1. Introduction
Citrus fruits, comprising various species within the genus
Citrus (family
Rutaceae), constitute one of the most extensively cultivated and economically significant crop groups globally. Renowned for their sensory qualities and rich nutritional profile, which includes vitamin C, dietary fiber, and phytochemicals, citrus fruits are widely linked to enhanced immune function, improved cardiovascular health, and reduced incidence of chronic diseases [
1,
2]. Global citrus production is estimated at approximately 22.5 million tonnes for the 2024/2025 season, with the Mediterranean region leading in the cultivation of oranges and lemons [
3].
More than 40% of the world’s citrus fruit production is processed into various products, including preserves, fresh juices, and flavorings [
4]. This process yields significant quantities of agro-industrial residues, primarily comprising peels, seeds, and residual pulp, which account for up to 50% of the initial fruit mass [
5]. The high organic content of this waste poses challenges for direct disposal as it can disrupt soil and water quality, highlighting the urgent need for sustainable valorization strategies.
Recent studies have emphasized the potential of citrus by-products as an excellent source of bioactive compounds suitable for applications in the nutraceutical and cosmeceutical fields [
6,
7]. Citrus peels and seeds contain significantly higher concentrations of polyphenols and specific flavonoids, including narirutin, hesperidin, and naringin, than the edible pulp [
8]. These phytochemicals have demonstrated a broad spectrum of biological activities that benefit human health [
2,
9].
Upcycling agricultural waste in cosmeceutical formulations represents a promising example of circular economy principles [
10]. Despite the well-documented bioactive profile of citrus waste, the use of these secondary streams for high-value applications remains limited. Addressing this gap through innovative technologies and green solvents can simultaneously reduce environmental impact and create new opportunities in the cosmeceutical market, thereby supporting sustainable development. In this context, natural deep eutectic solvents (NaDESs) have emerged as environmentally friendly and effective solvents for recovering valuable compounds [
11]. These methodologies align with circular economy principles and greatly decrease the environmental impacts of traditional solvents.
Skin aging is a complex and multifactorial phenomenon marked by structural degradation, reduced barrier function, and impaired regenerative capacity, primarily driven by oxidative stress. Reactive oxygen species (ROS) contribute to extracellular matrix degradation, cellular senescence, and diminished activity of fibroblasts and keratinocytes, ultimately leading to compromised collagen synthesis and delayed wound healing [
12]. Therefore, phytochemicals that can mitigate oxidative stress and support skin function are appealing candidates for cosmetic applications.
This study aims to investigate the biological properties of citrus waste-derived extracts obtained through NaDES-based green extraction, to develop sustainable, high-value ingredients for topical formulations. By upcycling citrus by-products, this research supports innovation in skin health and advances the circular cosmeceutical industry.
2. Materials and Methods
2.1. Chemicals
Phosphate-buffered saline (PBS) tabs (giving a 137 mM NaCl, 2.7 mM KCl phosphate-buffered solution, pH 7.4, final concentration 0.01 M), trypsin–EDTA, the antibiotic solution 100× (10,000 U/mL penicillin and 10 mg/mL streptomycin), Gallic acid, the Folin–Ciocalteu phenol reagent, Hydroxytyrosol, Fluorescein, 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH), Trolox, neocarzinostatin, Tween 20, bovine serum albumin (BSA), ethanol and the oxidative stress inducers lipopolysaccharide (LPS) and menadione were obtained from Sigma-Aldrich (St. Louis, MO, USA). An LPS stock solution (5 mg/mL) was prepared in PBS and stored at −20 °C. A menadione stock solution (100 mM) was prepared in DMSO and stored at −20 °C. Choline chloride (ChCl, ≥98%), Betaine (Bet, ≥98%), Urea (U, ≥99%), DL-malic acid (MA, ≥98%), Citric Acid (CA, ≥98%), L-(+)-Tartaric acid (TA, ≥99.5%), and D-(+)-Glucose (Glu, ≥99.5%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM) high glucose was acquired from Microgem (Naples, Italy), and fetal bovine serum (FBS) was acquired from Thermo Fisher Scientific (Waltham, MA, USA). The WST-8 and Cytotoxicity Lactate Dehydrogenase (LDH) Assay kits were acquired from Dojindo Molecular Technologies (Kumamoto, Japan). The chemiluminescent probe (AquaSpark™510 Peroxide Probe) was provided by Biosynth Carbosynth (Staad, Switzerland). A 10 mM mother solution was prepared by dissolving the probe in DMSO. A RNeasy Mini Kit was purchased from QIAGEN (Hilden, Germany). Primers for Real-Time PCR were purchased from IDT (Coralville, IA, USA). Analytical standards of hesperidin, hesperetin, naringin, and rutin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Isotopically labeled standards rutin-D3 were obtained from Santa Cruz Biotechnology (Dallas, TX, USA), hesperetin-13C-D4 from Sigma-Aldrich (St. Louis, MO, USA), and naringenin-D4 from MedChemExpress (Monmouth Junction, NJ, USA).
2.2. Raw Material
Oranges (Citrus sinensis) and lemon (Citrus limon) pomace, including peels and seeds, were kindly provided by Dr. Paolo Rapisarda (Acireale, CT, Italy). After collection, the pomace was freeze-dried for 24 h using a Labconco™ FreeZone™ (Labconco Corporation, Kansas City, MO, USA) 4.5 L and stored at −80 °C until the experiments were conducted.
2.3. Preparation and Characterization of NaDES Mixtures
NaDESs were prepared according to the methodology established by Dai et al. [
13], utilizing the molar ratios specified in
Table 1.
Briefly, Choline Chloride (ChCl) and Betaine (Bet) served as hydrogen bond acceptors (HBAs), while Urea (U), Glucose (G), Malic acid (MA), Citric acid (CA), and Tartaric acid (TA) acted as hydrogen bond donors (HBDs). The two components of the mixture were combined within a container, subjected to magnetic stirring, and heated to 70 °C until a transparent liquid was obtained. Subsequently, distilled water at 20 or 40 wt% was added to facilitate the formation of a homogeneous liquid phase. Following the synthesis, NaDESs were characterized based on their appearance, pH, and density, as reported in the
Supplementary Materials (Section S1).
2.4. Extraction Optimization Using Conventional Solvents
Two extraction methodologies were employed to compare efficiency and optimize the process. Extractions were performed using mixtures of water (H2O) and ethanol (EtOH) at ratios of 50:50 and 30:70 (v/v), with a solid–liquid ratio of 1:10 (i.e., 1 g of lemon waste (L) or orange waste (O) combined with 10 g of solvent). The extractions were conducted as follows:
- -
Mechanical agitation: Samples were stirred on a roller mixer for 3 h at room temperature (rt) in the dark to prevent photodegradation of bioactive compounds.
- -
Ultrasound-assisted extraction (UAE): Samples underwent alternating 5 min cycles in an ultrasonic bath followed by 1 min cooling on ice, for a total extraction time of 1 h, to minimize overheating and degradation.
After extraction, all mixtures were centrifuged for 10 min at 1200×
g, separated from the residual biomass, and filtered through a filter membrane (0.2 μm pores). Samples were stored in the dark at 4 °C. Each extraction was performed in triplicate. Following the production, the extracts were characterized based on their appearance and pH, as reported in the
Supplementary Materials (Section S2).
2.5. Evaluation of Total Phenolic Content
The total phenolic content (TPC) of extracts was assessed using the Folin–Ciocalteu colorimetric method following the procedure originally reported by Ainsworth et al. [
14]. This method relies on the redox reaction between phenolic compounds and the Folin–Ciocalteu reagent. In this process, phenolic compounds act as electron donors, reducing the reagent and forming a blue-colored complex. The color intensity, which correlates with the phenolic content of the sample, can be detected spectrophotometrically.
Briefly, each extract solution (100 μL) was combined with 200 µL of Folin–Ciocalteu reagent and 800 µL of sodium carbonate solution (700 mM). The reaction mixtures were incubated for 2 h at room temperature in the dark. Subsequently, 200 µL of each sample was transferred into a 96-well microplate, and absorbance was measured at 765 nm using a spectrophotometric reader. Distilled water was used as blank. Gallic acid (100–1000 µg/mL) served as the standard compound, and the TPC was expressed as μg of gallic acid equivalent (GAE)/mg citrus extract. Each measurement was performed in triplicate, and the data represent the mean of three independent experiments.
2.6. Citrus Waste Extracts Preparation Using NaDESs
O or L was mixed with NaDES at a solid–liquid ratio of 1:10 (w/w); i.e., 1 g of L/O was added to 10 g of diluted solvents. The suspension was stirred on a roller stirrer at rt, in the dark, for 3 h. After extraction, all mixtures were centrifuged for 10 min at 1200× g, separated from the residual biomass, and filtered through a filter membrane (0.2 μm pores). Finally, samples were stored in the dark at 4 °C. Each extraction was performed in triplicate. Sample nomenclature according to solvent used:
O(ChCl: U): extract obtained using ChCl: U starting from Orange Matrix;
L(ChCl: U): extract obtained using ChCl: U starting from Lemon Matrix;
O(ChCl: CA): extract obtained using ChCl: CA starting from Orange Matrix;
L(ChCl: CA): extract obtained using ChCl: CA starting from Lemon Matrix;
O(Bet: U): extract obtained using Bet: U starting from Orange Matrix;
L(Bet: U): extract obtained using Bet: U starting from Lemon Matrix.
Following the extractions, extracts were characterized based on appearance, including crystallization and precipitation phenomena, pH evaluation, as reported in the
Supplementary Materials (Section S3), and TPC.
2.7. Polyphenol Profile by HPLC-ESI-MS/MS
Liquid chromatography analysis was performed using an Agilent 1290 Infinity II ultra-high-performance liquid chromatography (UHPLC) system, coupled to an Agilent 6495 LC/TQ triple quadrupole mass spectrometer. The system featured an electrospray ionization (ESI) source configured for negative ionization mode and operated in multiple reaction monitoring (MRM) acquisition mode. Analytical separation was performed using Phenyl-Hexyl (1.7 µm, 150 mm × 2.1 mm i.d; Waters, Milford, MA, USA). The mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol) in gradient elution mode: B 5%, B 35% to 4 min and kept to 8 min, B 40% to 11 min, B 55% to 12 min, B 70% to 13 min and kept to 16 min, B 80% to 17 min and kept to 20 min. The equilibration time was 3 min, and the total run time was 23 min. The flow rate was 0.3 mL/min, the column temperature was maintained at 33 °C, and the injection volume was 2 μL.
The MS/MS parameters were optimized by directly infusing single analytes diluted in the mobile phase. Argon gas was employed as the collision gas, and nitrogen as the nebulizer and heater gas. The nitrogen flow rates were set to 14 L/min for nebulization and 12 L/min for desolvation. The ion source block and sheath gas temperature were set at 220 °C and 350 °C, respectively. Capillary and nozzle voltages were adjusted to 3.00 kV and 2.00 kV, respectively.
Standard solutions of five polyphenols were used to evaluate the polyphenol profiles of each extract. Single stock solutions (1 mg/mL) of rutin, naringin, naringenin, hesperetin, and hesperidin were prepared in ethanol or dimethyl sulfoxide (DMSO) and stored until use at −80 °C.
Isotopically labeled internal standards (ISs) were included. Rutin-d3, hesperetin-13C-d4, and naringenin-d4 were prepared in DMSO at 1 mg/mL and stored until use at −80 °C.
Table 2 reports the optimized MS/MS conditions for each analyte and its internal standard, detailing the selected precursor and product ions as well as the corresponding collision energies.
Standard solutions used for method validation were prepared by diluting stock solutions in the mobile phase. Spiked sample solutions (QC) used for matrix-matched calibration curve were obtained by adding diluted stock solution in the range 2–100 ng/mL to the solvent (ChCl: U; ChCl: CA, Bet: U).
The limit of detection (LOD) and limit of quantification (LOQ) were established based on signal-to-noise ratios of 3 and 10, respectively. Matrix effects for each compound were assessed by performing an ANOVA test to compare six-point calibration curves (2, 5, 10, 25, 50, and 100 ng/mL) prepared in mobile phase and in ChCl: U; ChCl: CA, Bet: U, respectively.
Selectivity was assessed by comparing the chromatograms obtained from standards, samples, and spiked sample solutions. ISs were employed for calibration curves at a fixed concentration of 10 ng/mL.
Accuracy was expressed as bias (%) = (STDm-STDs)/STDs, where STDm and STDs represent the mean measured concentration and spiked concentration, respectively. Precision was calculated as the coefficient of variation (CV%).
2.8. Human Keratinocytes Cellular Model
The human keratinocyte cell line (HaCaT), spontaneously immortalized and derived from adult epidermis, was used for all in vitro experiments. The cells were kindly provided by Prof. F. Martini (University of Ferrara, Italy) and originally purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS), 2.5 mM L-glutamine, and penicillin/streptomycin (100 U/mL/100 µg/mL) at 37 °C in a humidified incubator with 5% CO2. The culture medium was refreshed daily before experiments to promote cell growth and viability.
2.9. Cell Cytotoxicity and Cell Viability
Cellular cytotoxicity and viability were evaluated using two complementary colorimetric assays, based, respectively, on lactate dehydrogenase (LDH) release and WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] reduction. The combination of these methods allowed a comprehensive assessment of both membrane integrity and metabolic activity in HaCaT cells following treatment with the extracts.
The detection of LDH release from cells was evaluated in the culture medium using the Cytotoxicity LDH Assay Kit (Dojindo Molecular Technologies, Kumamoto, Japan). In the reaction, LDH catalyzes the oxidation of lactate to pyruvate, simultaneously reducing NAD+ to NADH. The generated NADH then drives a diaphorase-dependent conversion of a tetrazolium salt into a red formazan product. The amount of formazan, quantified spectrophotometrically at 490 nm, is directly proportional to the extent of cell membrane disruption.
For these experiments, HaCaT cells were seeded in 96-well plates (10 × 104 cells/well) and cultured at 37 °C with 5% CO2. After 24 h, the cells were treated for 24 h with different dilution of extracts O(ChCl: U), L(ChCl: U), O(ChCl: CA), L(ChCl: CA), O(Bet: U), and L(Bet: U), (dilution range of 1:10–1:1000) and then the culture medium was collected. The change in the absorbance between treated samples and controls was monitored at 37 °C using an Allsheng FlexA-200 Microplate Reader (Allsheng, Hangzhou, China). Each measurement was performed in triplicate, and the data represent the mean of three independent experiments.
Table S4 summarizes the calculated concentrations (µg/mL) of the major identified phenolic compounds for each extract and dilution, allowing direct comparison of dose levels across treatments.
To complement LDH results and estimate metabolic activity, cell viability was measured using the WST-8 assay. In this colorimetric bioassay, the salt WST8 is reduced by cellular dehydrogenases to orange, water-soluble formazan product, whose absorbance at 450 nm directly reflects the number of living cells.
For these experiments, HaCaT cells were seeded and treated under the same experimental conditions as described for the LDH using the extracts O(ChCl: U), L(ChCl: U), O(Bet: U), and L(Bet: U) in the dilution range of 1:100–1:1000. The decrease in absorbance between the samples and the control was monitored at 450 nm using an Allsheng FlexA-200 Microplate Reader at 37 °C. Each measurement was performed in triplicate, and the data represent the mean of three independent experiments.
2.10. Antioxidant Activity of the Extracts in HaCaT Cells
The intracellular H
2O
2 production was assessed in HaCaT cells through the chemiluminescent (CL) cell-based bioassay previously developed by our group [
15]. The working solution of the probe and the pro-oxidant stimuli (menadione or LPS) were prepared in PBS from their respective stock solutions. Before testing, calibration curves were obtained to verify the linear relationship between probe emission and H
2O
2 concentration in HaCaT cells exposed to increasing doses of the pro-oxidants, as reported in the
Supplementary Materials (Section S5).
Once the method was optimized in HaCaT cells, the antioxidant activities of the extracts were investigated. HaCaT cells (10 × 10
4 cells/well) were seeded in a 96-well black plate with a transparent bottom. The following day, the cells were treated for 24 h with extracts O
(ChCl: U), L
(ChCl: U), O
(Bet: U), and L
(Bet: U) (dilution range: 1:100–1:1000) and hydroxytyrosol (HT) (concentration range: 0.5–25 µM) as positive antioxidant control [
16]. Following treatment, the medium was replaced with 50 µL of probe working solution (final concentration of 5 μM), and the plates were incubated for 20 min at 37 °C. Subsequently, oxidative stress was induced by adding menadione (50 µM) or LPS (25 µg/mL) to the wells. CL emission was monitored for 40 min using a Varioskan Flash plate reader (Thermo Fisher Scientific, Waltham, MA, USA). The PBS solution was used as a negative control. Each measurement was performed in triplicate, and the data represent the mean of three independent experiments.
2.11. The Scratch Assay in HaCaT Cells
HaCaT cells (80 × 104 cells/well) were seeded into 6-well transparent plates and cultured until reaching approximately 80% confluence. A uniform scratch was then generated through the cell monolayer using a sterile 200 µL pipette tip. Detached cells and debris were removed by gentle washing with PBS, and fresh complete medium was added.
Images of the scratched area were captured immediately after the scratch (time 0) using an Olympus CL40 inverted microscope connected to an electron-multiplying charge-coupled device (EMCCD) camera (ImagEM-X2, Hamamatsu). After the scratch formation, the culture medium was supplemented with the various extracts (O
(ChCl: U), L
(ChCl: U), O
(Bet: U), L
(Bet: U)) at a dilution of 1:100. The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO
2 throughout the experiment. The wound area was re-examined and imaged at 24 h and 48 h post-treatment to assess the rate of wound closure. Untreated cells served as the negative control. Images were analyzed using ImageJ (version 1.54p; NIH, Bethesda, MD, USA). The wound zone was outlined, and its area measured (µm
2) at each time point. For each condition, three independent wound regions were imaged per well, and the average wound area was used for the calculation. Wound closure was quantified using ImageJ software and calculated according to Equation (1).
This formula quantifies the relative decrease in the wound area over time, providing a direct measure of the keratinocytes’ ability to restore the integrity of the cell monolayer under different treatment conditions [
17,
18]. Each measurement was performed in triplicate, and the data represent the mean of three independent experiments.
2.12. Protein Release
HaCaT cells (80 × 104 cells/well) were seeded for 24 h into 6-well transparent plates. Then, cells were pretreated with extracts (O(ChCl: U), L(ChCl: U), O(Bet: U), L(Bet: U)) at a dilution of 1:100 for 24 h. After treatment, the supernatants were collected and centrifuged to eliminate cellular debris. The concentrations of IL-1β, IL-10, IL-8, and IL-6 were quantified using commercial ELISA kits (Elabscience Biotechnology Co., Wuhan, China) following the manufacturer’s instructions. All determinations were performed in triplicate, and data represent the mean of three independent experiments.
2.13. Immunofluorescence Determination of 53BP1 Foci
HaCaT (25 × 10
4 cells/well) were seeded onto glass coverslips, pretreated with O
(Bet: U) (dilution 1:100) for 24 h. Subsequently, cells were exposed to the genotoxic agent neocarzinostatin (NCS) (0.13 μM) for 2 h to induce DNA damage, followed by a 24 h recovery period in fresh medium. Cells were then fixed in cold 70% ethanol for 10 min, rinsed once with PBS, and blocked for 30 min in 4% BSA prepared in PBS containing 0.1% Tween-20 (PBST). After blocking, samples were incubated for 1 h at room temperature with the primary antibody anti-53BP1 (Novus Biologicals, Littleton, CO, USA) diluted in 1% BSA-PBST buffer. Following three washes in PBST, slides were incubated for 1 h with Alexa Fluor 555-conjugated goat anti-rabbit secondary antibody (Cell Signaling Technology, Danvers, MA, USA) in 1% BSA–PBST. Nuclei were counterstained with Hoechst 33,342 (Thermo Fisher Scientific, Waltham, MA, USA), and slides were mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Images were acquired with an Olympus CL40 inverted microscope connected to an electron-multiplying charge-coupled device (EMCCD) camera (ImagEM-X2, Hamamatsu). 53BP1 foci detected within the nuclei were quantified and classified as follows: nuclei containing 0 ≤ foci < 5, 5 ≤ foci < 10, 10 ≤ foci < 20, and foci ≥ 20, in increasing order of DNA damage severity [
19]. The distribution of cells across these classes was expressed as percentage values for each treatment. Differences among distributions were assessed using the Chi-square test, while the percentage of cells with >20 foci (high-damage class) was analyzed by one-way ANOVA followed by Tukey’s post hoc test. Data are expressed as mean ± SD from three independent experiments, each performed in triplicate.
2.14. Statistical Analysis
Data are expressed as mean ± SD (unless otherwise indicated) from at least three independent biological replicates, each performed in triplicate. Statistical analyses were carried out using GraphPad Prism (GraphPad Prism v6.0, GraphPad Software Inc., La Jolla, CA, USA). Data normality was verified using the Shapiro–Wilk test. Differences among multiple groups were assessed by one-way ANOVA or two-way ANOVA, followed by appropriate post hoc tests (Tukey or Dunnett) as indicated in the figure legends. When appropriate, repeated-measures ANOVA was applied to evaluate changes over time. When appropriate, 95% confidence intervals (CI) were calculated to indicate data variability and the precision of mean estimates. IC50 values were determined from non-linear regression analysis of dose–response curves using a four-parameter logistic model. A p-value < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. NaDES Preparation and Characterization
Previous research highlighted that citrus waste is a sustainable source rich in bioactive compounds, especially flavonoids and phenolic acids. However, traditional extraction methods from plant matrices often rely on volatile and hazardous organic solvents, which pose significant environmental and safety concerns. To overcome these issues, in our study we exploited NaDES as a safer, eco-friendly alternative.
NaDES formulations were initially screened for stability, pH, and density. Among the tested formulations, ChCl: U, ChCl: CA, and Bet: U demonstrated the most favorable physicochemical properties and were selected for subsequent extraction experiments.
Detailed characterization data, including appearance, crystallization behavior, pH, and density values for all NaDES formulations, are provided in
Supplementary Materials (Table S1).
3.2. Optimization of Polyphenols Extraction by Using Conventional Solvents
Two distinct extraction methodologies were implemented to identify the more efficient technique and optimize the extraction process. The first method used mechanical agitation, while the second involved the use of UAE. Both extractions were carried out with a conventional solvent blend of H2O and EtOH in ratios of 50:50 and 30:70 (v/v). These solvent mixtures were selected for their ability to effectively dissolve polyphenolic compounds while prioritizing safety over more toxic alternatives, such as methanol and hexane.
All extracts were clear, homogeneous, and non-viscous, with lower pH values in more aqueous (50:50) mixtures and lemon samples due to higher acid content and increased acid dissociation (
Table S2). The extraction efficiency of UAE and roller agitation was quantitatively assessed via TPC using the Folin–Ciocalteu assay (
Table 3).
Two-way ANOVA (extraction method × extraction condition) revealed no statistically significant differences (
p > 0.05), indicating that UAE and roller agitation performed comparably across all matrices and solvent compositions. A trend toward higher TPC values was observed with 70% ethanol, consistent with improved solubilization of phenolic compounds under higher ethanol content [
20]. Given the comparable performance, lower energy requirements, simpler scale-up, and reduced environmental impact compared to UAE, roller agitation was selected for subsequent NaDES extractions.
3.3. Citrus Waste Extracts by Using NaDESs
Based on previous observations, ChCl: U, ChCl: CA, and Bet: U were selected for the extraction experiments. Next, extractions were performed using the roller agitation method, and the extracts were characterized.
All extracts were clear, homogeneous, free of particulates or crystallization, with slightly acidic pH (4.20–5.60); ChCl: CA extracts showed the lowest pH due to citric acid, yet all remained within acceptable ranges for further biological testing (
Table S2). Next, the extraction yield was evaluated by determining the TPC of the obtained extracts using the Folin–Ciocalteu assay. All extracts demonstrated a high polyphenol content (
Table 4). The extract O
(Bet: U) exhibited the highest TPC, with 11.97 ± 0.11 μg GAE/mg extract.
All extracts obtained with NaDESs showed TPC values ranging from 6.30 to 11.97 μg GAE/mg, generally higher than those from EtOH: H2O (5.51–7.65 μg GAE/mg). However, one-way ANOVA comparing NaDESs and conventional ethanol–water extracts revealed no significant differences in TPC (p > 0.05), although a clear trend toward improved extraction with NaDESs was observed. These results suggest that NaDESs may serve as a sustainable alternative to conventional ethanol–water mixtures without compromising extraction efficiency.
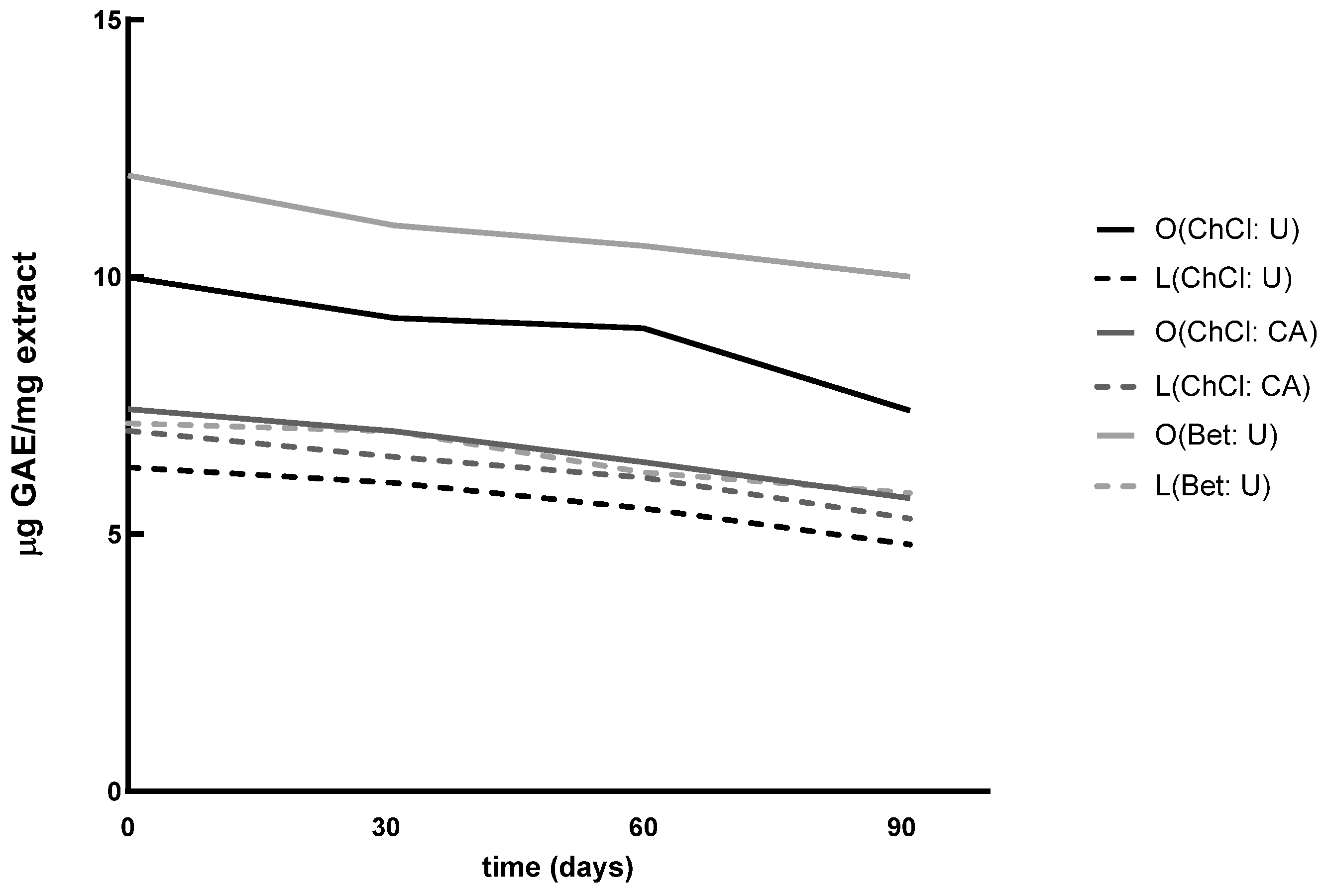
Next, TPC was assessed at 30, 60, and 90 days from the initial time point (t
0). A progressive reduction in phenolic content was observed (
Figure 1); however, statistical analysis by repeated-measures ANOVA revealed no significant differences over time (
p > 0.05). This suggests that, while natural degradation processes occurred, the extracts remained largely stable under the applied storage conditions (protected from light and kept at 4 °C).
3.4. Characterization of Citrus Waste Extracts Through HPLC-ESI-MS/MS Analysis
UHPLC-ESI MS/MS determined the polyphenol content in the three formulations. The method was validated using standard samples, QCs, and real samples.
Intra- and inter-day accuracy and precision were within 15% for all analytes at the three tested concentration levels (LOQ, 5× LOQ, and 10× LOQ), confirming the reliability and robustness of the developed method. The comparison of standard solutions, spiked samples, and blank matrices demonstrated good selectivity under MRM acquisition mode.
Due to matrix effects (p < 0.05), quantification was performed using matrix-matched calibration curves obtained by plotting the analyte/IS peak area ratio against the analyte concentration, with linear least-squares regression analysis. The determination coefficients (r2) of the analytical calibration curves were ≥0.990 for all analytes. LOD and LOQ for all the analytes were 0.1 ng/mL and 1 ng/mL, respectively.
Orange and lemon extracts obtained with the three solvents (ChCl: U, ChCl: CA, Bet: U) were analyzed in triplicate using UHPLC-MS/MS. Before analysis, the extracts were appropriately diluted, and a fixed amount of IS (10 ng/mL) was added to each sample to ensure accurate quantification.
Table 5 reports the results obtained.
The data obtained revealed the efficiency of different NaDES formulations in extracting bioactive compounds from citrus waste, both lemon and orange matrices. Each solvent exhibited varying extraction efficiencies, suggesting distinct affinities for specific classes of bioactive molecules.
Rutin was extracted more effectively from lemon waste than orange waste across all NaDES formulations. ChCl: U achieved the highest rutin concentration (23.42 μg/mL), outperforming all other combinations. Naringin was predominantly extracted from orange waste, with the highest yield observed using ChCl: CA (20.14 μg/mL), followed by Bet: U (15.41 μg/mL). In contrast, lemon waste yielded much lower concentrations (0.45–0.95 μg/mL) in all NaDES formulations. Across all samples, naringenin levels were below the limit of quantification (LOQ). This could indicate minimal availability of this aglycone in the analyzed waste materials or challenges in its extraction using these solvents. Hesperetin concentrations were generally low, with the highest levels observed in orange waste (Bet: U, 0.76 μg/mL). Hesperidin showed the highest extraction yield, with Bet: U from orange waste achieving a concentration of 1174.90 μg/mL, significantly surpassing all other combinations. Lemon waste also produced high levels of hesperidin, with yields ranging from 143.10 to 466.00 μg/mL.
The results underline the versatility and efficiency of NaDESs in extracting bioactive compounds from citrus waste. Bet: U emerged as the most promising solvent due to its dual polarity properties, which enable high yields of hesperidin. Further optimization of NaDES formulations could enhance their applications in the nutraceutical and cosmeceutical industries.
3.5. Safety Evaluation of Citrus Waste Extracts in HaCaT Cells
The biological activities of the extracts were assessed using the immortalized human keratinocyte cell line HaCaT, a widely recognized in vitro model for studying epidermal homeostasis and pathophysiology [
21]. Indeed, due to their stability and high proliferative capacity, HaCaT cells enable reproducible assessment of cellular responses to bioactive compounds.
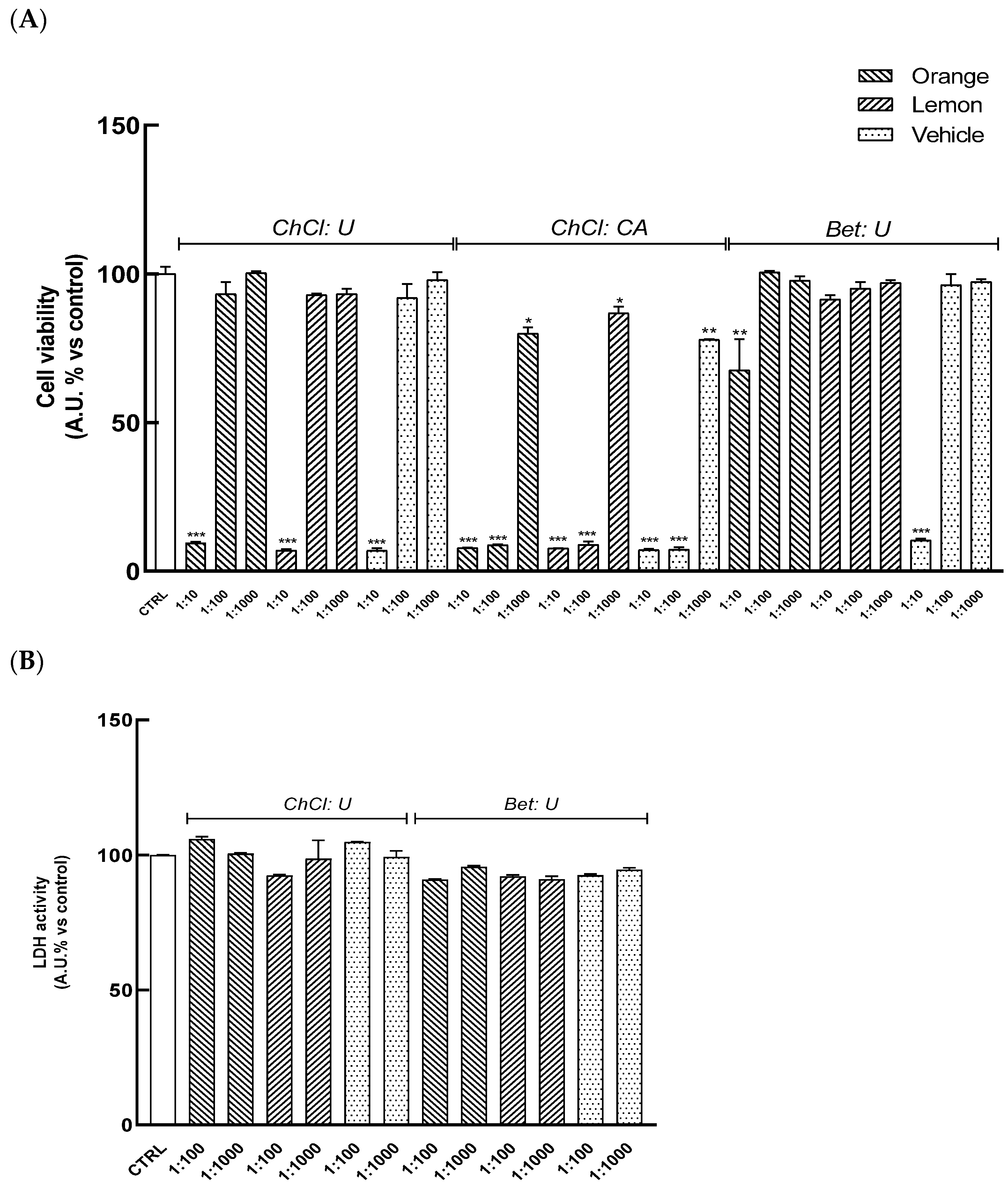
HaCaT cells were pretreated for 24 h with serial dilutions of extracts (range 1:10–1:1000) to evaluate their cytocompatibility. As shown in
Figure 2A, treatment with extracts O
(ChCl: CA), L
(ChCl: CA) results in a significant reduction in cell viability at all tested dilutions. Similarly, the highest concentration (1:10) of other extracts, O
(ChCl: U), L
(ChCl: U), and O
(Bet: U), L
(Bet: U), also leads to a significant decrease in formazan dye levels with respect to the control, indicating reduced cellular viability. Conversely, dilutions ranging from 1:100 to 1:1000 of O
(ChCl: U), L
(ChCl: U), and O
(Bet: U), L
(Bet: U) did not induce cell toxicity, as also confirmed by the absence of significant changes in LDH release relative to control cells (
Figure 2B), indicating these concentrations were safe in HaCat cells.
3.6. Evaluation of the Antioxidant Activity of Citrus Waste Extracts in HaCaT Cells
Human skin is consistently exposed to ROS arising from both environmental factors and endogenous processes. Excessive ROS levels damage lipids, proteins, and DNA within cells, impairing the skin’s structure and functions [
22]. To assess the antioxidant properties of the extracts, intracellular H
2O
2 levels were measured in HaCaT cells through a CL bioassay [
11].
Firstly, the correlation between the CL emission and intracellular H
2O
2 production was examined by exposing HaCaT cells to increasing concentrations of menadione or lipopolysaccharide (LPS), well-known oxidative stress inducers. Results demonstrated a linear correlation between the CL signal and the concentration of the pro-oxidant stimulus, both for menadione (R
2 = 0.976) and LPS (R
2 = 0.989) (
Figure S1), confirming the reliability of the method and its alignment with the expected physiological regulation of ROS production.
Next, the assay was applied to investigate the antioxidant capacity of the extracts. HaCaT cells were pretreated for 24 h with different dilutions of the extracts (dilution range: 1:100 to 1:1000), and then exposed to a fixed concentration of menadione (50 μM) or LPS (25 μg/mL). CL emission was recorded for 60 min, and PBS served as the negative control. A concentration-dependent decrease in the CL signal was observed for all extracts, indicating good IC
50 values, as shown in
Table 6.
All extracts effectively reduced ROS levels triggered by both LPS and menadione, as indicated by their IC
50 values under these conditions. These results suggest that citrus extracts may offer protection against various pro-oxidant challenges, likely due to the combined action of the polyphenolic constituents, which may modulate oxidative stress through multiple mechanisms [
23,
24].
In parallel, the antioxidant potential of the extracts was also evaluated using the oxygen radical absorbance capacity (ORAC) assay, as described in the
Supplementary Materials (Section S6). The ORAC results were consistent with the CL assay, further confirming the antioxidant capacity of the extracts.
3.7. Effect of the Extracts on Skin Wound Healing in HaCaT Cells
Tissue repair is a dynamic and finely regulated process involving coordinated cellular responses that restore the integrity and functionality of injured tissues. This process encompasses several stages, including inflammation, cell proliferation, and tissue remodeling, during which various cell lineages, signaling molecules, and growth factors collaborate to restore tissue architecture. Keratinocytes are crucial in this process, as they migrate towards the wound site, undergo proliferation, and produce extracellular matrix components to promote effective tissue regeneration [
25,
26].
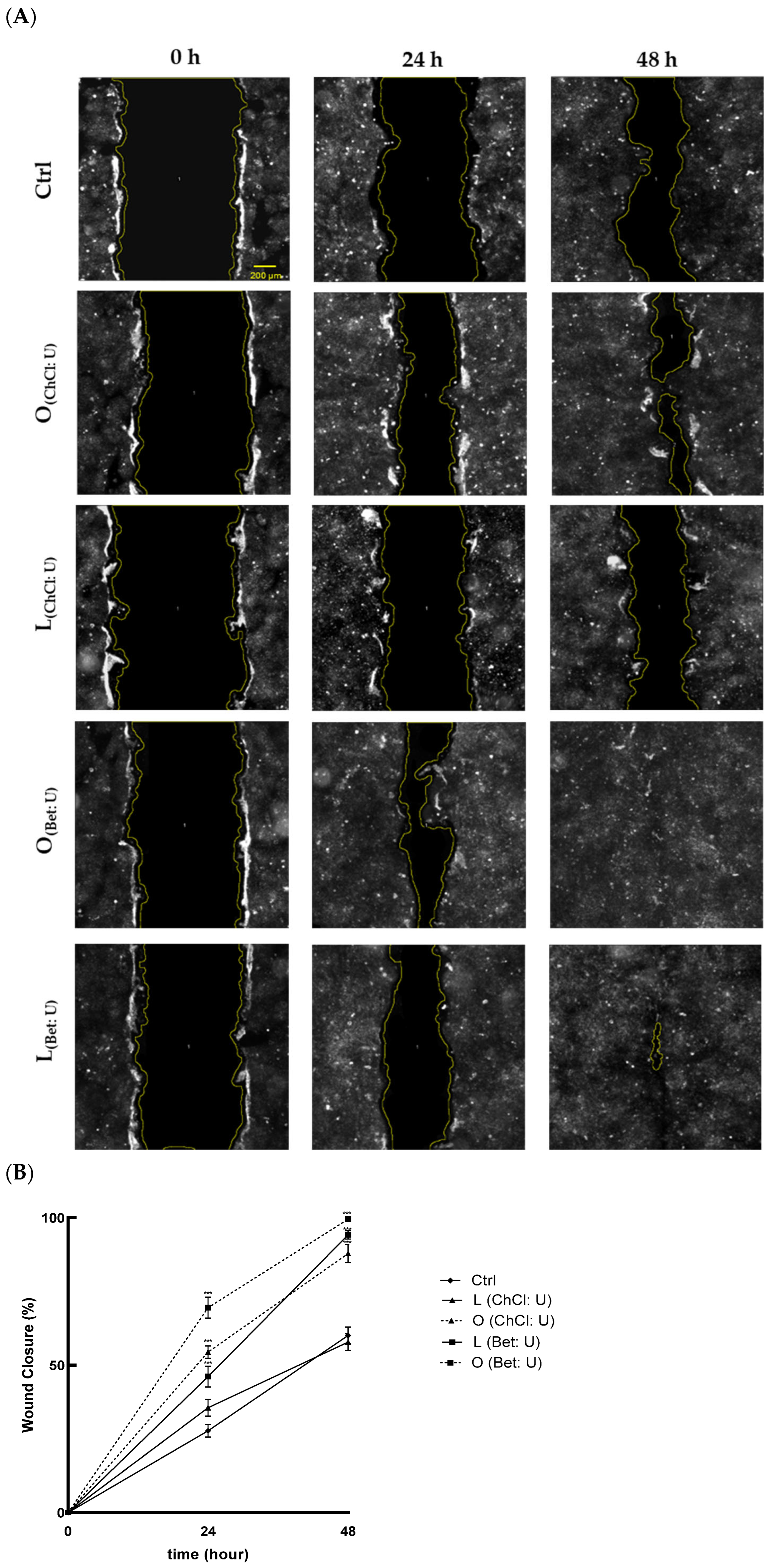
To evaluate the effects of the extracts on cell migration and wound healing, a scratch assay was used. As shown in
Figure 3A, treatments with O
(ChCl: U), O
(Bet: U), and L
(Bet: U) significantly enhanced wound closure at 24 and 48 h, compared with the control. Notably, treatment with O
(Bet: U) achieved near-complete closure, with 72% wound closure after just 24 h and full closure (100%) by 48 h (
Figure 3B). In contrast, L
(ChCl: U) did not promote significant improvement compared to the control, indicating lower efficacy in promoting keratinocyte migration.
Results indicate that extracts O
(ChCl: U), O
(Bet: U), and L
(Bet: U) significantly promote keratinocyte migration, suggesting enhanced regenerative potential. This wound-healing activity may be primarily linked to their enriched bioactive profiles, especially the high hesperidin content (497.50 μg/mL for O
(ChCl: U), 1175.90 μg/mL for O
(Bet: U), and 466.00 μg/mL for L
(Bet: U)). Indeed, several in vitro and in vivo studies have demonstrated that hesperidin, administered either topically or orally, accelerates cutaneous healing under various conditions, including chronic diabetic foot ulcers [
23,
27]. The antioxidant effect exerted by these extracts may shield keratinocytes from ROS that could otherwise impair the healing process by inducing oxidative stress and compromising cellular integrity [
28].
To further elucidate the molecular mechanisms underlying the observed wound-healing effects, RT-qPCR analyses were performed to assess the expression of genes involved in oxidative stress response, inflammation, and tissue remodeling, including TGF-β, NF-κB cofactors (p50, p52, p65), SOD1, and HO-1. As reported in the
Supplementary Materials (Section S7, Figure S2), treatment with O
(Bet: U) led to the upregulation of SOD1 and HO-1, suggesting activation of the Nrf2/ARE antioxidant pathway, together with a moderate increase in TGF-β, consistent with the promotion of tissue regeneration. Conversely, NF-κB cofactors were only mildly modulated under basal conditions, whereas pretreatment with O
(Bet: U) attenuated their overexpression induced by TNF-α, indicating a balanced control of inflammatory signaling. These transcriptional changes support the pro-healing and cytoprotective effects induced by O
(Bet: U).
3.8. Evaluation of IL-1β, IL-10, IL-8, and IL-6 Cell Release in HaCaT Cells
Wound healing is a carefully orchestrated, multi-phase event involving a balanced interplay between pro-inflammatory and regenerative mediators to achieve effective tissue repair. Cytokines play a central role in regulating keratinocyte and fibroblast proliferation, extracellular matrix remodeling, and immune recruitment of immune cells [
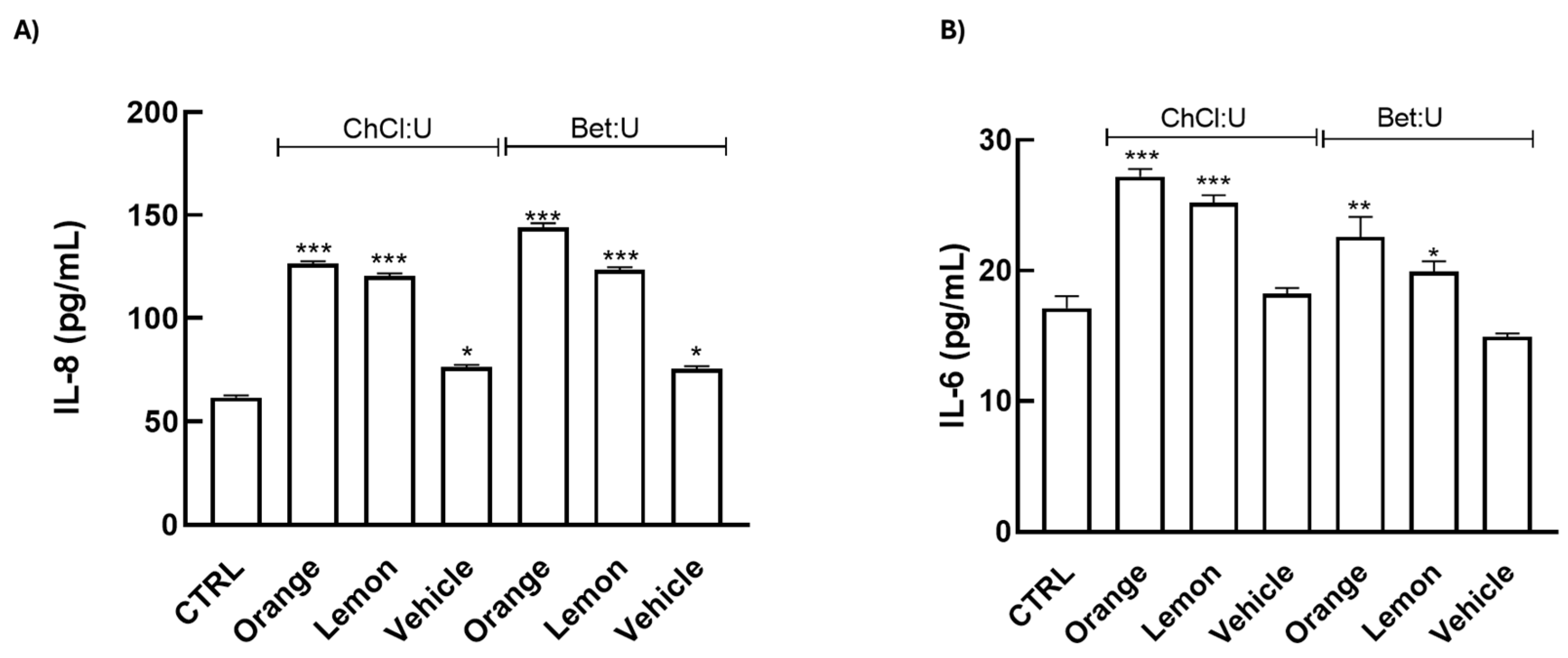
29]. To better explore the molecular mechanisms underlying the wound-healing effects of extracts, we measured the release of key interleukins (IL)-1β, -10, -8, and -6, known to modulate inflammatory and regenerative pathways in skin repair, in HaCaT cells treated for 24 h with the extracts using ELISA immunoassays.
Our data reveal that treatments induce a significant upregulation of IL-6 and IL-8, while IL-1β and IL-10 remained undetectable (
Figure 4). IL-6 is a primary early responder following skin injury, rapidly increasing at the wound site and facilitating immune cell recruitment and debris clearance, thereby driving the initial inflammatory phase [
30,
31]. IL-8, in turn, acts as a potent chemoattractant for neutrophils, enhancing microbial clearance and promoting angiogenesis, critical processes for wound re-epithelialization [
32,
33,
34]. Collectively, these results indicate a beneficial activation of the early inflammatory phase, essential for effective tissue repair.
These findings are noteworthy, as IL-6 and IL-8, traditionally considered pro-inflammatory, are increasingly recognized for their regulatory roles in tissue repair. Both cytokines facilitate keratinocyte migration, fibroblast activation, and neovascularization, essential for the proliferative phase of wound repair [
35,
36]. Notably, IL-6 also supports macrophage polarization toward a reparative M2 phenotype in the skin, thereby supporting extracellular matrix remodeling and tissue regeneration [
30,
37]. Consistent with this role, IL-6-deficient animal models display marked delays in wound re-epithelialization, angiogenesis, and collagen deposition [
38].
The treatments do not induce IL-1β expression, suggesting that the extracts do not elicit a strong acute inflammatory response, which may otherwise contribute to chronic inflammatory skin conditions [
39].
3.9. DNA Damage Assessment by 53BP1 Foci Formation in HaCaT Cells
The formation of 53BP1 foci is a well-established marker of DNA double-strand break response and is widely used to monitor cellular genotoxic stress. Maintaining genome integrity during skin regeneration is critical, as keratinocytes and fibroblasts undergo intense proliferation and oxidative stress during the repair process [
40]. Persistent accumulation of 53BP1 foci has been linked to cellular senescence and defective DNA-repair mechanisms under chronic oxidative or inflammatory stress [
41]. In the skin context, such genomic instability may contribute to delayed tissue regeneration and impaired wound closure [
42].
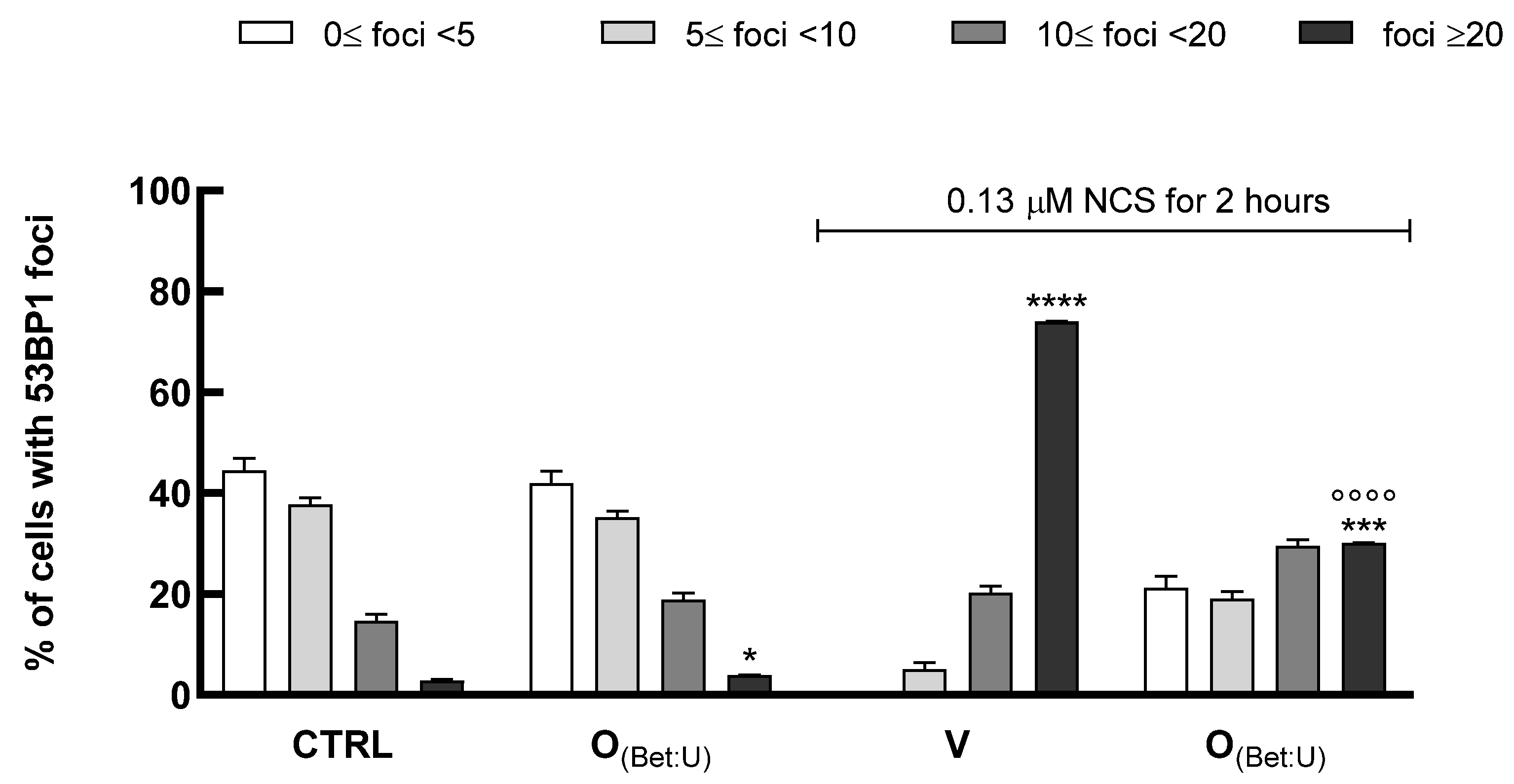
Therefore, to assess whether the most promising formulation investigated (O
(Bet: U)) could counteract DNA damage, we performed immunofluorescence analysis of 53BP1 to evaluate its ability to protect keratinocytes from genotoxic stress. In this assay, HaCaT cells were exposed to NCS, in the presence or absence of 24 h pretreatment with O
(Bet: U) (dilution 1:100). The number of 53BP1 foci per nucleus was quantified as an indicator of DNA damage levels. As shown in
Figure 5, under control conditions, most nuclei exhibited fewer than 10 foci reflecting basal genome stability. In contrast, exposure to NCS markedly shifted the cell population toward nuclei bearing >20 foci, consistent with extensive DNA double-strand breaks. Treatment with O
(Bet: U) alone did not alter the distribution compared with control cells, confirming the absence of intrinsic genotoxicity. However, the pretreatment with O
(Bet: U) prior to NCS exposure significantly reduced the proportion of cells with >20 foci, suggesting partial protection from NCS-induced DNA damage. Statistical analysis substantiated these findings, with Chi-square test revealing significant differences in the overall distribution of foci among treatments (
p < 0.001) driven by the NCS-induced damage, whereas control and O
(Bet: U) displayed comparable foci distributions (
p > 0.05). Consistently, the one-way ANOVA applied to the high-damage category (>20 foci per nucleus) confirmed a marked reduction in severely damaged cells after O
(Bet: U) pretreatment (
p < 0.001). Collectively, these results demonstrate that O
(Bet: U) markedly attenuates the accumulation of DNA double-strand breaks, plausibly by strengthening cellular resilience to genotoxic stress through the enhancement of antioxidant and cytoprotective pathways, as also indicated by the concomitant upregulation of SOD1 and HO-1, as reported in the
Supplementary Materials (Section S7).
4. Conclusions
To summarize, this study introduces an innovative and environmentally sustainable approach for valorizing citrus by-products, addressing waste management challenges and meeting the increasing demand for eco-friendly solutions within the cosmetics industry. Using NaDESs, especially ChCl: U and Bet: U, enabled the development of a highly effective extraction method that surpassed traditional ethanol–water systems in isolating important polyphenols such as hesperidin, naringin, and rutin. These findings underscore NaDESs’ ability to extract and concentrate valuable phytochemicals from agricultural waste streams.
According to our data, the extracts showed strong antioxidant activity, significantly improved keratinocyte migration and wound closure, and enhanced cellular protection against genotoxic stress, suggesting their possible use as active ingredients in skin repair and anti-aging products [
43]. Notably, the use of biodegradable, cosmetic-grade solvents aligns with green chemistry principles and supports a circular economy. The extraction process, performed at room temperature with a basic roller mixer, offers notable advantages in scalability, energy efficiency, and cost-effectiveness, making industrial applications more scalable.
However, several limitations should be acknowledged. The chemical profile of these extracts remains partially characterized; a more comprehensive analysis using advanced multi-omics approaches (including untargeted metabolomics and proteomics) is needed to elucidate the full spectrum of bioactive molecules and potential synergistic interactions. Furthermore, such analyses are also crucial for verifying the potential presence of contaminants or undesired compounds that may develop during the extraction process and could compromise safety. Additionally, while in vitro tests provide initial insights, they cannot fully reproduce the structural and physiological complexity of human skin. Consequently, ex vivo human skin or advanced models (i.e., reconstructed human epidermis, 3D cultures, organ-on-chip systems) are required to verify the effectiveness and safety in more complex biological systems. Another important consideration concerns formulation stability and delivery. Future studies should investigate the incorporation of NaDES-based extracts into dermatologically relevant systems, such as hydrogels, emulsions, or nanocarriers, to preserve bioactivity, improve skin permeation, and ensure long-term stability and cosmetic compatibility.
In conclusion, this study lays a strong foundation for the sustainable valorization of citrus by-products using environmentally friendly extraction techniques, offering notable environmental and economic advantages. The integration of comprehensive molecular characterization, in vivo validation, and advanced formulation design will be crucial to translating these promising in vitro findings into effective, safe, and market-ready cosmeceutical products. This strategy not only supports circular economy principles but also emphasizes the potential of green chemistry to drive innovation in the cosmeceutical industry.