Novel Scale for Clinical Identification of Adverse Magnesium/Calcium Imbalances: Applications and Perspectives
Abstract
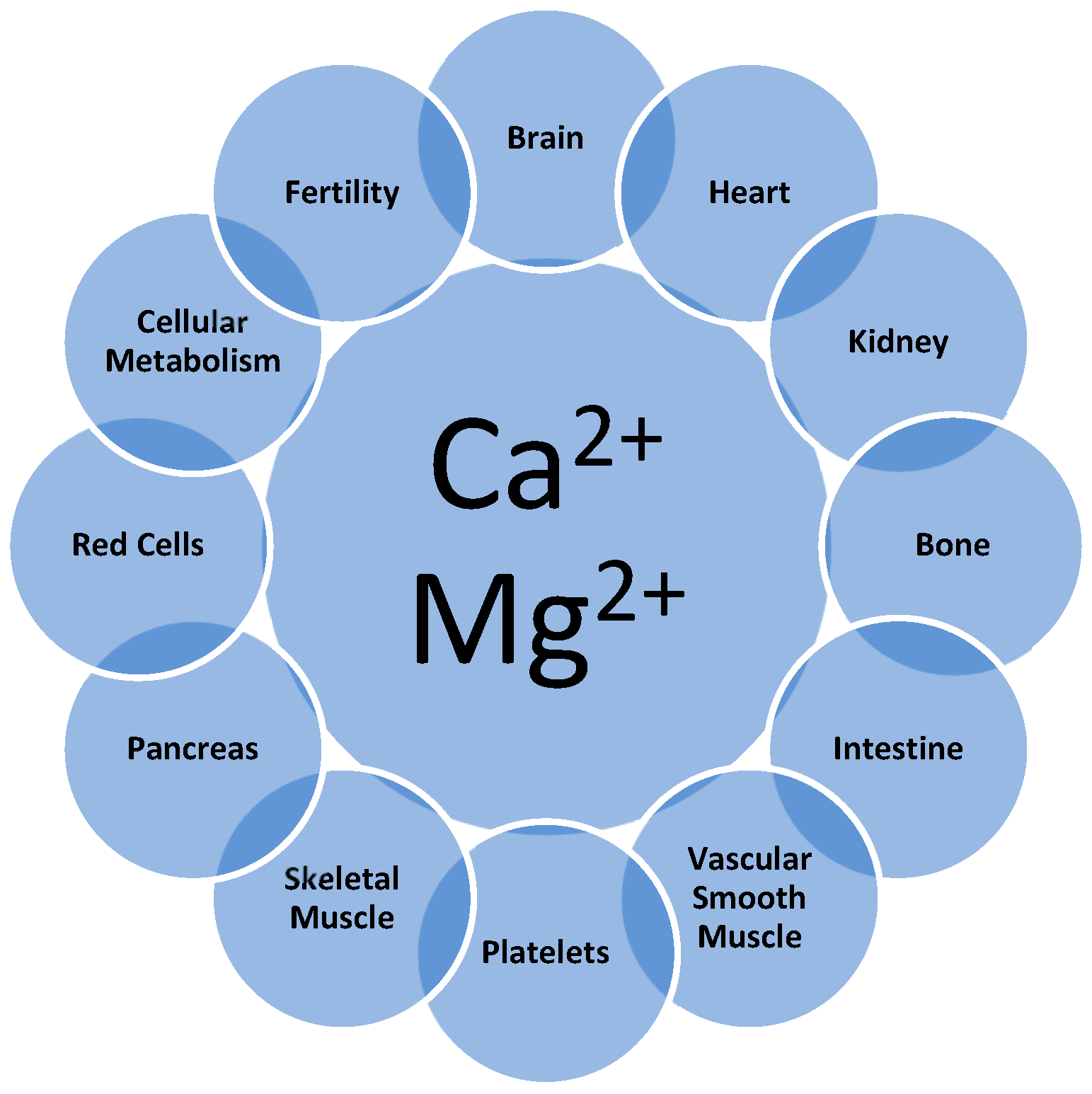
1. Introduction
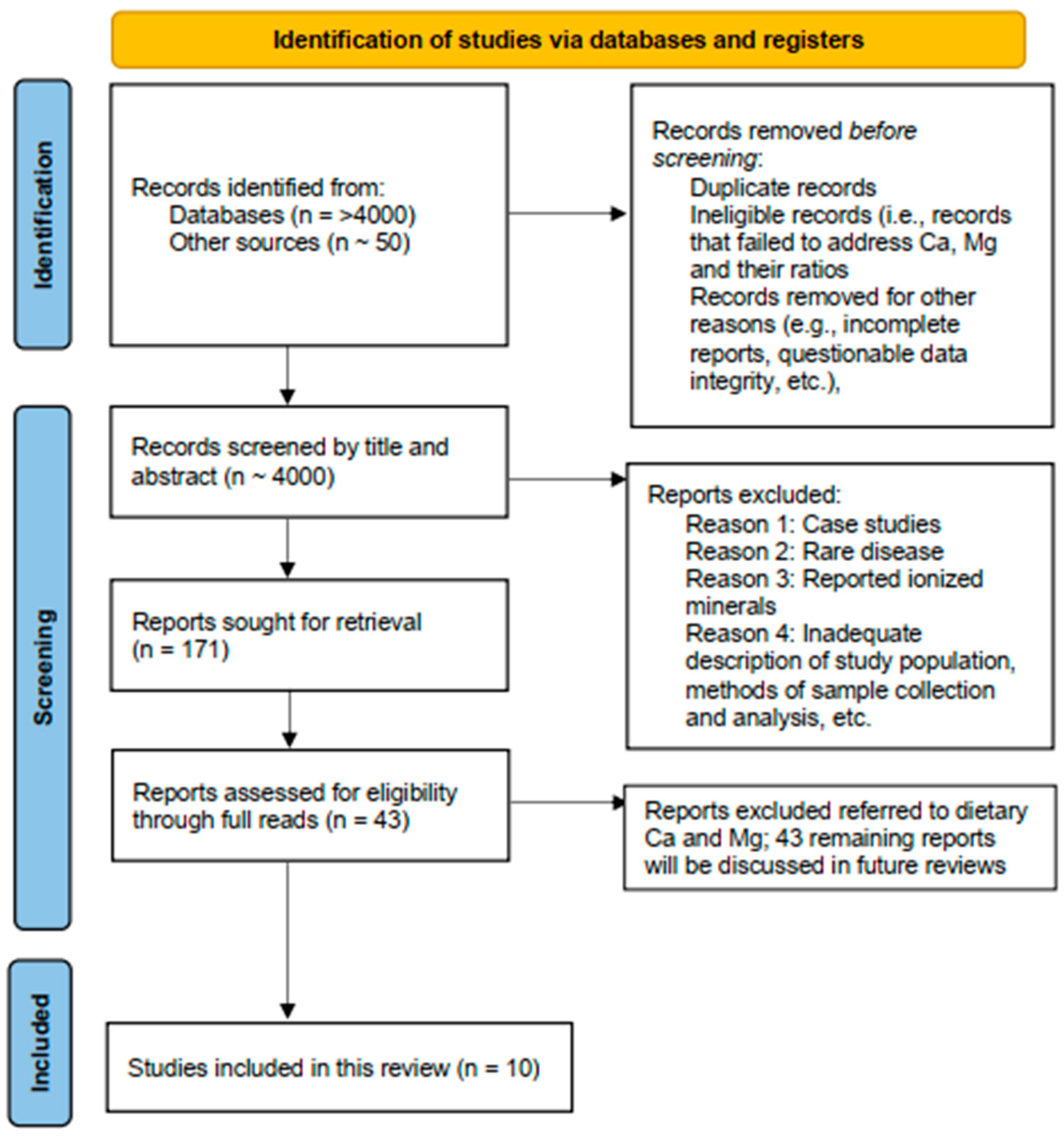
2. Materials and Methods
2.1. Selection of Studies
2.2. Application of the Novel Serum Mg/Ca–Ca/Mg Scale
3. Results
3.1. Ca/Mg and Metabolic Syndrome
3.2. Ca/Mg and Glucose Regulation in Patients with Coronary Artery Disease
3.3. Mg/Ca and Ischemic Stroke
3.4. Ca/Mg and Cardiac Arrythmias
3.5. Mg/Ca and Chronic Obstructive Pulmonary Disease
3.6. Ca/Mg and Cystic Fibrosis
3.7. Ca/Mg and Risk of Prostate Cancer
3.8. Ca/Mg and Periodontitis
3.9. Ca and Mg and Kidney Stones
3.10. Ca/Mg and Sickle Cell Disease
Magnesium homeostasis is altered in SCD patients, and Mg levels are lower in HbSS patients. Although serum calcium/magnesium ratio is significantly higher in SCD patients compared with healthy controls, there is no significant difference between patients with HbSS and HbSC genotypes. Taken together, the data suggest Mg supplementation may be required in sickle cell patients.(p. 547)
4. Discussion
- Setting 1: Patients with obesity. Patients consuming high-fat diets will excrete both Ca and Mg in urine, reducing the serum levels in ways not considered in scale development [48]. Serum minerals will also be altered if the patient with obesity has diabetes. These factors indicate the need to assess the proposed scale in this special population.
- Setting 2: Patients with renal insufficiency/chronic kidney disease (CKD). Patients with CKD, particularly those in advanced stages and/or using renal replacement therapy, will have serum Ca and Mg levels that have been modified by physiological adjustments that attempt to compensate for mineral imbalances, use of phosphate binders that alter mineral balances, and use of other therapies and therapeutics that alter mineral balances [49,50]. Because of these factors, the scale’s utility may be lower in patients with renal insufficiency/CKD and needs to be specifically studied in this special population.
- Setting 3: Pregnant women and new mothers. Data concerning the changes in serum Ca and Mg during pregnancy and lactation are sparse. Pregnant women and new mothers experience Ca and Mg mobilization from the maternal skeleton throughout pregnancy and during lactation [51,52]. Mineral mobilization may alter Ca/Mg in this special population, requiring specific testing of the reliability of the scale.
- Setting 4: Patients who have both low serum Mg and low serum Ca but sufficient Mg/Ca balance. Under these conditions, the patient’s body may adapt to the lower physiological concentrations of the two minerals. Nonetheless, if the patient is experiencing physiological stress (e.g., rapid growth, recovery from a bone fracture, or pregnancy), supplementation of both minerals may prove beneficial [53]. The scale needs to be specifically tested in these special populations to ensure its reliability.
5. Conclusions
The complex interaction between Ca and Mg at the cellular level necessitates a delicate balance to enhance their effects, and merely having each mineral within its respective normal range fails to capture this dynamic relationship. Ultimately, given the cost-effectiveness and accessibility of serum Ca and Mg measurements in most clinical laboratories along with the Ca: Mg ratio’s value in capturing their combined homeostatic impact, we advocate for incorporating Ca: Mg ratio monitoring into routine clinical practice to support tailored interventions for high risk individuals.(p. 8 of 10)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| Ca | calcium |
| CAD | coronary artery disease |
| CF | cystic fibrosis |
| CI | confidence interval |
| CLMD | chronic latent Mg deficiency |
| COPD | chronic obstructive pulmonary disease |
| CVD | cardiovascular disease |
| ECG | electrocardiography |
| eGFR | estimated glomerular filtration rate |
| FBG | fasting blood glucose |
| HbA1c | glycated hemoglobin |
| HBB | hemoglobin subunit β |
| HbS | hemoglobin allele βS |
| HbSC | hemoglobin SC |
| HbSS | hemoglobin SS |
| HDL | high-density lipoprotein |
| HGI | hemoglobin glycation index |
| HR | hazard ratio |
| hs-CRP | high-sensitivity C-reactive protein |
| iMg | ionized Mg |
| LDL | low-density lipoprotein |
| MetS | metabolic syndrome |
| Mg | magnesium |
| NIHSS | National Institutes of Health Stroke Scale |
| NMHS | Nashville Men’s Health Study |
| OR | odds ratio |
| PIN | prostate intraepithelial neoplasia |
| PMN | polymorphonuclear |
| PTH | parathyroid hormone |
| QBB | Qatar Biobank |
| RCT | randomized controlled (clinical) trial |
| RR | risk ratio |
| SCD | sickle cell disease |
| SHIP-0 | Study of Health in Pomerania (Germany) |
| tCa | total Ca |
| tMg | total Mg |
| TyG | triglyceride-glucose index |
References
- Elin, R.J. Magnesium metabolism in health and disease. Dis. Mon. 1988, 34, 161–218. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Kröse, J.L.; de Baaij, J.H.F. Magnesium biology. Nephrol. Dial. Transplant. 2024, 39, 1965–1975. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Colaneri-Day, S.; Rosanoff, A. Clinical guideline for detection and management of magnesium deficiency in ambulatory care. Nutrients 2025, 17, 887. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Dietary magnesium and chronic disease. Adv. Chronic. Kidney Dis. 2018, 25, 230–235. [Google Scholar] [CrossRef]
- Touyz, R.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Magnesium disorders. N. Engl. J. Med. 2024, 390, 1998–2009. [Google Scholar] [CrossRef]
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM 2018, 111, 759–763. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium status and stress: The vicious circle concept revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef]
- Berkelhammer, C.; Bear, R.A. A clinical approach to common electrolyte problems: 4. Hypomagnesemia. Can. Med. Assoc. J. 1985, 132, 360–368. [Google Scholar] [PubMed]
- Fanelli, S.M.; Jonnalagadda, S.S.; Pisegna, J.L.; Kelly, O.J.; Krok-Schoen, J.L.; Taylor, C.A. Poorer diet quality observed among US adults with a greater number of clinical chronic disease risk factors. J. Prim. Care Community Health 2020, 11, 2150132720945898. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Chen, C.; Lu, L.; Shechter, M.; Rosanoff, A.; He, K. Magnesium levels in relation to rates of preterm birth: A systematic review and meta-analysis of ecological, observational, and interventional studies. Nutr. Rev. 2021, 79, 188–199. [Google Scholar] [CrossRef]
- Rosanoff, A.; Capron, E.; Barak, P.; Mathews, B.; Nielsen, F. Edible plant tissue and soil calcium:magnesium ratios: Data too sparse to assess implications for human health. Crop Pasture Sci. 2015, 66, 1265–1277. [Google Scholar] [CrossRef]
- Thomas, D. A study on the mineral depletion of the foods available to us as a nation over the period 1940 to 1991. Nutr. Health 2003, 17, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Rosanoff, A.; West, C.; Elin, R.J.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The case for an evidence-based reference interval for serum magnesium: The time has come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Drach, G.W. Contribution to therapeutic decisions of ratios, absolute values and other measures of calcium, magnesium, urate or oxalate balance in stone formers. J. Urol. 1976, 116, 338–340. [Google Scholar] [CrossRef]
- Liebscher, D.H.; Liebscher, D.E. About the misdiagnosis of magnesium deficiency. J. Am. Coll. Nutr. 2004, 23, 730s–731s. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status. Clin. Chem. 1987, 33, 1965–1970. [Google Scholar] [CrossRef]
- Rosanoff, A.; von Ehrlich, B.; Nelson, D. A Proposed Scale to Assess Magnesium Status Using Serum Calcium and Magnesium Ratios. Nutrients, 2025; in press. [Google Scholar]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-kidney-metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
- Kisters, S.; Kisters, K.; Werner, T.; Vormann, J.; Tokmak, F.; Westhoff, T.; Gröber, U.; Predel, H.G.; Reuter, H. Positive effects of magnesium supplementation in metabolic syndrome. Int. J. Clin. Pharmacol. Ther. 2024, 62, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Shugaa Addin, N.; Niedermayer, F.; Thorand, B.; Linseisen, J.; Seissler, J.; Peters, A.; Rospleszcz, S. Association of serum magnesium with metabolic syndrome and the role of chronic kidney disease: A population-based cohort study with Mendelian randomization. Diabetes Obes. Metab. 2024, 26, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, R.; Aldulaimi, H.; Hinawi, R.; Al-Sadi, F.; Al-Baker, A.; Alkuwari, A.; Sameer, M.; Al-Abdulla, G.; Shi, Z.; Rathnaiah Babu, G. Association of serum magnesium and calcium with metabolic syndrome: A cross-sectional study from the Qatar-biobank. Nutr. Metab. 2025, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hu, P.; Wang, J.; Lu, N. Serum calcium and magnesium were inversely associated with high sensitivity C-reactive protein in Chinese adults with coronary artery disease. Curr. Med. Res. Opin. 2023, 39, 497–503. [Google Scholar] [CrossRef]
- Moia, M.N.; Lima, S.; da Silva Nunes, F.L.; Queiroz, S.; Marchioni, D.M.L.; Pedrosa, L.F.C.; Barbosa, F., Jr.; de Oliveira Lyra, C.; Sena-Evangelista, K.C.M. Plasma levels of magnesium, calcium, calcium to magnesium ratio, and associations with metabolic syndrome and cardiometabolic risk factors. Biol. Trace Elem. Res. 2024, 202, 5307–5318. [Google Scholar] [CrossRef]
- Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium deficiency and cardiometabolic disease. Nutrients 2023, 15, 2355. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke epidemiology and risk factor management. Continuum (Minneap. Minn) 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Ortiz, J.F.; Ruxmohan, S.; Saxena, A.; Morillo Cox, Á.; Bashir, F.; Tambo, W.; Ghani, M.R.; Moya, G.; Córdova, I. Minocycline and magnesium as neuroprotective agents for ischemic stroke: A systematic review. Cureus 2020, 12, e12339. [Google Scholar] [CrossRef]
- Feng, P.; Niu, X.; Hu, J.; Zhou, M.; Liang, H.; Zhang, Y.; Tong, W.; Xu, T. Relationship of serum magnesium concentration to risk of short-term outcome of acute ischemic stroke. Blood Press 2013, 22, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, L.; Dong, Y.; Xu, J.; Wei, Y.; Yu, D.; Xu, J.; Zhang, W. The effect of magnesium intake on stroke incidence: A systematic review and meta-analysis with trial sequential analysis. Front. Neurol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Vierling, W.; Liebscher, D.H.; Micke, O.; von Ehrlich, B.; Kisters, K. [Magnesium deficiency and therapy in cardiac arrhythmias: Recommendations of the German Society for Magnesium Research]. Dtsch. Med. Wochenschr. 2013, 138, 1165–1171. [Google Scholar] [CrossRef]
- Fairley, J.L.; Zhang, L.; Glassford, N.J.; Bellomo, R. Magnesium status and magnesium therapy in cardiac surgery: A systematic review and meta-analysis focusing on arrhythmia prevention. J. Crit. Care 2017, 42, 69–77. [Google Scholar] [CrossRef]
- Curran, J.; Ross-White, A.; Sibley, S. Magnesium prophylaxis of new-onset atrial fibrillation: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292974. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kong, X.J.; Ji, Y.Y.; Fan, J.; Ji, C.C.; Chen, X.M.; Ma, Y.D.; Tang, A.L.; Cheng, Y.J.; Wu, S.H. Serum electrolyte concentrations and risk of atrial fibrillation: An observational and mendelian randomization study. BMC Genom. 2024, 25, 280. [Google Scholar] [CrossRef]
- Ruljancic, N.; Popovic-Grle, S.; Rumenjak, V.; Sokolic, B.; Malic, A.; Mihanovic, M.; Cepelak, I. COPD: Magnesium in the plasma and polymorphonuclear cells of patients during a stable phase. COPD 2007, 4, 41–47. [Google Scholar] [CrossRef]
- Greer, R.M.; Buntain, H.M.; Potter, J.M.; Wainwright, C.E.; Wong, J.C.; O’Rourke, P.K.; Francis, P.W.; Bell, S.C.; Batch, J.A. Abnormalities of the PTH-vitamin D axis and bone turnover markers in children, adolescents and adults with cystic fibrosis: Comparison with healthy controls. Osteoporos. Int. 2003, 14, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Motley, S.S.; Smith, J.A., Jr.; Concepcion, R.; Barocas, D.; Byerly, S.; Fowke, J.H. Blood magnesium, and the interaction with calcium, on the risk of high-grade prostate cancer. PLoS ONE 2011, 6, e18237. [Google Scholar] [CrossRef]
- Fowke, J.H.; Koyama, T.; Dai, Q.; Zheng, S.L.; Xu, J.; Howard, L.E.; Freedland, S.J. Blood and dietary magnesium levels are not linked with lower prostate cancer risk in black or white men. Cancer Lett. 2019, 449, 99–105. [Google Scholar] [CrossRef]
- Salvi, G.E.; Lawrence, H.P.; Offenbacher, S.; Beck, J.D. Influence of risk factors on the pathogenesis of periodontitis. Periodontol. 2000 1997, 14, 173–201. [Google Scholar] [CrossRef]
- Salvi, G.E.; Carollo-Bittel, B.; Lang, N.P. Effects of diabetes mellitus on periodontal and peri-implant conditions: Update on associations and risks. J. Clin. Periodontol. 2008, 35, 398–409. [Google Scholar] [CrossRef]
- Meisel, P.; Pink, C.; Nauck, M.; Jablonowski, L.; Voelzke, H.; Kocher, T. Magnesium/calcium ratio in serum predicts periodontitis and tooth loss in a 5-year follow-up. JDR Clin. Trans. Res. 2016, 1, 266–274. [Google Scholar] [CrossRef]
- Oreopoulos, D.G.; Soyannwo, M.A.; McGeown, M.G. Magnesium-calcium ratio in urine of patients with renal stones. Lancet 1968, 2, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Boasiako, C.; Kusi-Mensah, Y.A.; Hayfron-Benjamin, C.; Aryee, R.; Dankwah, G.B.; Kwawukume, L.A.; Darkwa, E.O. Total serum magnesium levels and calcium-to-magnesium ratio in sickle cell disease. Medicina 2019, 55, 547. [Google Scholar] [CrossRef]
- Bradley, M.; Melchor, J.; Carr, R.; Karjoo, S. Obesity and malnutrition in children and adults: A clinical review. Obes. Pillars 2023, 8, 100087. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.; Abdel-Rahman, E.M.; Boykin, H.; Song, M.K. A narrative review of management strategies for common symptoms in advanced CKD. Kidney Int. Rep. 2021, 6, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.S.; Kaple, M.; Hingway, S. A brief review of diagnostic techniques and clinical management in chronic kidney disease. Cureus 2023, 15, e49030. [Google Scholar] [CrossRef]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.C. Minerals in pregnancy and their impact on child growth and development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed]
- Seelig, M.S. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review). J. Am. Coll. Nutr. 1994, 13, 429–446. [Google Scholar] [CrossRef] [PubMed]
|
|
| Values in mg/dL | Calculation: | |
| Mg Status | Serum Mg/Ca Weight Ratio | Serum Ca/Mg Weight Ratio |
| Adequate | ≥0.24 | ≤4.17 |
| Mild Mg depletion | 0.218–0.24 | 4.17–4.59 |
| Moderate Mg depletion | <0.218 | >4.59 |
| Serious Mg depletion | ≤0.18 | ≥5.55 |
| Values in mmol/L or mEq/L | Calculation: | |
| Mg status | Serum Mg/Ca | Serum Ca/Mg |
| Adequate | ≥0.4 | ≤2.5 |
| Mild Mg depletion | 0.36–0.4 | 2.5–2.78 |
| Moderate Mg depletion | <0.36 | >2.78 |
| Serious Mg depletion | ≤0.3 | ≥3.33 |
| MetS (n = 7724) | Non-MetS (n = 1929) | |
|---|---|---|
| Mean serum Mg, mmol/L * | 0.81 ± 0.08 | 0.83 ± 0.06 |
| Mg status using the typical serum Mg reference range | Adequate | Adequate |
| Mean serum Ca, mmol/L | 2.33 ± 0.09 | 2.30 ± 0.08 |
| Mean Ca/Mg molar ratio | 2.92 ± 0.36 | 2.77 ± 0.23 |
| Mg status using the novel serum Mg/Ca–Ca/Mg scale | Moderate to serious Mg depletion | Mild Mg depletion |
| Recommendation based on the novel scale | Increased Mg likely to reduce both acute and long-term risks of MetS | Increased Mg likely to reduce long-term risks of MetS |
| Quartile | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Mean serum Mg, mmol/L * | 0.77 ± 0.04 | 0.83 ± 0.01 | 0.88 ± 0.01 | 0.97 ± 0.12 |
| Mg status using the typical serum Mg reference range | Adequate | Adequate | Adequate | Adequate |
| Mean serum Ca, mmol/L | 2.14 ± 0.08 | 2.24 ± 0.02 | 2.31 ± 0.02 | 2.41 ± 0.02 |
| Mean Mg/Ca molar ratio | 0.33 ± 0.02 | 0.37 ± 0.01 | 0.39 ± 0.01 | 0.44 ± 0.07 |
| Mg status using the novel serum Mg/Ca–Ca/Mg scale | Moderate to serious Mg depletion | Mild Mg depletion | Almost adequate | Adequate |
| Recommendation based on the novel scale | Increased Mg likely to reduce both acute and long-term risks of CAD | Increased Mg likely to reduce long-term risks of CAD | Increased Mg may prevent development of Mg deficiency | No changes to Mg intake recommended |
| Quartile | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Serum Mg, mmol/L | <0.83 | 0.83–0.88 | 0.89–0.97 | >0.98 |
| Mg status using the typical reference range | Adequate | Adequate | Adequate | Adequate |
| Serum Ca, mmol/L * | 2.3 (2.2–2.4) | 2.3 (2.2–2.4) | 2.3 (2.2–2.4) | 2.3 (2.2–2.4) |
| Mean Mg/Ca molar ratio | 0.36 | 0.37 | 0.40 | 0.43 |
| Mg status using the novel serum Mg/Ca–Ca/Mg scale | Mild Mg depletion | Mild Mg depletion | Adequate | Adequate |
| Recommendation based on the novel scale | Increased Mg likely to reduce long-term risks of ischemic stroke | Increased Mg may prevent development of Mg deficiency | No changes recommended | No changes recommended |
| Clinical diagnosis using Wu et al. [38] data and a reference range for serum Mg (0.7–1.1 mmol/L [1.7–2.67 mg/dL]) | ||||||
| Electrolyte | Percentile | Range, mg/dL | p Value of the Hazard Ratio | Clinical Diagnosis 1 | ||
| Unadjusted | Adjusted Model 1 | Adjusted Model 2 | ||||
| Mg | <5th | 0.50–1.39 | <0.001 | <0.001 | <0.001 | Moderate to serious Mg deficiency; supplemental Mg indicated |
| 5 to <20th | 1.40–1.49 | 0.17 | <0.001 | 0.004 | ||
| 20 to <40th | 1.50–1.59 | 0.93 | 0.90 | 0.80 | ||
| 40 to <60th | 1.60–1.69 | 1.00 | 1.00 | 1.00 | Mg sufficient | |
| 60 to <80th | 1.70–1.79 | 0.96 | 0.31 | 0.59 | ||
| 80 to <95th | 1.80–1.89 | 0.09 | 0.01 | 0.11 | ||
| >95th | 1.90–3.10 * | 0.90 | 0.64 | 0.78 | ||
| Clinical diagnosis based on use of the reference range (Diagnosis 1) or the novel serum Mg/Ca–Ca/Mg scale (Diagnosis 2) | ||||||
| Mineral | Percentile | Mg, mg/dL (mmol/L) | Clinical Diagnosis 1 | Ca/Mg Molar Ratio | Clinical Diagnosis 2 | |
| Range | Average | |||||
| Mg | <5th | 0.50–1.39 | 0.95 (0.4) | Mg deficient; supplemental Mg indicated | 6.18 | Serious Mg deficiency; supplemental Mg indicated |
| 5 to <20th | 1.40–1.49 | 1.45 (0.60) | 4.12 | |||
| 20 to <40th | 1.50–1.59 | 1.55 (0.64) | 3.86 | |||
| 40 to <60th | 1.60–1.69 | 1.65 (0.70) | Mg in range; supplemental Mg not supported | 3.53 | Mg deficient; supplemental Mg indicated | |
| 60 to <80th | 1.70–1.79 | 1.75 (0.72) | 3.43 | |||
| 80 to <95th | 1.80–1.89 | 1.85 (0.76) | 3.25 | Moderate Mg depletion; supplemental Mg indicated | ||
| >95th | 1.90–3.10 * | 2.50 (1.04) | 2.38 | Mg sufficient | ||
| Ca | 9.89 (2.47) | — | ||||
| Patients with COPD | Healthy Smokers | Healthy Non-Smokers | |
|---|---|---|---|
| Serum Mg, mmol/L * | 0.85 (0.83) [0.57–1.03] | 0.85 (0.87) [0.75–1.07] | 0.88 (0.86) [0.64–1.03] |
| Serum Ca, mmol/L * | 2.42 (2.42) [2.20–2.64] | 2.28 (2.31) [2.10–2.50] | 2.31 (2.36) [2.15–3.32] |
| Mean Ca/Mg molar ratio * | 2.89 (2.91) [2.15–3.86] | 2.58 (2.67) [2.26–3.24] | 2.65 (2.70) [2.19–3.44] |
| Mg status using the typical serum Mg reference range | Adequate | Adequate | Adequate |
| Mg status using the novel serum Mg/Ca–Ca/Mg scale | Moderate to serious Mg depletion | Nearly adequate | Nearly adequate |
| Recommendation based on the novel scale | Increased Mg likely to reduce both acute and long-term risks of COPD | Increased Mg mainly preventive and likely to reduce long-term risks of COPD | Increased Mg mainly preventive and likely to reduce long-term risks of COPD |
| Control Subjects (n = 142) | Subjects with CF (n = 149) | |||
|---|---|---|---|---|
| Adolescents (n = 92) | Adults (n = 50) | Adolescents (n = 87) | Adults (n = 62) | |
| Mean serum Mg, mmol/L | 0.86 ± 0.05 | 0.84 ± 0.06 | 0.80 ± 0.08 | 0.73 ± 0.08 |
| Mg status using the typical serum Mg reference range | Adequate | Adequate | Adequate | Adequate |
| Mean serum Ca, mmol/L * | 2.42 ± 0.09 | 2.38 ± 0.08 | 2.43 ± 0.07 | 2.34 ± 0.09 |
| Mean Ca/Mg molar ratio | 2.81 | 2.83 | 3.04 | 3.21 |
| Mg status using the novel serum Mg/Ca–Ca/Mg scale | Moderate Mg depletion | Moderate Mg depletion | Moderate to serious Mg depletion | Near serious Mg depletion |
| Recommendation based on the novel scale | Increased Mg likely to reduce both acute and long-term risks of CF | Increased Mg likely to reduce both acute and long-term risks of CF | Increased Mg likely to reduce both acute and long-term risks of CF | Increased Mg likely to reduce both acute and long-term risks of CF |
| Data from Dai et al. Study [41] | |||||
| Group | n | Mean Values * | Ca/Mg, by Weight | Mg Status Using the Scale | |
| Mg (ng/mL) | Ca (ng/mL) | ||||
| Age adjusted | |||||
| Control | 163 | 2.16 | 9.70 | 4.52 | Mg adequate to mild depletion Mild to moderate depletion |
| Prostate intraepithelial neoplasia (PIN) | 133 | 2.18 | 9.73 | 4.52 | |
| Low-grade cancer | 99 | 2.14 | 9.66 | 4.57 | |
| High-grade cancer | 98 | 2.09 ^ | 9.82 ^^ | 4.78 | Moderate Mg depletion |
| Fully adjusted * | |||||
| Control | 163 | 2.09 | 9.81 | 4.75 | Moderate Mg depletion |
| PIN | 133 | 2.09 | 9.87 | 4.79 | |
| Low-grade cancer | 99 | 2.07 | 9.76 | 4.78 | |
| High-grade cancer | 98 | 2.03 ** | 9.91 | 4.96 *** | Moderate to serious Mg depletion |
| Data from Fowke et al. Study [42] | |||||
| Race | |||||
| Black | 1322 | 2.3 | 9.8 | 4.3 | Mild Mg depletion to adequate |
| White | 2.4 | 9.7 | 4.1 | ||
| Quartile | |||
|---|---|---|---|
| Q1 | Q2–Q3 | Q4 | |
| Mean serum Mg, mmol/L * | 0.70 ± 0.04 | 0.77 ± 0.04 | 0.88 ± 0.09 |
| Mg status using the typical serum Mg reference range | Nearly adequate | Adequate | Adequate |
| Mean serum Ca, mmol/L * | 2.48 ± 0.10 | 2. 41 ± 0.10 | 2.34 ± 0.11 |
| Mean Mg/Ca molar ratio * | 0.28 ± 0.01 | 0.32 ± 0.01 | 0.38 ± 0.04 |
| Recommendations based on the novel serum Mg/Ca–Ca/Mg scale | Serious Mg depletion; increased Mg warranted | Moderate to serious Mg depletion; increased Mg warranted | Nearly adequate Mg status; maintain with diet and supplements, if needed |
| Normal Range | Patient Value | |
|---|---|---|
| Mean serum Mg, mEq/L | 2.0 | 1.76 |
| Mg status using the typical serum Mg reference range | Adequate | Adequate |
| Mean serum Ca, mg/dL | 9.55 | 9.81 |
| Mean Mg/Ca weight ratio | 0.25 | 0.217 |
| Recommendation based on the novel serum Mg/Ca–Ca/Mg scale | Adequate | Moderate Mg depletion; increased Mg likely to reduce both acute and long-term risks of stones |
| Electrolyte | Healthy Subjects (n = 48) | Patients with Sickle Cell Disease (SCD) (n = 120) | Clinical Diagnosis Using Serum Mg Reference Range | Clinical Diagnosis Using Novel Scale | |
| Mean Mg, mmol/L * | 0.90 ± 0.11 | 0.80 ± 0.24 | Both groups Mg sufficient | Healthy subjects: Mg adequate Patients with SCD: mild to moderate Mg deficit; initiate or continue Mg supplementation | |
| Mean Ca, mmol/L | 2.28 ± 0.53 | 2.11 ± 0.38 | |||
| Mean Ca/Mg molar ratio | 2.54 ± 0.89 | 2.80 ± 0.72 | |||
| Electrolyte | Healthy subjects (n = 48) | Patients with SCD (by subtype) | Clinical diagnosis using serum Mg reference range | Clinical diagnosis using novel scale | |
| HbSS (n = 79) | HbSC (n = 41) | ||||
| Mean Mg, mmol/L | 0.90 ± 0.11 | 0.79 ± 0.25 | 0.82 ± 0.21 | All 3 groups Mg sufficient | Patients with SCD: mild to moderate Mg deficit; initiate or continue Mg supplementation |
| Mean Ca, mmol/L | 2.28 ± 0.53 | 2.07 ± 0.39 | 2.17 ± 0.36 | ||
| Mean Ca/Mg molar ratio | 2.54 ± 0.89 | 2.79 ± 0.71 | 2.82 ± 0.76 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, D.J.; Rosanoff, A.; von Ehrlich, B. Novel Scale for Clinical Identification of Adverse Magnesium/Calcium Imbalances: Applications and Perspectives. Nutrients 2025, 17, 3662. https://doi.org/10.3390/nu17233662
Nelson DJ, Rosanoff A, von Ehrlich B. Novel Scale for Clinical Identification of Adverse Magnesium/Calcium Imbalances: Applications and Perspectives. Nutrients. 2025; 17(23):3662. https://doi.org/10.3390/nu17233662
Chicago/Turabian StyleNelson, Deanna J., Andrea Rosanoff, and Bodo von Ehrlich. 2025. "Novel Scale for Clinical Identification of Adverse Magnesium/Calcium Imbalances: Applications and Perspectives" Nutrients 17, no. 23: 3662. https://doi.org/10.3390/nu17233662
APA StyleNelson, D. J., Rosanoff, A., & von Ehrlich, B. (2025). Novel Scale for Clinical Identification of Adverse Magnesium/Calcium Imbalances: Applications and Perspectives. Nutrients, 17(23), 3662. https://doi.org/10.3390/nu17233662






