The Effect of Short-Term Rhodiola rosea Supplementation on Simulated Game Time, Perceived Fatigue, and Performance in Basketball Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
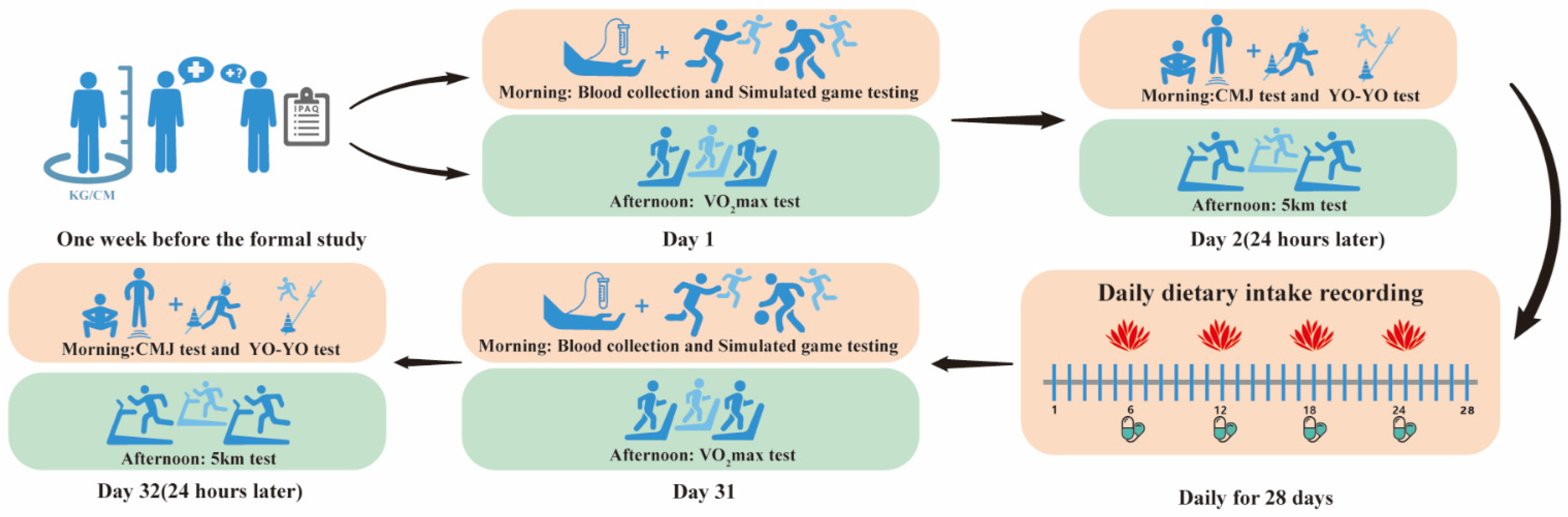
2.2. Experimental Procedure
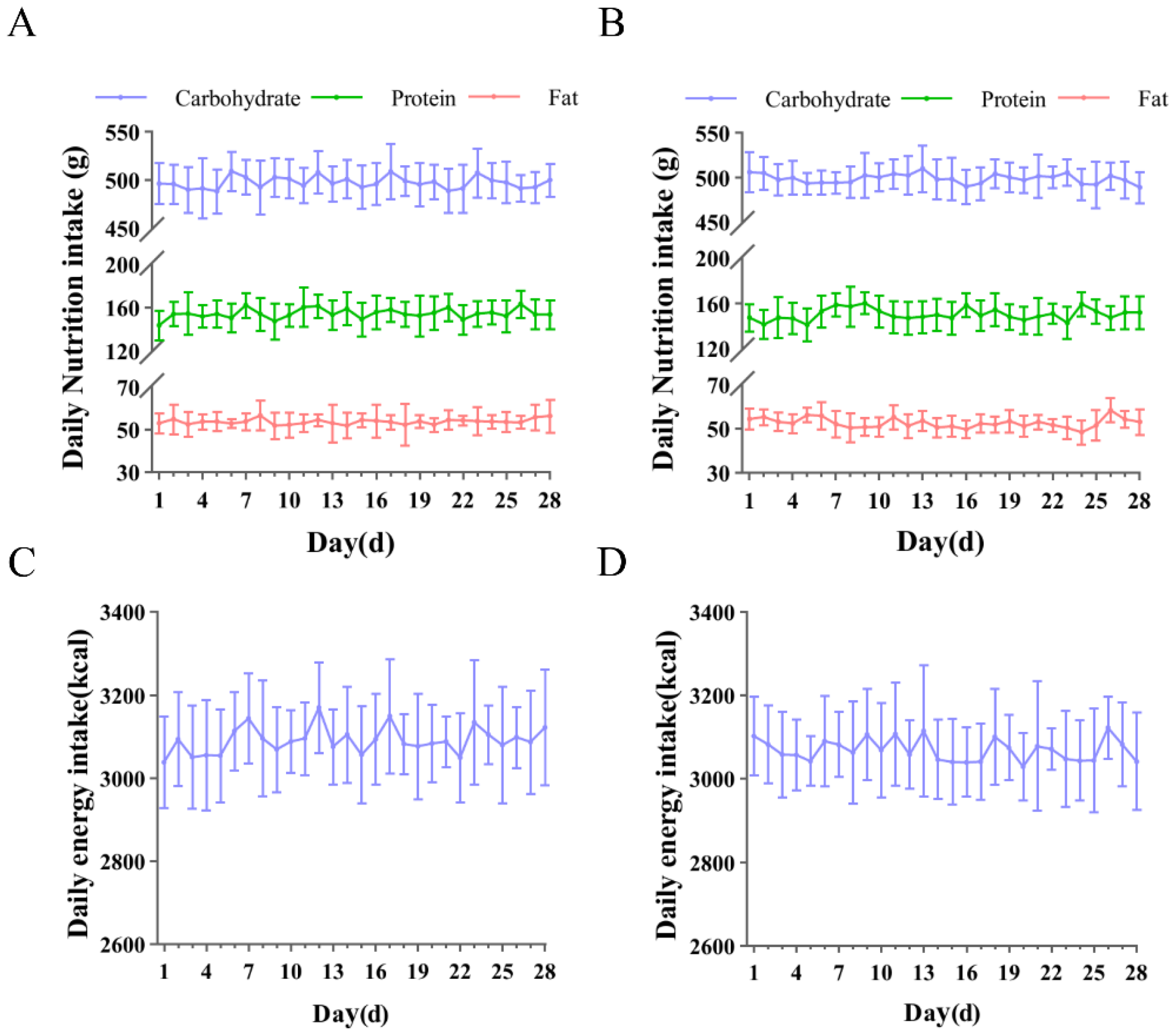
2.3. Daily Dietary Intake Recording
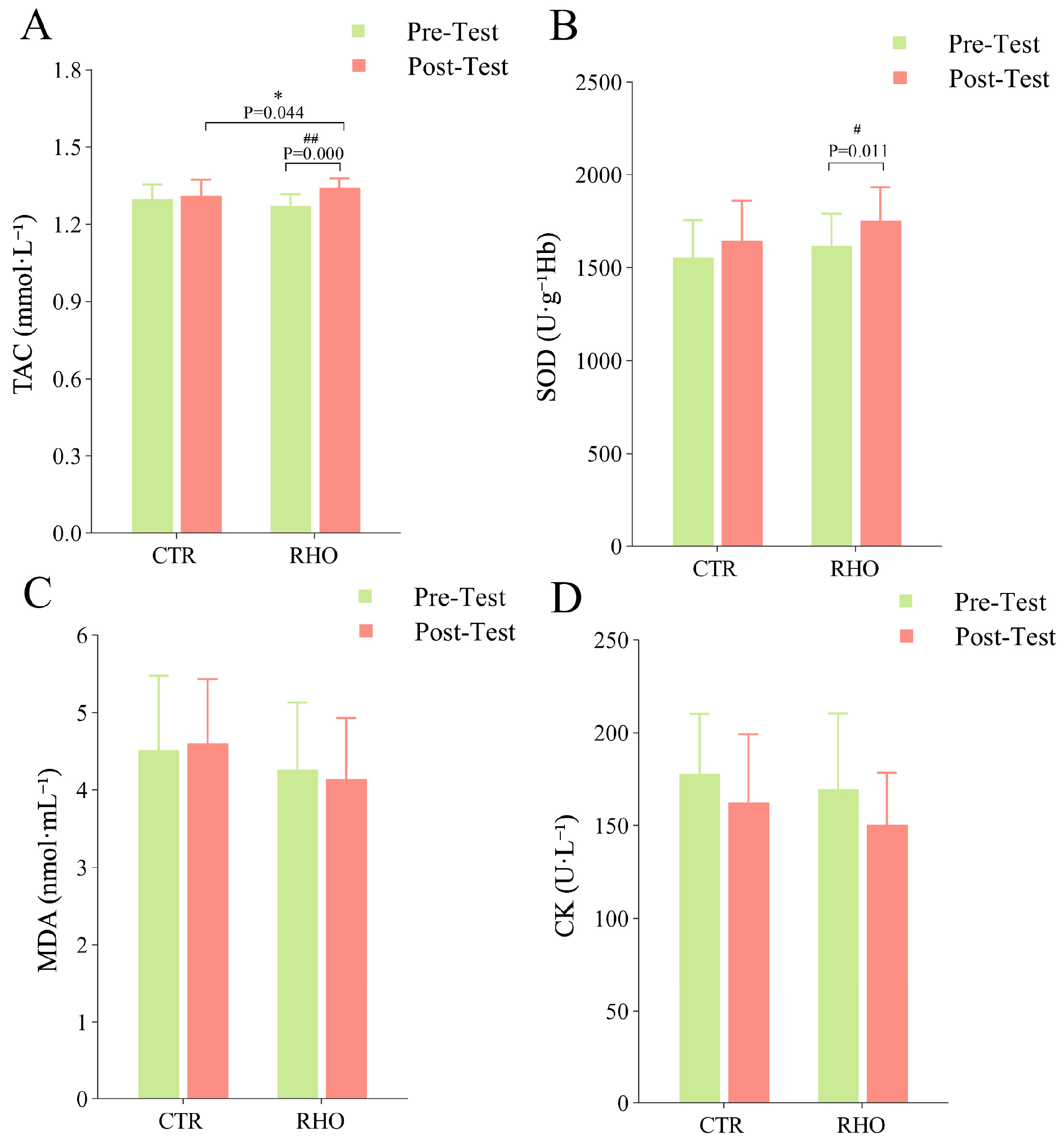
2.4. Blood Collection
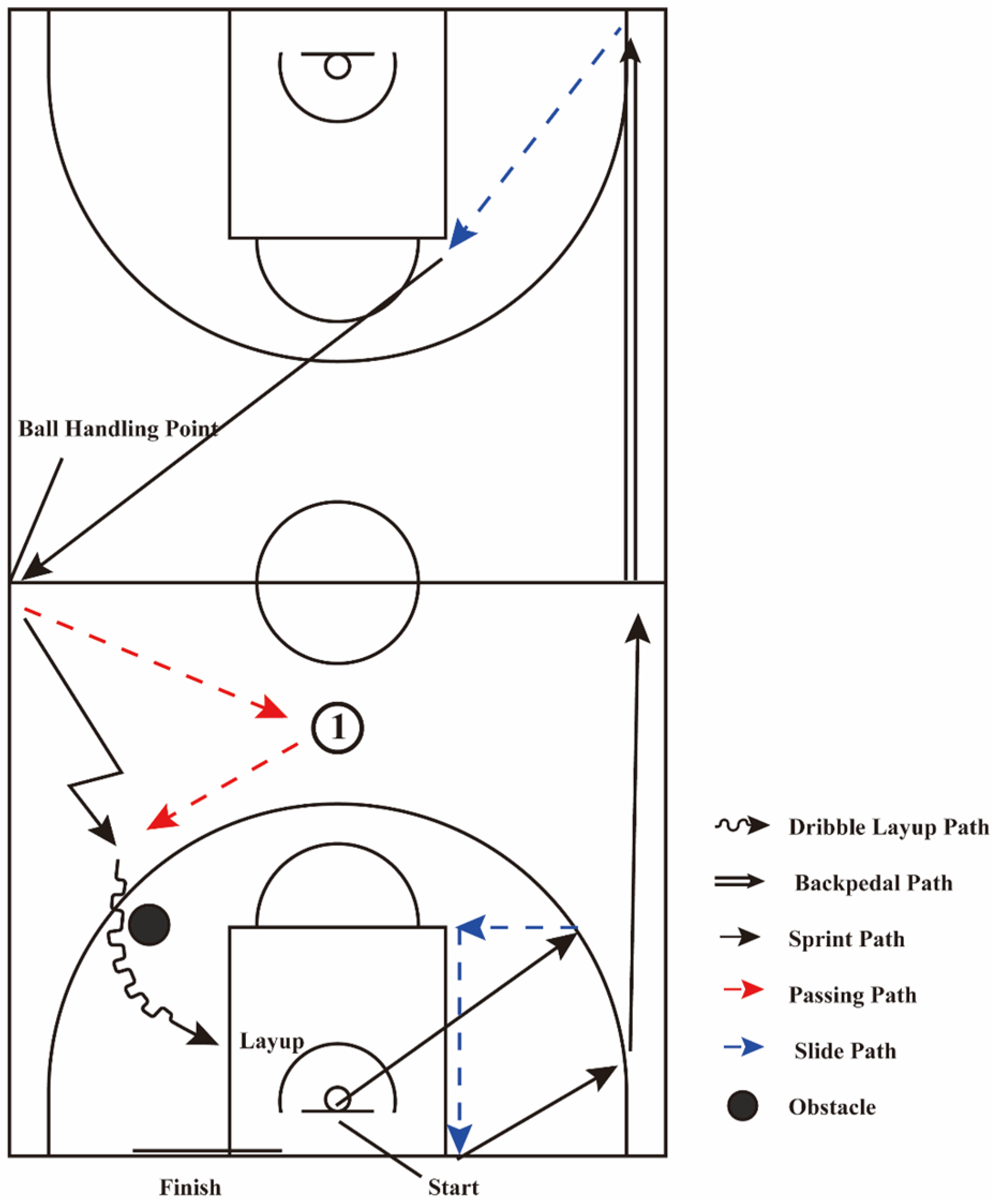
2.5. Simulated Game Testing
- Part 1:
- Participants performed a standing vertical jump at the center of the hoop (only during the first run of the test). After landing, two instructors started timing. Participants completed sprints (indicated by a black solid single arrow in Figure 2), defensive slides (indicated by a blue dashed arrow in Figure 2), and backpedaling (indicated by two black solid arrows in Figure 2) at maximal speed. This part included 4 sprints (sprint 1, sprint 2, sprint 3, sprint 4), 3 defensive slides (slide 1, slide 2, slide 3), and 1 backpedal.
- Part 2:
- An assistant stands at the left sideline midcourt with the ball. Upon receiving it, the participant passes the ball to an assistant at the top of the three-point arc (as depicted in Figure 2, Position ①, where the red dashed line represents the basketball passing path), then quickly moves to receive the return pass and completes a dribble layup, with the player’s movement path is indicated by a polyline, and the dribble-layup path is shown as a wavy line.
- Part 3:
- After the layup, participants moved to the baseline for a 5 s rest. They then started from directly under the hoop. These three parts constituted one set, with a total of three sets. During the test, instructors recorded the time for each activity path. After the simulated game, they recorded the ratings of perceived exertion (RPE).
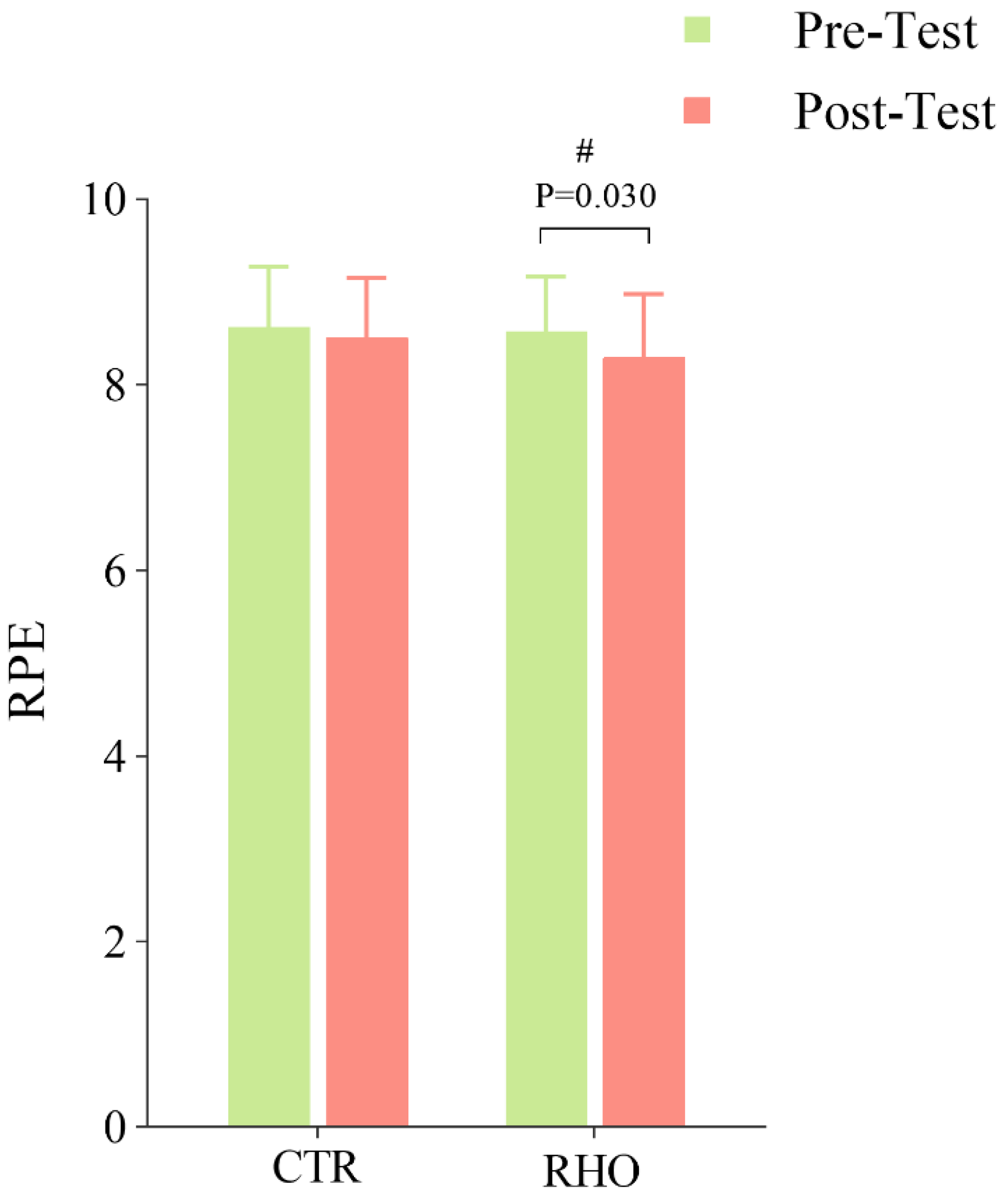
2.6. RPE Assessment
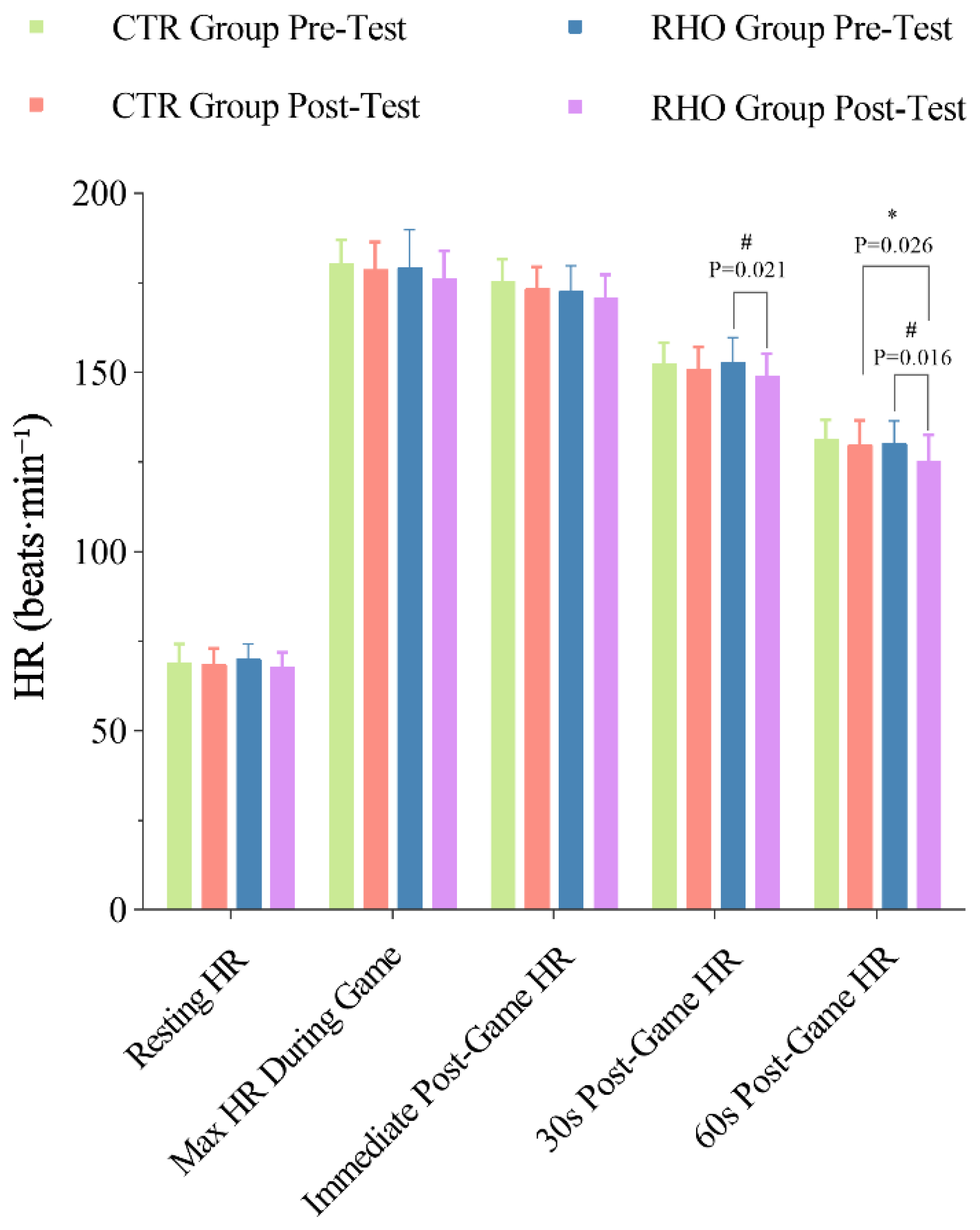
2.7. HR Test
2.8. YO-YO Test
2.9. CMJ Test
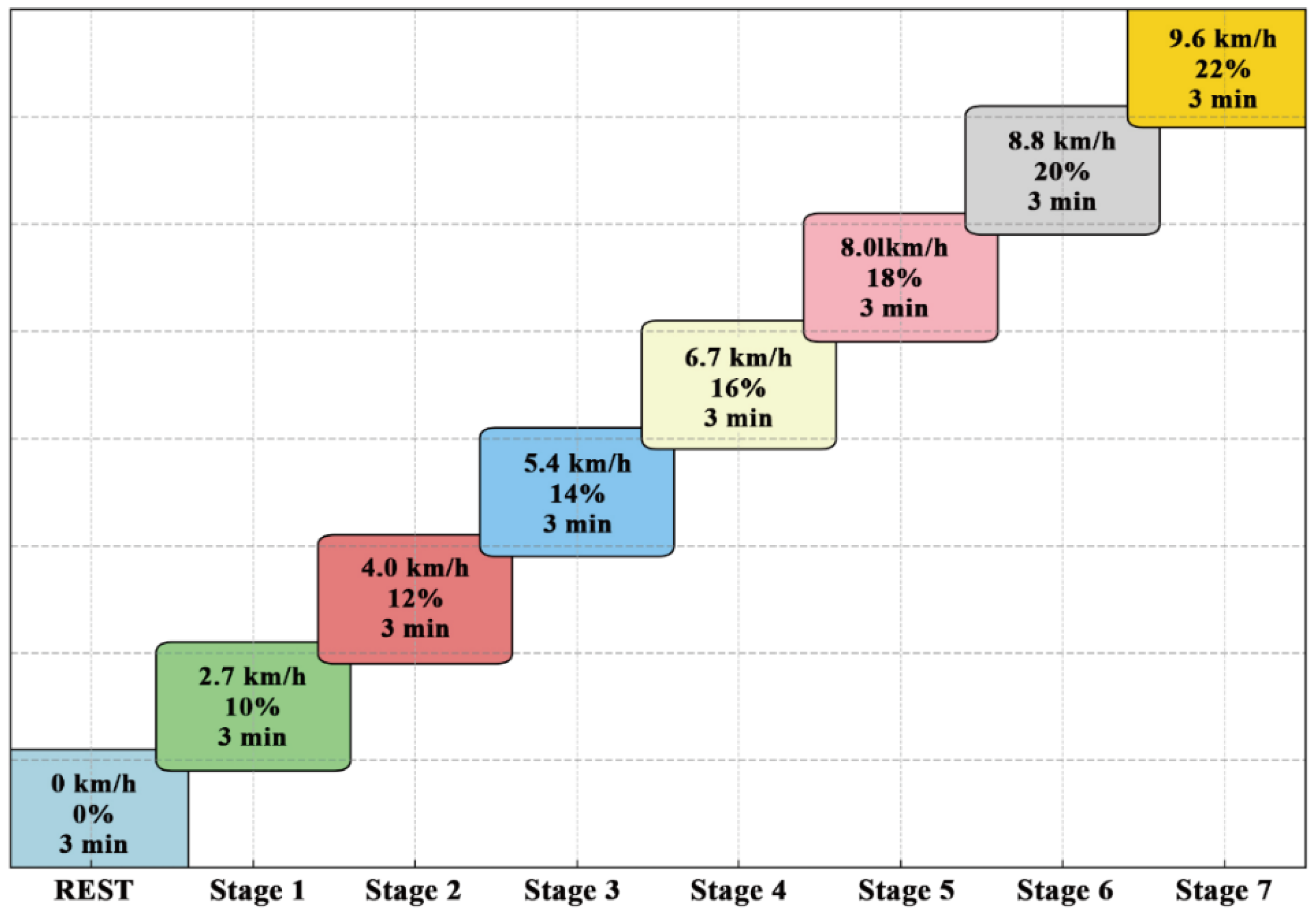
2.10. VO2max Test
2.11. 5 km Test
2.12. Data Analysis
3. Results
3.1. Daily Recorded Intake of Carbohydrates, Proteins, Fats, and Energy
3.2. Blood Analysis Results
3.3. Results of the Simulated Game Tests for Athletes
3.3.1. Completion Time of Each Stage of the Simulated Game
3.3.2. Changes in Athletes’ HR During Simulated Game
3.3.3. Changes in Athletes’ Perceived Fatigue After Simulated Game
3.4. Test Results of Basketball Players’ Athletic Performance
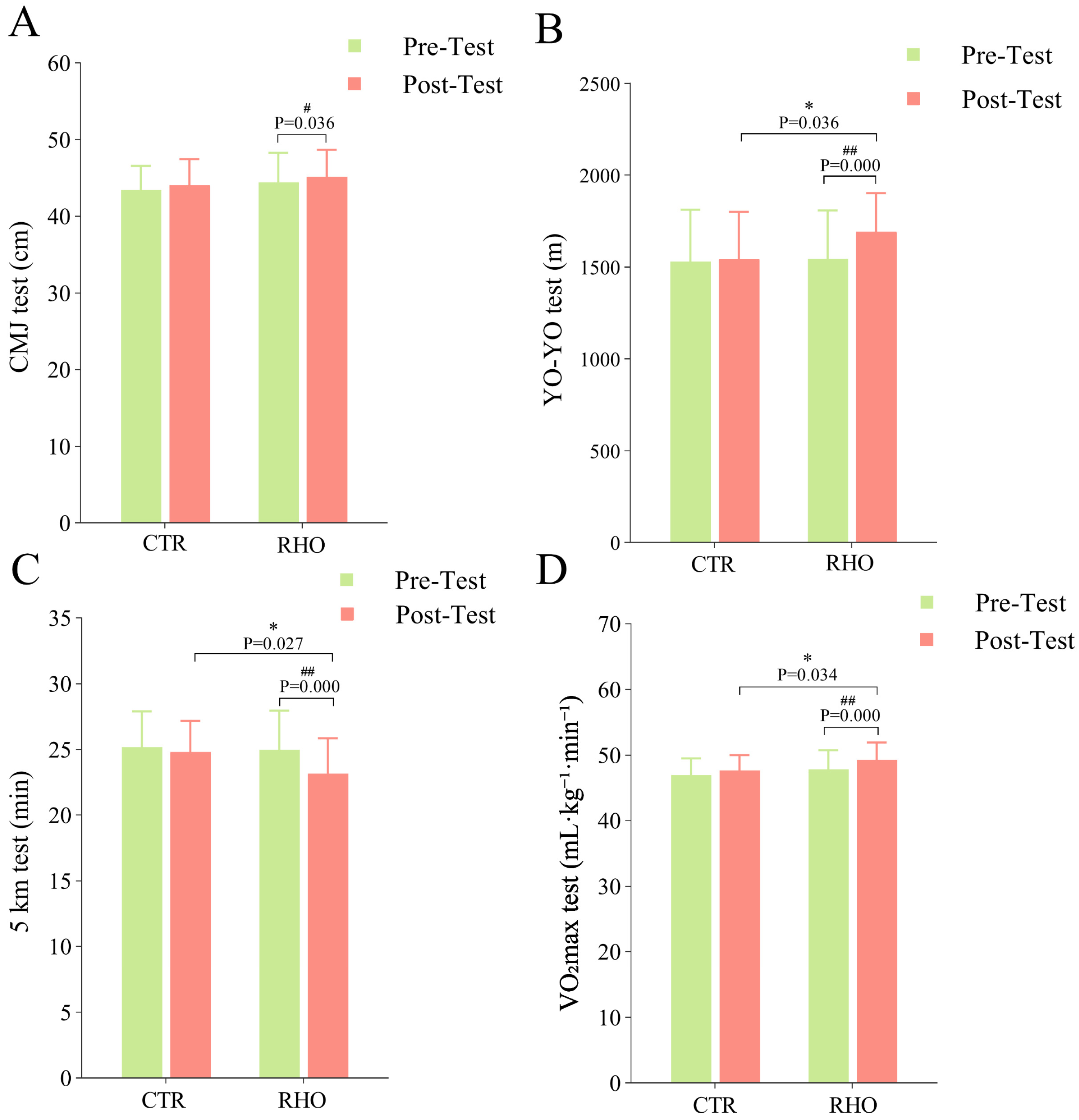
3.4.1. CMJ Test Results
3.4.2. YO-YO Test Results
3.4.3. 5 km Test Results
3.4.4. VO2max Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, J.; Wundersitz, D.; Bini, R.; Kingsley, M. Effect of Player Role and Competition Level on Player Demands in Basketball. Sports 2021, 9, 38. [Google Scholar] [CrossRef]
- Calleja-González, J.; Terrados, N.; Mielgo-Ayuso, J.; Delextrat, A.; Jukic, I.; Vaquera, A.; Torres, L.; Schelling, X.; Stojanovic, M.; Ostojic, S.M. Evidence-based post-exercise recovery strategies in basketball. Physician Sportsmed. 2016, 44, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Schelling, X.; Torres, L. Accelerometer Load Profiles for Basketball-Specific Drills in Elite Players. J. Sports Sci. Med. 2016, 15, 585–591. [Google Scholar]
- Russell, S.; Halson, S.L.; Jenkins, D.G.; Rynne, S.B.; Roelands, B.; Kelly, V.G. Thinking About Elite Performance: The Experience and Impact of Mental Fatigue in Elite Sport Coaching. Int. J. Sports Physiol. Perform. 2023, 18, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Griffiths, P.C.; Mellalieu, S.D. Training Load and Fatigue Marker Associations with Injury and Illness: A Systematic Review of Longitudinal Studies. Sports Med. 2017, 47, 943–974. [Google Scholar] [CrossRef]
- Conte, D.; Kamarauskas, P.; Ferioli, D.; Scanlan, A.T.; Kamandulis, S.; Paulauskas, H.; Lukonaitienė, I. Workload and well-being across games played on consecutive days during in-season phase in basketball players. J. Sports Med. Phys. Fit. 2021, 61, 534–541. [Google Scholar] [CrossRef]
- Chan, C.C.; Yung, P.S.; Mok, K.M. The Relationship between Training Load and Injury Risk in Basketball: A Systematic Review. Healthcare 2024, 12, 1829. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-Based Supplements for the Enhancement of Athletic Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.A.; Tovar, A.P.; Richardson, C.E.; Cortez, A.N.; Davis, B.A. Energy Availability, Macronutrient Intake, and Nutritional Supplementation for Improving Exercise Performance in Endurance Athletes. Curr. Sports Med. Rep. 2018, 17, 215–223. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine Int. J. Phytother. Phytopharm. 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Yan, Y.; Yang, W.; Chen, S.; Song, G.; Li, X.; Gu, Y.; Yun, H.; Li, Y. Synergistic Effect of Rhodiola rosea and Caffeine Supplementation on the Improvement of Muscle Strength and Muscular Endurance: A Pilot Study for Rats, Resistance Exercise-Untrained and -Trained Volunteers. Nutrients 2023, 15, 582. [Google Scholar] [CrossRef]
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions-Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef]
- Olsson, E.M.; von Schéele, B.; Panossian, A.G. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009, 75, 105–112. [Google Scholar] [CrossRef]
- Kang, D.Z.; Hong, H.D.; Kim, K.I.; Choi, S.Y. Anti-Fatigue Effects of Fermented Rhodiola rosea Extract in Mice. Prev. Nutr. Food Sci. 2015, 20, 38–42. [Google Scholar] [CrossRef]
- Punja, S.; Shamseer, L.; Olson, K.; Vohra, S. Rhodiola rosea for mental and physical fatigue in nursing students: A randomized controlled trial. PLoS ONE 2014, 9, e108416. [Google Scholar] [CrossRef]
- De Bock, K.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 298–307. [Google Scholar] [CrossRef]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielecka, E. The influence of supplementation with Rhodiola rosea L. extract on selected redox parameters in professional rowers. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 186–199. [Google Scholar] [CrossRef]
- Ferioli, D.; Alcaraz, P.E.; Freitas, T.T.; Trimarchi, F.; Conte, D.; Formica, L.; Chung, L.H.; Scanlan, A.T. The reliability and discriminant validity of physical, technical, and perceptual-physiological measures during a game-specific basketball activity simulation protocol. Front. Psychol. 2024, 15, 1414339. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C. Rating of perceived exertion (RPE). J. Physiother. 2012, 58, 62. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, C.G.; Maze, S.B.; Wells, A.C.; Marshall, M.M.; Rogers, R.R. Effects of short-term Rhodiola rosea (Golden Root Extract) supplementation on anaerobic exercise performance. J. Sports Sci. 2019, 37, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Crendal, F.; Grachev, S.; Seifulla, R.; Ziegenfuss, T. Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles. Bull. Exp. Biol. Med. 2003, 136, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef]
- Walker, T.B.; Altobelli, S.A.; Caprihan, A.; Robergs, R.A. Failure of Rhodiola rosea to alter skeletal muscle phosphate kinetics in trained men. Metab. Clin. Exp. 2007, 56, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Wikman, G.K.; Mandrikov, V.B.; Mironova, I.A.; Neumoin, V.V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine Int. J. Phytother. Phytopharm. 2000, 7, 85–89. [Google Scholar] [CrossRef]
- van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef]
- Panossian, A.; Wagner, H. Stimulating effect of adaptogens: An overview with particular reference to their efficacy following single dose administration. Phytother. Res. PTR 2005, 19, 819–838. [Google Scholar] [CrossRef]
- Hickey, M.S.; Franke, W.D.; Herbert, W.G.; Walberg-Rankin, J.; Lee, J.C. Opioid antagonism, perceived exertion and tolerance to exercise-thermal stress. Int. J. Sports Med. 1992, 13, 326–331. [Google Scholar] [CrossRef]
- Sgherza, A.L.; Axen, K.; Fain, R.; Hoffman, R.S.; Dunbar, C.C.; Haas, F. Effect of naloxone on perceived exertion and exercise capacity during maximal cycle ergometry. J. Appl. Physiol. 2002, 93, 2023–2028. [Google Scholar] [CrossRef]
- Lee, F.T.; Kuo, T.Y.; Liou, S.Y.; Chien, C.T. Chronic Rhodiola rosea extract supplementation enforces exhaustive swimming tolerance. Am. J. Chin. Med. 2009, 37, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Bang, V.M.J.; Aranão, A.L.C.; Nogueira, B.Z.; Araújo, A.C.; Bueno, P.; Barbalho, S.M.; de Souza, M.; Guiguer, E.L. Effects of Rhodiola rosea and Panax ginseng on the Metabolic Parameters of Rats Submitted to Swimming. J. Med. Food 2019, 22, 1087–1090. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hou, C.W.; Bernard, J.R.; Chen, C.C.; Hung, T.C.; Cheng, L.L.; Liao, Y.H.; Kuo, C.H. Rhodiola crenulata- and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt. Med. Biol. 2014, 15, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Dun, Y.; Liu, S.; Zhang, W.; Xie, M.; Qiu, L. Exercise Combined with Rhodiola sacra Supplementation Improves Exercise Capacity and Ameliorates Exhaustive Exercise-Induced Muscle Damage through Enhancement of Mitochondrial Quality Control. Oxidative Med. Cell. Longev. 2017, 2017, 8024857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.; Zhang, Z.X.; Guo, A.J.; Bi, C.W.; Zhu, K.Y.; Xu, S.L.; Zhan, J.Y.; Lau, D.T.; Dong, T.T.; Choi, R.C.; et al. Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: A signaling via blocking the degradation pathway in kidney and liver cells. Eur. J. Pharmacol. 2012, 679, 34–39. [Google Scholar] [CrossRef]
- Yun, H.; Lu, B.; Su, W.; Wang, J.; Zheng, J.; Wang, J.; Wang, Z.; Li, Y.; Sun, Y.; Liu, C. Combined effects of Rhodiola rosea and caffeine supplementation on aerobic endurance and muscle explosiveness: A synergistic approach. Front. Nutr. 2024, 11, 1335950. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Bunn, H.F. Erythropoietin. Cold Spring Harb. Perspect. Med. 2013, 3, a011619. [Google Scholar] [CrossRef] [PubMed]

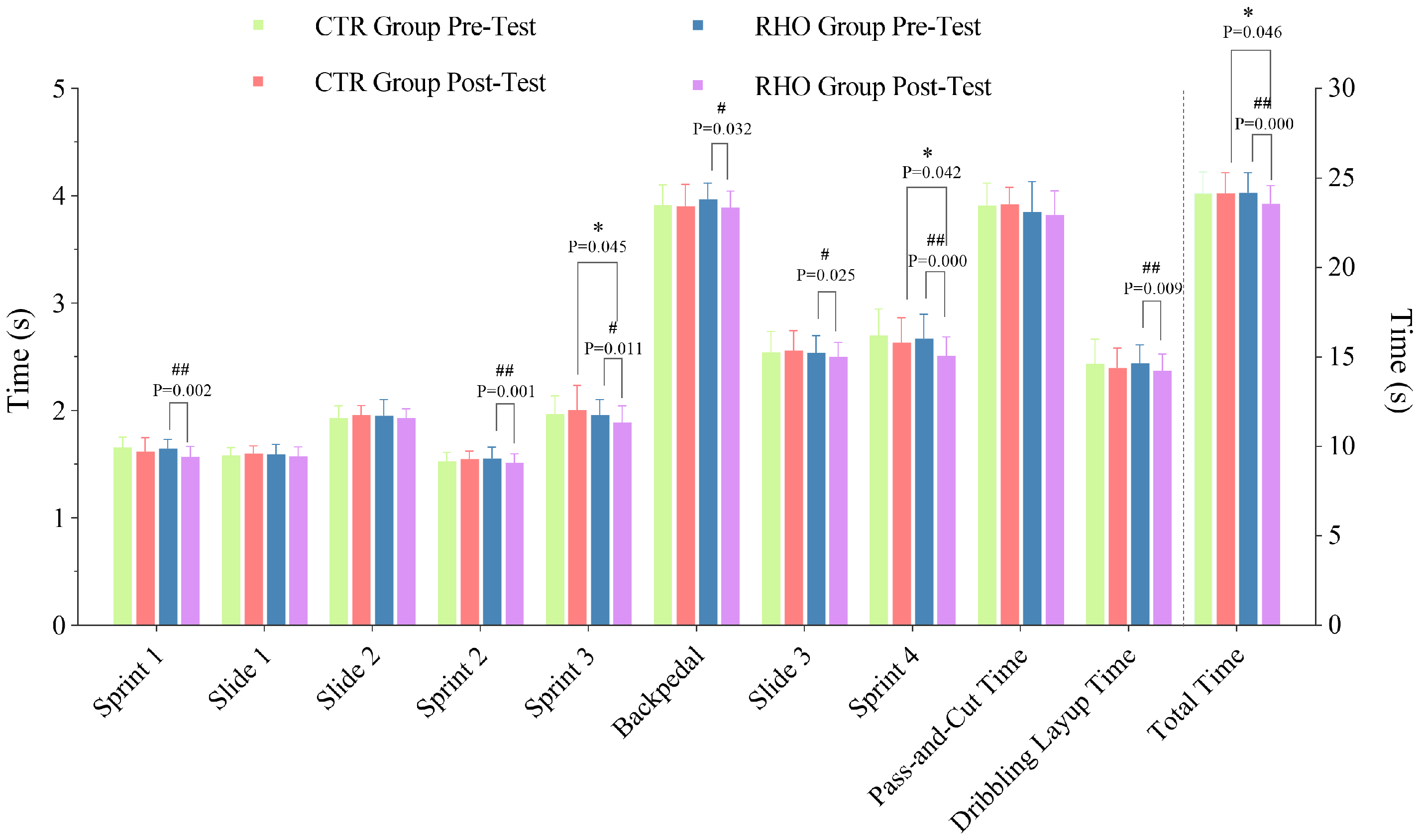
| Body Characteristics | CTR Group (n = 24) | RHO Group (n = 24) |
|---|---|---|
| Age (years) | 20.33 ± 1.34 | 19.46 ± 2.02 |
| Height (cm) | 186.50 ± 6.11 | 188.29 ± 7.09 |
| Weight (kg) | 88.22 ± 6.05 | 91.33 ± 5.74 |
| Body mass index (kg/m2) | 25.37 ± 1.48 | 25.76 ± 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, H.; Yu, L.; Zhao, K.; Jiang, W.; Liu, S.; Dai, J.; Xu, L.; Sun, P.; Yun, H.; et al. The Effect of Short-Term Rhodiola rosea Supplementation on Simulated Game Time, Perceived Fatigue, and Performance in Basketball Players. Nutrients 2025, 17, 3694. https://doi.org/10.3390/nu17233694
Wang J, Zhao H, Yu L, Zhao K, Jiang W, Liu S, Dai J, Xu L, Sun P, Yun H, et al. The Effect of Short-Term Rhodiola rosea Supplementation on Simulated Game Time, Perceived Fatigue, and Performance in Basketball Players. Nutrients. 2025; 17(23):3694. https://doi.org/10.3390/nu17233694
Chicago/Turabian StyleWang, Jing, Haotian Zhao, Longqi Yu, Kai Zhao, Wei Jiang, Shuning Liu, Jin Dai, Lina Xu, Peng Sun, Hezhang Yun, and et al. 2025. "The Effect of Short-Term Rhodiola rosea Supplementation on Simulated Game Time, Perceived Fatigue, and Performance in Basketball Players" Nutrients 17, no. 23: 3694. https://doi.org/10.3390/nu17233694
APA StyleWang, J., Zhao, H., Yu, L., Zhao, K., Jiang, W., Liu, S., Dai, J., Xu, L., Sun, P., Yun, H., & Liu, C. (2025). The Effect of Short-Term Rhodiola rosea Supplementation on Simulated Game Time, Perceived Fatigue, and Performance in Basketball Players. Nutrients, 17(23), 3694. https://doi.org/10.3390/nu17233694






