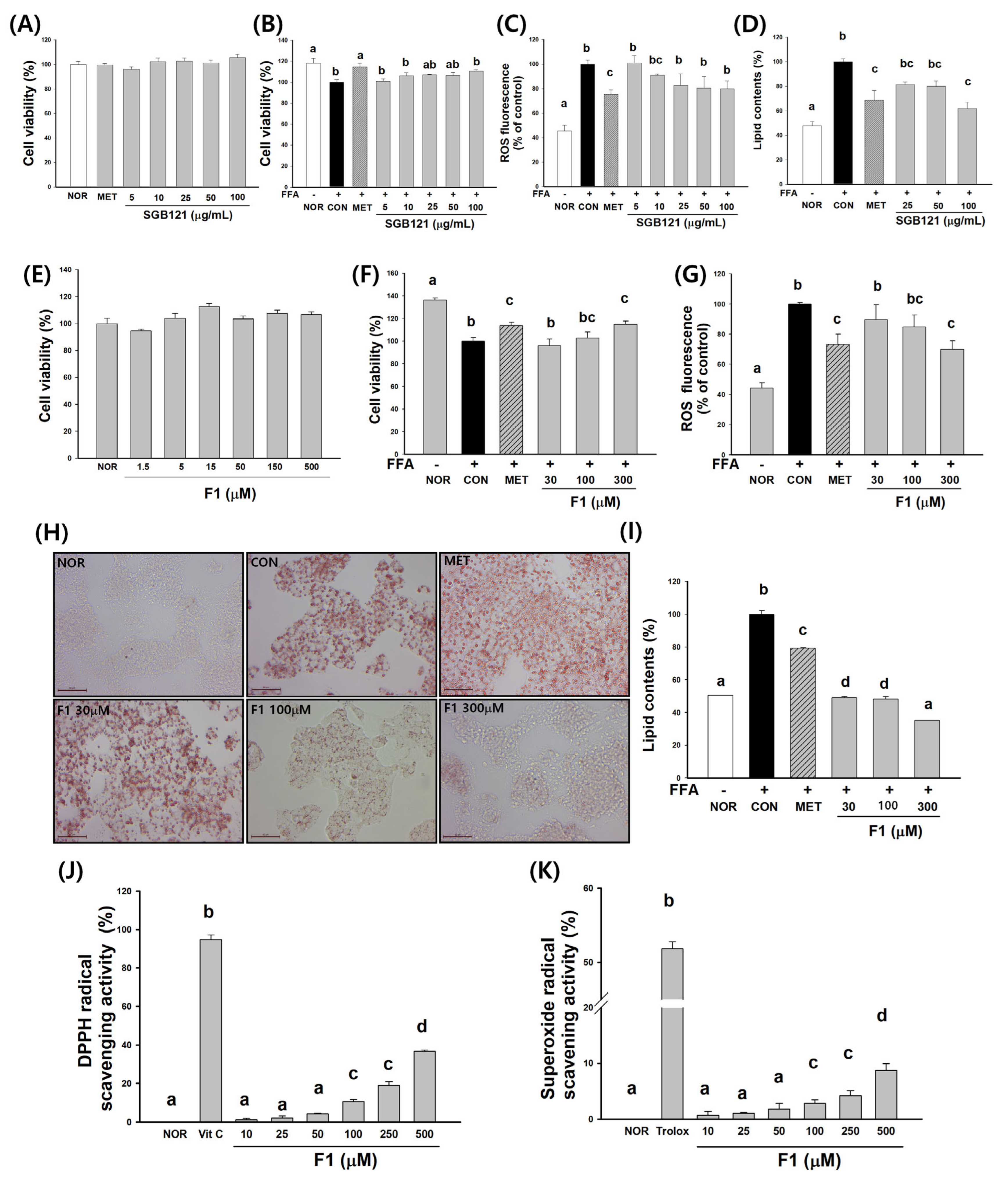
3.2. SGB121 Enhances Antioxidant Capacity and Attenuates Lipid Accumulation in HepG2 Cells
3.3. Ginsenoside F1 Enhances Antioxidant Capacity and Attenuates Lipid Accumulation in HepG2 Cells
3.3.1. Cytotoxicity and Cell Viability
3.3.2. Protection Against Oxidative Stress
3.3.3. Inhibition of Lipid Accumulation
3.3.4. Radical Scavenging Activity
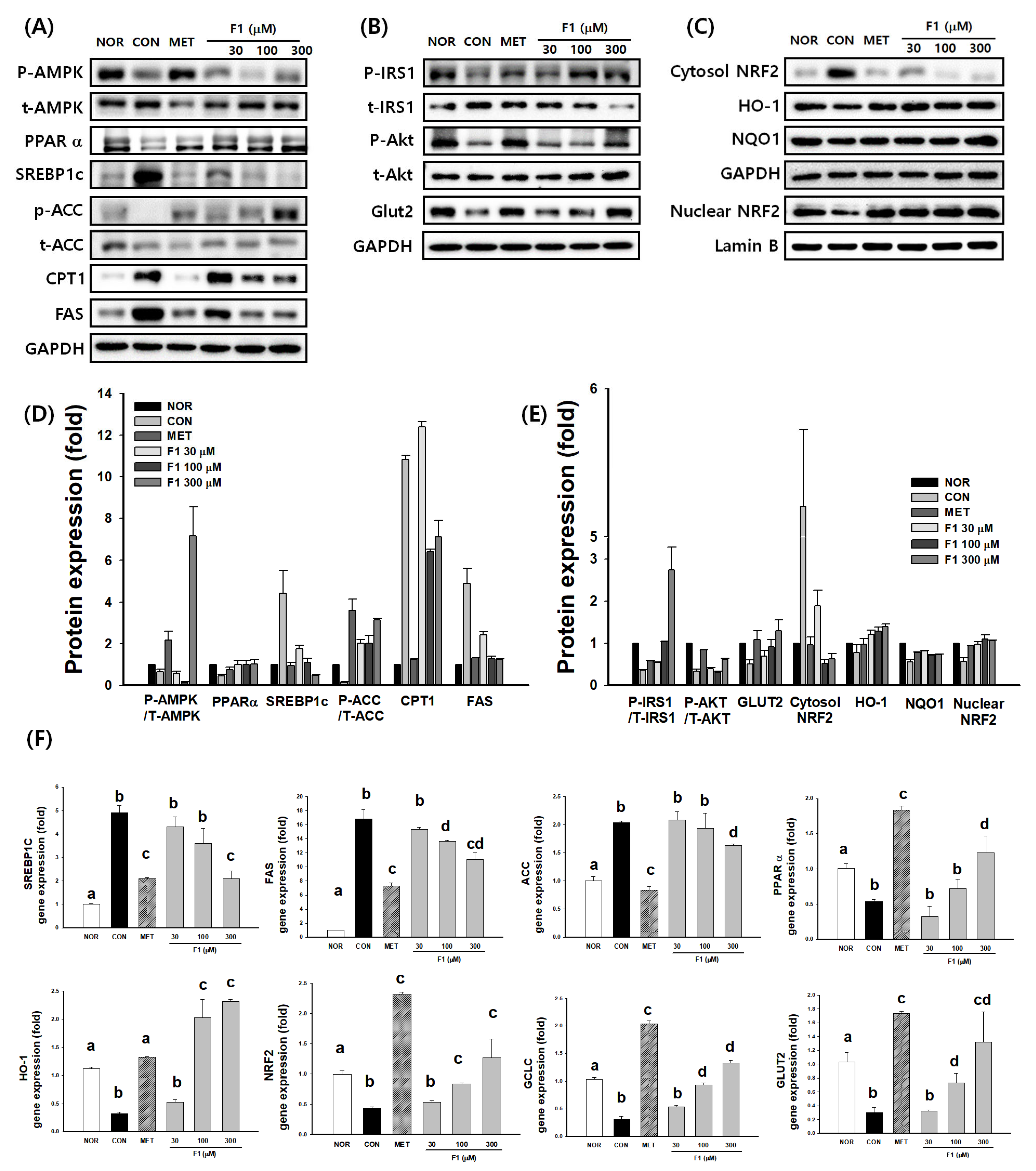
3.4. F1 Activates AMPK and Modulates Lipid Metabolism-Related Proteins
3.4.1. Regulation of Lipid Metabolism by F1 Through AMPK Activation
3.4.2. F1 Improves Insulin Signaling
3.4.3. F1 Promotes NRF2-Mediated Antioxidant Responses
3.4.4. F1 Regulates the Expression of Genes Involved in Lipid Metabolism and Antioxidant Defense
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAFLD | metabolic dysfunction-associated fatty liver disease |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| PPT | protopanaxatriol |
| HFD | high-fat diet |
| HFHC | high-fat, high-carbohydrate |
| BSA | bovine serum albumin |
| DMSO | dimethyl sulfoxide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HRP | horseradish peroxidase |
| DEXA | dual-energy X-ray absorptiometry |
| MDA | malondialdehyde |
| TBA | thiobarbituric acid |
| GSH | glutathione |
| TG | triglyceride |
| TC | total cholesterol |
| AST | aspartate amino transferase |
| ALT | alanine aminotransferase |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
References
- Dong, H.; Zhao, Y.; Teng, H.; Jiang, T.; Yue, Y.; Zhang, S.; Fan, L.; Yan, M.; Shao, S. Pueraria lobata antioxidant extract ameliorates non-alcoholic fatty liver by altering hepatic fat accumulation and oxidative stress. J. Ethnopharmacol. 2024, 333, 118468. [Google Scholar] [CrossRef]
- Jiang, M.; Butt, A.S.; Cua, I.H.; Pan, Z.; Al-Busafi, S.A.; Méndez-Sánchez, N.; Eslam, M. MAFLD vs. MASLD: A year in review. Expert. Rev. Endocrinol. Metab. 2025, 20, 267–278. [Google Scholar] [CrossRef]
- Dayal, U.; Soni, U.; Bansal, S.; Aggarwal, K.; Chennupati, C.; Kanagala, S.G.; Gupta, V.; Munjal, R.S.; Jain, R. MAFLD: Exploring the Systemic Effects Beyond Liver. J. Community Hosp. Intern. Med. Perspect. 2025, 15, 42–48. [Google Scholar] [CrossRef]
- Acierno, C.; Barletta, F.; Caturano, A.; Nevola, R.; Sasso, F.C.; Adinolfi, L.E.; Rinaldi, L. Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights. Nutrients 2025, 17, 2229. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Soares, P.; Chavarria, D.; Benfeito, S.; Cagide, F.; Teixeira, J.; Oliveira, P.J.; Borges, F. Decreasing the burden of non-alcoholic fatty liver disease: From therapeutic targets to drug discovery opportunities. Eur. J. Med. Chem. 2024, 277, 116723. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Maity, D.K.; Kumar, A.; Sarkar, S.; Bhattacharya, T.; Sahu, A.; Sreedhar, R.; Arumugam, S. Beyond obesity: Lean metabolic dysfunction-associated steatohepatitis from unveiling molecular pathogenesis to therapeutic advancement. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 13647–13665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Zhang, Z.; Song, Z.; Xu, J.; Zhang, M.; Gong, M. Overview of Panax ginseng and its active ingredients protective mechanism on cardiovascular diseases. J. Ethnopharmacol. 2024, 334, 118506. [Google Scholar] [CrossRef]
- Bastola, T.; Pariyar, R.; Jeon, B.-M.; Baek, J.-I.; Chang, B.Y.; Kim, S.-C.; Kim, S.Y.; Seo, J. Protective effects of SGB121, ginsenoside F1-enriched ginseng extract, on scopolamine-induced cytotoxicity and memory impairments. J. Funct. Foods 2020, 74, 104165. [Google Scholar] [CrossRef]
- Cui, C.H.; Jeon, B.M.; Fu, Y.; Im, W.T.; Kim, S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl. Microbiol. Biotechnol. 2019, 103, 7003–7015. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Shi, J.; Wang, R.; Yang, Y.; Yang, L.; Zhao, S.; Wang, Z. Biosynthesis of rare 20(R)-protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with uridine diphosphate glycosyltransferase genes. J. Ginseng Res. 2019, 43, 116–124. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Mei, Y.; Liu, L.; Li, L.; Yang, L.; Wang, Z. Rare ginsenosides: A unique perspective of ginseng research. J. Adv. Res. 2024, 66, 303–328. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Chen, J.; Xie, D.; Wang, Y.; Zhou, J. Tea Polyphenol Epigallocatechin Gallate and the Gut–Health Axis: Unraveling Structural Characteristics, Metabolic Pathways, and Systemic Benefits. Adv. Nutr. 2025, 16, 100545. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 107, 154449. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.L.; Lee, S.; Park, K.H.; Park, C.; Huh, Y.; Jeong, N.Y.; Jung, J. Protective and therapeutic effect of (S)-ginsenoside F1 on peripheral nerve degeneration targeting Schwann cells: A pharmaco-neuroanatomical approach. Anat. Sci. Int. 2022, 97, 79–89. [Google Scholar] [CrossRef]
- Pillai, J.R.; Wali, A.F.; Shivappa, P.; Talath, S.; Attia, S.M.; Nadeem, A.; Rehman, M.U. Evaluating the anti-cancer potential and pharmacological in-sights of Physalis angulata Root Extract as a strong candidate for future research. J. Genet. Eng. Biotechnol. 2024, 22, 100410. [Google Scholar] [CrossRef]
- Hou, J.; Jeon, B.; Baek, J.; Yun, Y.; Kim, D.; Chang, B.; Kim, S.; Kim, S. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J. Ginseng Res. 2022, 46, 79–90. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Youn, K.; Jun, M. Protective effect of sargahydroquinoic acid against Aβ25–35-evoked damage via PI3K/Akt mediated Nrf2 antioxidant defense system. Biomed. Pharmacother. 2021, 144, 112271. [Google Scholar] [CrossRef]
- Almalki, R.S.; Gazzaz, M.; Miski, S.F.; Fayed, H.M.; Mohamed, B.M.S.A.; Hessin, A.F.; Afifi, S.M.; Esatbeyoglu, T.; Korany, R.M.S.; Elbaset, M.A. Repositioning gemigliptin for the alleviation of thioacetamide-induced liver fibrosis in rats: Targeting TLR4/MAPK, SIRT1/AMPK/Nrf2, PI3K/AKT/mTOR axis and apoptosis. Eur. J. Pharm. Sci. 2025, 212, 107192. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 1979, 254, 7558–7560. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-J.; Jung, D.S.; Shin, J.M.; Erdenebileg, S.; Nho, C.W. Heracleum dissectum Ledeb. ethanol extract attenuates metabolic syndrome symptoms in high-fat diet-induced obese mice by activating adiponectin/AMPK signaling. J. Funct. Foods 2021, 84, 104581. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Jeon, B.M.; Baek, J.I.; Kim, S.C.; Kim, S.Y. SGL 121 Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism Through AMPK Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 4534. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Chen, S.; Ren, Q.; Chen, J.; Liu, X.; Huang, R.; Huang, B.; Wei, N.; Wu, J. Anle138b improves insulin resistance in mice and its possible signal pathways. Med. Drug Discov. 2025, 28, 100232. [Google Scholar] [CrossRef]
- Riva, G.; Villanova, M.; Cima, L.; Ghimenton, C.; Bronzoni, C.; Colombari, R.; Crestani, M.; Sina, S.; Brunelli, M.; D’Errico, A.; et al. Oil Red O Is a Useful Tool to Assess Donor Liver Steatosis on Frozen Sections During Transplantation. Transplant. Proc. 2018, 50, 3539–3543. [Google Scholar] [CrossRef]
- Lalhminghlui, K.; Jagetia, G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA 2018, 4, Fso272. [Google Scholar] [CrossRef]
- Dobashi, Y.; Itoh, K.; Tohei, A.; Amao, H. Screening for intestinal microflora influencing superoxide dismutase activity in mouse cecal mucosa. J. Vet. Med. Sci. 2014, 76, 453–456. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Luo, S.; Jia, H.; Xu, Q.; Chang, R.; Dong, Z.; Gao, S.; Song, Q.; Dong, H.; et al. Nuclear factor erythroid 2-related factor 2 protects bovine mammary epithelial cells against free fatty acid-induced mitochondrial dysfunction in vitro. J. Dairy. Sci. 2021, 104, 12830–12844. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Heimann, M.; Elashry, M.I.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. Optimizing the Adipogenic Induction Protocol Using Rosiglitazone Improves the Physiological Parameters and Differentiation Capacity of Adipose Tissue-Derived Mesenchymal Stem Cells for Horses, Sheep, Dogs, Murines, and Humans. Animals 2023, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H. A Simple Outline of Methods for Protein Isolation and Purification. Endocrinol. Metab. 2017, 32, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.F.; Flowers, S.; Bhattacharya, R.; Faibish, D.; Behl, Y.; Kotton, D.N.; Gerstenfeld, L.; Moran, E.; Graves, D.T. FOXO1 modulates osteoblast differentiation. Bone 2011, 48, 1043–1051. [Google Scholar] [CrossRef]
- Abbasi, E.; Khodadadi, I. High-fat diet may increase the risk of insulin resistance by inducing dysbiosis. Metab. Open 2025, 27, 100381. [Google Scholar] [CrossRef]
- Wang, H.; Shen, H.; Seo, W.; Hwang, S. Experimental models of fatty liver diseases: Status and appraisal. Hepatol. Commun. 2023, 7, e00200. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, D.; Mao, R.; Yao, Z.; Wu, Q.; Lv, W. Global burden of MAFLD, MAFLD related cirrhosis and MASH related liver cancer from 1990 to 2021. Sci. Rep. 2025, 15, 7083. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, M.; Zhang, X.; Tang, X.; Zhang, Y.; Yang, X.; Xia, G. Development and validation of a nomogram model for predicting the risk of MAFLD in the young population. Sci. Rep. 2024, 14, 9376. [Google Scholar] [CrossRef]
- Xin, X.; Chen, C.; Xu, X.; Lv, S.; Sun, Q.; An, Z.; Chen, Y.; Xiong, Z.; Hu, Y.; Feng, Q. Caffeine ameliorates metabolic-associated steatohepatitis by rescuing hepatic Dusp9. Redox Biol. 2025, 80, 103499. [Google Scholar] [CrossRef]
- Nishikawa, S.; Sugimoto, J.; Okada, M.; Sakairi, T.; Takagi, S. Gene expression in livers of BALB/C and C57BL/6J mice fed a high-fat diet. Toxicol. Pathol. 2012, 40, 71–82. [Google Scholar] [CrossRef]
- Sugimoto, K.; Shinagawa, T.; Kuroki, K.; Toma, S.; Hosomi, R.; Yoshida, M.; Fukunaga, K. Dietary Bamboo Charcoal Decreased Visceral Adipose Tissue Weight by Enhancing Fecal Lipid Excretions in Mice with High-Fat Diet-Induced Obesity. Prev. Nutr. Food Sci. 2023, 28, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, Y.; Yang, X.; Qiao, Y.; Yi, G.; Fan, W.; Liu, H.; Tong, M. Effect of Trichinella spiralis-Derived Antigens on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice. ACS Pharmacol. Transl. Sci. 2024, 7, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxidative Med. Cell. Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Kojima, S.; Kuo, T.F.; Tatsukawa, H.; Hirose, S. Induction of cross-linking and silencing of Sp1 by transglutaminase during liver injury in ASH and NASH via different ER stress pathways. Dig. Dis. 2010, 28, 715–721. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Ding, R.B.; Tian, K.; Cao, Y.W.; Bao, J.L.; Wang, M.; He, C.; Hu, Y.; Su, H.; Wan, J.B. Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J. Agric. Food Chem. 2015, 63, 2413–2422. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, X.-l.; Liu, T.; Wang, M.-j.; Kang, J.-s.; Yang, J.; Mi, N. Lactucin ameliorates FFA-induced steatosis in HepG2 cells by modulating mitochondrial homeostasis through the SIRT1/PGC-1α signaling axis. Heliyon 2024, 10, e39890. [Google Scholar] [CrossRef]
- Służały, P.; Paśko, P.; Galanty, A. Natural Products as Hepatoprotective Agents-A Comprehensive Review of Clinical Trials. Plants 2024, 13, 1985. [Google Scholar] [CrossRef]
- Deja, S.; Fletcher, J.A.; Kim, C.-W.; Kucejova, B.; Fu, X.; Mizerska, M.; Villegas, M.; Pudelko-Malik, N.; Browder, N.; Inigo-Vollmer, M.; et al. Hepatic malonyl-CoA synthesis restrains gluconeogenesis by suppressing fat oxidation, pyruvate carboxylation, and amino acid availability. Cell Metab. 2024, 36, 1088–1104.e1012. [Google Scholar] [CrossRef]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, S.; Fan, P.; Li, L.; Feng, S.; Xiao, H.; Zhu, L. The Roles of Liver Inflammation and the Insulin Signaling Pathway in PM2.5 Instillation-Induced Insulin Resistance in Wistar Rats. Dis. Markers 2021, 2021, 2821673. [Google Scholar] [CrossRef]
| NOR | CON | MET | SGB121 (mg/kg) | |||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| Hepatic TG (mg/g liver) | 6.7 ± 0.6 a | 62.8 ± 7.6 b | 36.6 ± 4.9 c | 48.4 ± 3.8 d | 49.8 ± 5.3 d | 27.9 ± 4.9 c |
| Serum TG (mg/dL) | 46.4 ± 9.4 a | 101.5 ± 2.9 b | 51.8 ± 7.3 c | 85.4 ± 10.7 d | 76.8 ± 14.0 cd | 76.9 ± 7.4 cd |
| Hepatic TC (mg/g liver) | 3.0 ± 0.2 a | 8.17 ± 1.07 b | 5.15 ± 0.5 c | 7.07 ± 0.80 b | 6.22 ± 0.64 b | 3.74 ± 0.30 c |
| Serum TC (mg/dL) | 152.8 ± 5.7 a | 242.3 ± 13.5 b | 215.9 ± 6.8 c | 227.4 ± 9.4 b | 238.5 ± 13.3 bc | 203.9 ± 11.3 c |
| ALT (unit/mL) | 32.3 ± 4.5 a | 61.0 ± 7.0 b | 41.2 ± 5.0 c | 56.2 ± 6.5 b | 52.4 ± 5.1 bc | 53.8 ± 5.3 b |
| AST (unit/mL) | 61.6 ± 6.7 a | 91.3 ± 8.0 b | 67.2 ± 3.5 a | 113.4 ± 4.5 d | 100.0 ± 19.0 b | 71.1 ± 9.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.Y.; Kim, I.; Park, H.; Kim, S.; Kim, S.Y. Protective Role of Ginsenoside F1-Enriched Extract (SGB121) in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Nutrients 2025, 17, 3693. https://doi.org/10.3390/nu17233693
Chang BY, Kim I, Park H, Kim S, Kim SY. Protective Role of Ginsenoside F1-Enriched Extract (SGB121) in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Nutrients. 2025; 17(23):3693. https://doi.org/10.3390/nu17233693
Chicago/Turabian StyleChang, Bo Yoon, In Kim, Hyungmin Park, Sunchang Kim, and Sung Yeon Kim. 2025. "Protective Role of Ginsenoside F1-Enriched Extract (SGB121) in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)" Nutrients 17, no. 23: 3693. https://doi.org/10.3390/nu17233693
APA StyleChang, B. Y., Kim, I., Park, H., Kim, S., & Kim, S. Y. (2025). Protective Role of Ginsenoside F1-Enriched Extract (SGB121) in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Nutrients, 17(23), 3693. https://doi.org/10.3390/nu17233693







